Abstract
Purpose
Respiratory-induced motion of oesophageal tumours and lymph nodes can influence positron-emission tomography/computed tomography (PET/CT). The aim was to compare standard three-dimensional (3D) and motion-compensated PET/CT regarding standardized uptake value (SUV), metabolic tumour volume (MTV) and detection of lymph node metastases.
Methods
This prospective observational study (NCT02424864) included 37 newly diagnosed oesophageal cancer patients. Diagnostic PET/CT was reconstructed in 3D and motion-compensated PET/CT. MTVs of the primary tumour were calculated using an automated region-growing algorithm with SUV thresholds of 2.5 (MTV2.5) and ≥ 50% of SUVmax (MTV50%). Blinded for reconstruction method, a nuclear medicine physician assessed all lymph nodes showing 18F‑fluorodeoxyglucose uptake for their degree of suspicion.
Results
The mean (95% CI) SUVmax of the primary tumour was 13.1 (10.6–15.5) versus 13.0 (10.4–15.6) for 3D and motion-compensated PET/CT, respectively. MTVs were also similar between the two techniques. Bland–Altman analysis showed mean differences between both measurements (95% limits of agreement) of 0.08 (−3.60–3.75), −0.26 (−2.34–1.82), 4.66 (−29.61–38.92) cm3 and −0.95 (−19.9–18.0) cm3 for tumour SUVmax, lymph node SUVmax, MTV2.5 and MTV50%, respectively. Lymph nodes were classified as highly suspicious (30/34 nodes), suspicious (20/22) and dubious (66/59) for metastases on 3D/motion-compensated PET/CT. No additional lymph node metastases were found on motion-compensated PET/CT. SUVmax of the most intense lymph nodes was similar for both scans: mean (95% CI) 6.6 (4.3–8.8) and 6.8 (4.5–9.1) for 3D and motion-compensated, respectively.
Conclusion
SUVmax of the primary oesophageal tumour and lymph nodes was comparable on 3D and motion-compensated PET/CT. The use of motion-compensated PET/CT did not improve lymph node detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most patients with oesophageal cancer present with locally advanced disease or with distant metastases [1]. 18F‑fluorodeoxyglucose (FDG) positron-emission tomography with computed tomography (PET/CT) has an important role in staging oesophageal cancer patients and therefore in selecting patients for potentially curative treatment. In the Netherlands, potentially curative treatment consists of neoadjuvant chemoradiotherapy followed by surgery for operable patients and definitive chemoradiotherapy for inoperable patients or patients with irresectable tumours [2, 3].
PET/CT is increasingly incorporated in radiation treatment preparation. Fusion of PET images with those of the planning CT scan facilitates demarcation of the oesophageal gross tumour volume (GTV) and helps to identify regional lymph node metastases that should be included in the radiation volume [4,5,6,7]. To improve delineation accuracy, automatic threshold-based delineation has been suggested [8]. Using a threshold with a certain standardized uptake value (SUV) or a predefined percentage of the maximum (SUVmax) has been described [6, 8]. The influence of motion-compensated PET/CT techniques on these PET tumour segmentation volumes is unknown.
Furthermore, detecting all lymph node metastases before the start of treatment is of crucial importance, to increase chances of treatment success. Combined PET/CT can reveal lymph node metastases, but its sensitivity is low (approximately 51%) and its specificity is moderate (84%) [9]. Possible explanations for this suboptimal performance are 1) a small proportion (0–32%) of oesophageal tumours are non-FDG-avid [5], 2) small lymph nodes can give a metabolic signal that remains below the detection level and 3) respiratory motion can cause image blurring of the lesions, which can fade out the signal of the lymph node metastases. The technology to reduce the effects of tumour motion induced by breathing was first studied in lung cancer [10,11,12,13]. The four-dimensional (4D) PET/CT can be reconstructed in a time-averaged motion-compensated (MC) PET/CT. Compared to 3D PET/CT, image blurring was significantly reduced, resulting in better characterization of lung lesions and lymph nodes. Also for liver lesions, improved diagnostic accuracy was seen with the motion-compensated PET/CT technique [14, 15].
Like lung tumours and liver metastases, oesophageal tumours and mediastinal lymph nodes are subject to substantial respiratory-induced motion [16, 17]. Furthermore, lymph node metastases of tumours at the distal oesophagus or gastroesophageal junction are expected to be located around the distal oesophagus or celiac trunk where a larger motion is expected than at the upper mediastinum [17]. As a result of the motion-induced image blurring, we anticipate that quantitative measurements are influenced. The metabolic signal spreads during image acquisition and, consequently, we hypothesize that SUVmax will be higher and metabolic tumour volume (MTV) more confined in images acquired on a motion-compensated PET/CT. As yet, the role of motion-compensated PET/CT in oesophageal cancer is not yet defined and the influence on lymph node assessment has not been studied. The aims of this prospective study were to compare 3D and motion-compensated PET/CT for measurement of the SUVmax and MTV of the primary tumour and for detection of lymph node metastases in patients with oesophageal cancer.
Methods
Between April 2014 and December 2016, this single-centre prospective cohort study (NCT02424864) included newly diagnosed oesophageal cancer patients undergoing diagnostic workup with PET/CT in the Netherlands Cancer institute. Patients of 18 years or older, with pathologically proven oesophageal cancer or tumour highly suspicious for oesophageal cancer who were planned for FDG PET-CT scan for staging purposes, could be included in the study. Written informed consent was obtained from all patients according to the International Conference of Harmonisation/Good Clinical Practise (ICH/GCP) and national and local regulations. This study was approved by the institute’s medical ethical committee.
FDG PET/CT scans were acquired using a combined PET/CT scanner (Gemini TF/Big Bore; Philips Medical Systems, Cleveland Ohia, USA). A PET with low-dose CT scan from skull base to thighs was acquired 60 ± 10 min after injection of FDG (190–260 MBq) at 2 min per bed position in 3D mode. Two bed positions centred around the oesophageal tumour were acquired in 4D mode at 4 min per bed position. Subsequently, the 4D CT of the thorax and upper abdominal region was acquired and reconstructed with a slice thickness of 3 mm. The reconstruction voxel size of the PET data was 4 × 4 × 4 mm, while the voxel size of the CT data was 1 × 1 × 3 mm. Motion of the tumour was determined by assessing the displacement during the breathing cycle in 10 phases in left–right (LR), craniocaudal (CC) and anteroposterior (AP) directions with in-house developed software. The 10 breathing phases were used to reconstruct a time-averaged mid position CT and attenuation-corrected motion-compensated PET scan; in addition, the 3D CT scan was used to reconstruct the 3D attenuation-corrected PET scan [11]. Details on the 4D PET/CT acquisition and reconstruction are provided in the supplementary material.
The 3D and motion-compensated PET/CT reconstructions were pseudonymised. Observers (EV, FV) were blinded for technique. The nuclear medicine physician (EV) assessed the quality of the scan on a five-point scale (very good, good, acceptable, poor and very poor).
The tumour was manually indicated and a volume of interest (VOI) was subsequently computed around the tumour using an automatic region-growing algorithm based on a minimum SUV threshold using Osirix software (Pixmeo SARL, Geneva, Switzerland). SUVmax was measured. Automatic segmentations with an SUV threshold of 2.5 and a threshold of 50% of the SUVmax were generated [6, 18]. When these segmentations extended into other FDG-avid organs (e.g. heart or liver) they were manually corrected using the anatomical boundaries on CT. Subsequently, of these segmented volumes, the SUVmean, metabolic tumour volume (MTV) and craniocaudal extension were measured and compared between 3D and motion-compensated reconstructions. Craniocaudal tumour length on PET/CT was also compared with the endoscopic tumour length.
An experienced gastrointestinal nuclear medicine physician (EV) scored the degree of suspicion of the lymph nodes on a four-point scale (highly suspicious, suspicious, dubious or unlikely) based on the intensity of the metabolic signal. The two most suspicious lymph nodes were localized and SUVmax per node was measured. The numbers and locations of highly suspicious, suspicious, dubious or unlikely nodes were compared between 3D and motion-compensated PET/CT.
Measurements between the two groups (3D versus motion-compensated scans) were compared using a paired Student’s t‑test, Wilcoxon signed-rank test and Bland–Altman analysis. Pearson correlation coefficients were calculated. P-values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS (version 24; IBM Corp., Armonk, NY, USA).
Results
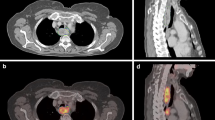
Thirty-nine patients were prospectively recruited for this study. In 2 patients, there was technical failure during 4D reconstruction, resulting in a total of 37 patients available for analysis. The patient characteristics are shown in Table 1. Based on all staging procedures combined, most patients had a clinically node-positive adenocarcinoma located in the distal oesophagus or gastroesophageal junction. Figure 1 shows images of a patient with a node-positive gastroesophageal junction tumour. After staging, patients were treated with neoadjuvant chemoradiotherapy (n = 23), definitive chemoradiotherapy (n = 9), neoadjuvant chemotherapy (n = 1) or palliative treatment (n = 4).
Motion-compensated (MC) and three-dimensional (3D) positron-emission tomography/computed tomography (PET/CT) of a patient with a gastroesophageal junction tumour. Fused PET/CT (a,d,g,j) and PET only (b,e,h,k) transversal slices, and maximum intensity projection (c,f,i,l) images of the primary tumour are shown in the upper panels (a–f), and a highly suspicious lymph node at the celiac trunk is shown in the lower panels (g–l). The primary tumour showed a maximum standardized uptake value (SUVmax) of 12.0 on 3D PET/CT and 14.7 on MC PET/CT. The pathological lymph node showed a SUVmax of 6.6 on 3D PET/CT versus 7.6 on MC PET/CT
Scan quality was rated as good in 65 and 68% of the 3D and motion-compensated PET/CT scans, respectively. Motion amplitude of the oesophageal tumour was largest in the CC direction and CC motion varied between 0 and 21 mm with a median of 6.0 mm. In 6 patients the CC motion amplitude was > 10 mm. There was no correlation between CC amplitude and 3D/motion-compensated differences in SUVmax (r = −0.18, p = 0.29), MTV2.5 (r = 0.05, p = 0.78) or MTV50% (r = −0.01, p = 0.94; see also Fig. 2).
Difference in maximum standardized uptake value (SUVmax) of the tumour between three-dimensional (3D) and motion-compensated (MC), plotted as a function of peak-to-peak craniocaudal amplitude of the tumour. The horizontal dashed line represents difference = 0. The vertical dashed line represents craniocaudal amplitude = 1.0 cm. (CT computed tomography)
The results of the quantitative analyses are shown in Table 2 and Fig. 3. In 60% of patients the automatically segmented MTVs based on SUV ≥ 2.5 (MTV2.5) had to be edited manually, because they extended into surrounding organs (such as heart, kidneys or liver). The extension of the volume in the oesophagus or stomach was not edited, because tumour extension and oesophagitis or gastritis could not be distinguished.
Bland–Altman plot of maximum standardized uptake value (SUVmax) of the tumour (a), SUVmax of the most intense lymph node (b), MTV2.5 (c) and MTV50% (d) measurements on three-dimensional (3D) positron-emission tomography/computed tomography (PET/CT) and motion-compensated (MC) PET/CT. The x‑axis shows the mean of the measurement on 3D PET/CT and motion-compensated PET/CT and the y‑axis shows the difference between the measurements. The dotted lines show the mean differences (3D – MC) between both methods. The solid lines show the upper and lower 95% limits of agreement. (MTV2.5 metabolic tumour volume with SUV threshold of 2.5, MTV50% metabolic tumour volume with threshold 50% of SUVmax)
The primary oesophageal tumour showed a mean (95% CI) SUVmax of 13.1 (10.6–15.5) on 3D versus 13.0 (10.4–15.6) on motion-compensated PET/CT (p = 0.809). The most intense lymph node showed a mean (95% CI) SUVmax of 6.6 (4.3–8.8) on 3D versus 6.8 (4.6–9.1) on motion-compensated PET/CT (p = 0.176). MTV2.5s were not significantly different between 3D and motion-compensated PET/CT. Also, MTVs based on SUVmax ≥ 50% (MTV50%) were similar on both scans (p = 0.554). The Bland–Altman analysis showed mean differences between 3D and motion-compensated measurement (and corresponding 95% limits of agreement) of 0.08 (−3.60–3.75), −0.26 (−2.34–1.82), 4.66 (−29.61–38.92) cm3 and −0.95 (−19.9–18.0) cm3 for tumour SUVmax, lymph node SUVmax, MTV2.5, and MTV50%, respectively (Fig. 3). Percentage differences of 3D and motion-compensated MTV50% showed an interquartile range (IQR) of 92–109%. The two outliers (Figs. 3d and 4 and Supplementary Fig. 1D) were patients with a relatively low SUVmax (≤8) of the primary tumour, resulting in differences in MTV50% between −81% and +160% on the 3D and 4D scans.
Image of a patient with a distal oesophageal tumour with motion-compensated (MC) and three-dimensional (3D) reconstruction. The primary oesophageal tumour showed a maximum standardized uptake value (SUVmax) of 8.0 on MC positron-emission tomography/computed tomography (PET/CT) and 6.9 on 3D PET/CT. The corresponding metabolic tumour volume (green volume) of ≥ 50% SUVmax was 5.89 cm3 on MC PET/CT versus 12.38 cm3 on 3D PET/CT. This was probably caused by extension of the volume of interest (VOI) to an area of oesophagitis due to the lower SUVmax on 3D PET/CT
The median craniocaudal length of the MTV2.5 was 94 mm (range 44–204 mm) on 3D PET/CT and 88 mm (range 56–200 mm) on motion-compensated PET/CT (p = 0.336). The median craniocaudal length of the MTV50% was 44 mm (range 12–120 mm) on 3D and also 44 mm (12–124 mm) on motion-compensated PET/CT (p = 0.645). A comparison of the length of the tumour on endoscopy and the length of the MTVs is presented in Fig. 5. The MTV-based tumour lengths showed poor correlation with the endoscopic tumour length, endoscopic length with MTV2.5 lengths (3D: r = 0.12, p = 0.48, MC: r = 0.07, p = 0.69) and endoscopic length with MTV50% (3D: r = 0.15, p = 0.39, MC: r = 0.07, p = 0.69).
Tumour length (mm) in craniocaudal direction as measured during endoscopy (black circles), length of MTV2.5 (grey squares) and length of MTV50% (black crosses). Patients are ordered by endoscopic tumour length. (3D three-dimensional, SUVmax maximum standardized uptake value, MTV2.5 metabolic tumour volume with SUV threshold of 2.5, MTV50% metabolic tumour volume with threshold 50% of SUVmax)
The degree of suspicion for lymph node metastases based on the 3D and motion-compensated method was classified as follows: 30 and 34 nodes highly suspicious, 20 and 22 suspicious and 66 and 59 dubious, respectively. A more detailed analysis was performed in a subgroup of patients in whom there was a discrepancy in grade of suspicion that would have a clinical impact. In 8/37 (22%) patients, lymph nodes were scored as unlikely to contain metastases on 3D while scored as dubious or suspicious on motion-compensated PET/CT or vice versa. These nodes were correlated with the assessment at endoscopic ultrasound (EUS) and pathology if the nodes were investigated with EUS-guided fine-needle aspiration (EUS-FNA). In 7 of 8 discrepant cases, the nodes defined at motion-compensated PET/CT as “unlikely” nodes were negative at EUS/pathology and “dubious” or “suspicious” nodes were positive at EUS/pathology, while this was conversely so for 3D PET/CT. In one patient the dubious node at motion-compensated PET/CT was suspicious at EUS, but FNA was not representative. These cases with discrepancies were reassessed on both scans. Direct comparison of the 3D with the motion-compensated PET/CT showed no clear differences in the intensity or clarity of these discrepant nodes. If those nodes were to be reclassified, they would be scored with an equal degree of suspicion on 3D and motion-compensated PET/CT.
Discussion
In this study, motion-compensated PET/CT revealed no clear clinical benefit compared to 3D PET/CT imaging in oesophageal cancer patients. SUVmax of the primary tumour and lymph nodes was similar between 3D and motion-compensated PET/CT. The MTV2.5 and MTV50% were also similar on both scans for the complete group. In a subgroup of patients (SUVmax of the primary tumour of ≤ 8), large differences of the MTV50% were observed between 3D and motion-compensated PET/CT, probably caused by uptake around a SUV of 4 in inflammatory tissue surrounding the tumour, with the result that minor differences in SUVmax could show a large impact on the MTV50%. In addition, threshold-based and endoscopic-based tumour lengths were poorly correlated. Furthermore, the motion-compensated technique did not improve the detection rate of lymph node metastases.
Prior studies on 4D PET/CT in oesophageal cancer focused on the primary tumour volumes only. Guo et al. evaluated the clinical target volumes (CTV) and planning target volumes (PTV) based on 3D CT, 4D CT and 3D PET/CT [19]. Volumes generated with 3D PET with 4D CT were significantly larger than those generated with 3D CT or 4D CT. Direct comparison of 3D PET/CT with 4D PET/CT was, however, not performed. Wang et al. determined GTV volumes on the average 4D PET/CT with auto-contouring methods using eight different thresholds and compared these PET-derived GTVs with a CT-derived GTVs [20]. SUV ≥ 2.5 and SUV ≥ 20% volumes correlated best with tumour length on CT. However, 4D PET/CT was not compared with 3D PET/CT. Scarsbrook et al. compared PTVs on 3D PET/4D CT with 4D PET/CT in 15 oesophageal cancer patients [21]. Volumes on both scans were similar, but overlap analysis demonstrated a median Dice similarity coefficient of 0.88 between both scans, leading to chance of under-coverage. However, the lack of pathology confirmation in this study obviates the clinical interpretation when this part of the volume is excluded from the PTV.
To our knowledge, the presented study is the first study in which the influence of motion-compensated PET/CT on the detection rate of pathological lymph nodes in oesophageal cancer was studied. Unfortunately, motion compensation did not improve the lymph node detection rate. PET/CT is superior to CT in the detection of distant metastases. However, for the identification of regional lymph node metastases, the sensitivity of PET/CT is insufficient [9, 22]. Moreover, the location of suspicious lymph nodes may influence radiation target volumes and/or choice of surgical approach. Suspicious nodes are included in the radiation target volumes [4], but larger radiation volumes increase the chance of toxicity [23, 24]. Postoperative complications increase with extended mediastinal lymph node dissection; a possible long-term benefit should thus be carefully weighed against the risk of a more complicated postoperative course [25]. Preferably, suspicious nodes are cytologically confirmed by EUS-FNA [26].
In this study, the additional value of motion-compensated PET/CT for the metabolic characterisation of oesophageal cancer was limited. There are several possible explanations for this finding. Firstly, the amplitude of the tumour motion was relatively small; on average 6.0 mm in craniocaudal direction. Zhao et al. described an average oesophageal tumour motion of 8.7 mm in craniocaudal direction using 4D CT [16]. The modest motion amplitude in our study might be explained by the 1 h resting phase prior to PET/CT acquisition, which is not applicable in the abovementioned CT studies, resulting in more relaxed patients with a more superficial breathing pattern. Kruis et al. investigated the influence of motion amplitude on 4D PET/CT in lung tumours and liver metastases [11]. For targets with a CC motion of less than 5 mm the benefit of motion-compensated PET/CT was limited, while a clear effect was seen with amplitudes of more than 10 mm. This effect was not observed in our study, possibly since only 6 patients had a peak-to-peak amplitude of > 10 mm. Furthermore, a limitation of the study is the spatial resolution. The full-width half maximum was 4.9 mm. Consequently, detection of differences with amplitudes of ≤ 10 mm is expected to be difficult. Conversely, Kruis et al. showed that for objects with a small volume, e.g. lymph nodes, motion-compensated PET/CT can improve the distinctiveness even with small motion amplitudes [11].
Secondly, the identification of the borders of the oesophageal tumour on PET/CT imaging may also be difficult. Oesophageal tumours frequently show submucosal spread along the oesophagus and proximal stomach, which might complicate demarcation of the tumour boundaries [27]. Also, oesophagitis and gastritis can give an increased metabolic signal and are often seen in oesophageal cancer patients, limiting differentiation of tumour from inflammation. In patients with tumours with relatively low FDG avidity, this can be even more difficult. These factors hamper demarcation of the tumour, even if the blurring due to the respiratory signal has been compensated for. In this study, the craniocaudal extension of MTVs showed large differences with the tumour length as determined during endoscopy.
And lastly, the accuracy of threshold-based delineation is not only influenced by object motion, but also by other factors, such as metabolic activity of the tumour, homogeneity of uptake, voxel size and signal-to-noise ratio [28]. In this study, we only investigated the influence of object motion.
The threshold-based volumes in this study showed low agreement with tumour length on endoscopy. Currently, semi-quantitative PET analyses are increasingly investigated in oesophageal cancer [29, 30]. Threshold-based volumes are proposed for delineation purposes and SUVmax or metabolic changes are proposed for response assessment or as prognostic factors. However, current segmentation methods show shortcomings [28]. Advanced image segmentation algorithms are emerging that can cope with such challenges [31]. Promising results have been shown with algorithms (e.g. voxel-specific threshold method) for delineation that can cope with tumour heterogeneity by using tumour reference value and a background value instead of a simple SUV threshold [32]. Also, different methodologies for segmentation or combined thresholds can contribute to the robustness of semi-quantitative volumes. However, most of these new segmentation methods have not yet been validated and the most reliable and robust delineation method remains to be found.
The limitations of our study are the relatively low motion amplitude of both tumour and lymph nodes during PET/CT acquisition, prohibiting proper analysis of motion-compensated PET/CT in patients with larger amplitude motion. Furthermore, cytological confirmation of lymph node involvement was only available for a limited number of patients and pathological assessment of the actual pretreatment tumour length was not possible due to neoadjuvant chemoradiotherapy or a non-surgical approach. Despite these limitations, the prospective study design with a relatively large patient sample contributes to the currently available literature.
In the future, the impact of PET-based measurements may increase even further. In the prospective dose-escalation phase I/II study of Yu et al., definitive chemoradiotherapy was given with an escalated simultaneous integrated boost to the volume of ≥ 50% SUVmax [33]. Encouraging local control rates and acceptable toxicity were seen. Unfortunately, the PET scan technique and segmentation algorithm were not described in the manuscript. For interpretation and implementation of results of studies using PET-volume guided treatment, uniform segmentation methods and PET technique are necessary.
Conclusion
Metabolic tumour volumes of the primary tumour and the SUVmax of the primary tumour and lymph nodes metastases were similar between 3D and motion-compensated PET/CT in the majority of patients. Motion compensation during PET/CT in oesophageal cancer patients did not improve the detection rate of lymph node metastases. Automatic segmentation should be used with caution, especially in patients with a primary tumour with a relatively low SUVmax.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Gwynne S, Hurt C, Evans M, Holden C, Vout L, Crosby T (2011) Definitive chemoradiation for oesophageal cancer—a standard of care in patients with non-metastatic oesophageal cancer. Clin Oncol 23(3):182–188. https://doi.org/10.1016/j.clon.2010.12.001
Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten KFJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A, group Cs (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16(9):1090–1098. https://doi.org/10.1016/S1470-2045(15)00040-6
Wu AJ, Bosch WR, Chang DT, Hong TS, Jabbour SK, Kleinberg LR, Mamon HJ, Thomas CR Jr., Goodman KA (2015) Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys 92(4):911–920. https://doi.org/10.1016/j.ijrobp.2015.03.030
Muijs CT, Beukema JC, Pruim J, Mul VE, Groen H, Plukker JT, Langendijk JA (2010) A systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancer. Radiother Oncol 97(2):165–171. https://doi.org/10.1016/j.radonc.2010.04.024
Konski A, Doss M, Milestone B, Haluszka O, Hanlon A, Freedman G, Adler L (2005) The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys 61(4):1123–1128. https://doi.org/10.1016/j.ijrobp.2004.07.717
Nowee ME, Voncken FEM, Kotte A, Goense L, van Rossum PSN, van Lier A, Heijmink SW, Aleman BMP, Nijkamp J, Meijer GJ, Lips IM, Dutch National Platform for Radiotherapy of Gastrointestinal Tumours g (2019) Gross tumour delineation on computed tomography and positron emission tomography-computed tomography in oesophageal cancer: a nationwide study. Clin Transl Radiat Oncol 14:33–39. https://doi.org/10.1016/j.ctro.2018.10.003
Zaidi H, El Naqa I (2010) PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur J Nucl Med Mol Imaging 37(11):2165–2187. https://doi.org/10.1007/s00259-010-1423-3
van Westreenen HL, Westerterp M, Bossuyt PM, Pruim J, Sloof GW, van Lanschot JJ, Groen H, Plukker JT (2004) Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 22(18):3805–3812. https://doi.org/10.1200/JCO.2004.01.083
Garcia Vicente AM, Castrejon AS, Leon Martin AA, Garcia BG, Pilkington Woll JP, Munoz AP (2011) Value of 4‑dimensional 18F-FDG PET/CT in the classification of pulmonary lesions. J Nucl Med Technol 39(2):91–99. https://doi.org/10.2967/jnmt.110.082719
Kruis MF, van de Kamer JB, Houweling AC, Sonke JJ, Belderbos JS, van Herk M (2013) PET motion compensation for radiation therapy using a CT-based mid-position motion model: methodology and clinical evaluation. Int J Radiat Oncol Biol Phys 87(2):394–400. https://doi.org/10.1016/j.ijrobp.2013.06.007
Nehmeh SA, Erdi YE, Pan T, Pevsner A, Rosenzweig KE, Yorke E, Mageras GS, Schoder H, Vernon P, Squire O, Mostafavi H, Larson SM, Humm JL (2004) Four-dimensional (4D) PET/CT imaging of the thorax. Med Phys 31(12):3179–3186. https://doi.org/10.1118/1.1809778
Sindoni A, Minutoli F, Pontoriero A, Iati G, Baldari S, Pergolizzi S (2016) Usefulness of four dimensional (4D) PET/CT imaging in the evaluation of thoracic lesions and in radiotherapy planning: review of the literature. Cancer Treat Res 96:78–86. https://doi.org/10.1016/j.lungcan.2016.03.019
Crivellaro C, De Ponti E, Elisei F, Morzenti S, Picchio M, Bettinardi V, Versari A, Fioroni F, Dziuk M, Tkaczewski K, Ahond-Vionnet R, Nodari G, Todde S, Landoni C, Guerra L (2018) Added diagnostic value of respiratory-gated 4D 18F-FDG PET/CT in the detection of liver lesions: a multicenter study. Eur J Nucl Med Mol Imaging 45(1):102–109. https://doi.org/10.1007/s00259-017-3795-0
Kruis MF, van de Kamer JB, Sonke JJ, Jansen EP, van Herk M (2013) Registration accuracy and image quality of time averaged mid-position CT scans for liver SBRT. Radiother Oncol 109(3):404–408. https://doi.org/10.1016/j.radonc.2013.08.047
Zhao KL, Liao Z, Bucci MK, Komaki R, Cox JD, Yu ZH, Zhang L, Mohan R, Dong L (2007) Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother Oncol 84(3):283–289. https://doi.org/10.1016/j.radonc.2007.07.008
Schaake EE, Rossi MM, Buikhuisen WA, Burgers JA, Smit AA, Belderbos JS, Sonke JJ (2014) Differential motion between mediastinal lymph nodes and primary tumor in radically irradiated lung cancer patients. Int J Radiat Oncol Biol Phys 90(4):959–966. https://doi.org/10.1016/j.ijrobp.2014.07.038
Zhong X, Yu J, Zhang B, Mu D, Zhang W, Li D, Han A, Song P, Li H, Yang G, Kong FM, Fu Z (2009) Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 73(1):136–141. https://doi.org/10.1016/j.ijrobp.2008.04.015
Guo YL, Li JB, Shao Q, Li YK, Zhang P (2015) Comparative evaluation of CT-based and PET/4DCT-based planning target volumes in the radiation of primary esophageal cancer. Int J Clin Exp Med 8(11):21516–21524
Wang YC, Hsieh TC, Yu CY, Yen KY, Chen SW, Yang SN, Chien CR, Hsu SM, Pan T, Kao CH, Liang JA (2012) The clinical application of 4D 18F-FDG PET/CT on gross tumor volume delineation for radiotherapy planning in esophageal squamous cell cancer. J Radiat Res 53(4):594–600. https://doi.org/10.1093/jrr/rrs009
Scarsbrook A, Ward G, Murray P, Goody R, Marshall K, McDermott G, Prestwich R, Radhakrishna G (2017) Respiratory-gated (4D) contrast-enhanced FDG PET-CT for radiotherapy planning of lower oesophageal carcinoma: feasibility and impact on planning target volume. BMC Cancer 17(1):671. https://doi.org/10.1186/s12885-017-3659-9
van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD (2008) Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 98(3):547–557. https://doi.org/10.1038/sj.bjc.6604200
Koeter M, Kathiravetpillai N, Gooszen JA, van Berge Henegouwen MI, Gisbertz SS, van der Sangen MJ, Luyer MD, Nieuwenhuijzen GA, Hulshof MC (2017) Influence of the extent and dose of radiation on complications after neoadjuvant chemoradiation and subsequent esophagectomy with gastric tube reconstruction with a cervical anastomosis. Int J Radiat Oncol Biol Phys 97(4):813–821. https://doi.org/10.1016/j.ijrobp.2016.11.054
Wang SL, Liao Z, Vaporciyan AA, Tucker SL, Liu H, Wei X, Swisher S, Ajani JA, Cox JD, Komaki R (2006) Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 64(3):692–699. https://doi.org/10.1016/j.ijrobp.2005.08.002
Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ (2002) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347(21):1662–1669. https://doi.org/10.1056/NEJMoa022343
Kappelle WFW, Van Leerdam ME, Schwartz MP, Bulbul M, Buikhuisen WA, Brink MA, Sie-Go D, Pullens HJM, Nikolakopoulos S, Van Diest PJ, Leenders M, Moons LMG, Bogte A, Siersema PD, Vleggaar FP (2018) Rapid on-site evaluation during endoscopic ultrasound-guided fine-needle aspiration of lymph nodes does not increase diagnostic yield: a randomized, multicenter trial. Am J Gastroenterol 113(5):677–685. https://doi.org/10.1038/s41395-018-0025-8
Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, Wang X, Gao G, Wu TT, Komaki R, Chang JY (2007) Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys 67(2):389–396. https://doi.org/10.1016/j.ijrobp.2006.09.015
Lee JA (2010) Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol 96(3):302–307. https://doi.org/10.1016/j.radonc.2010.07.003
Stiekema J, Vermeulen D, Vegt E, Voncken FE, Aleman BM, Sanders J, Boot H, van Sandick JW (2014) Detecting interval metastases and response assessment using 18F-FDG PET/CT after neoadjuvant chemoradiotherapy for esophageal cancer. Clin Nucl Med 39(10):862–867. https://doi.org/10.1097/RLU.0000000000000517
Tandberg DJ, Cui Y, Rushing CN, Hong JC, Ackerson BG, Marin D, Zhang X, Czito BG, Willett CW, Palta M (2018) Intratreatment response assessment with 18F-FDG PET: correlation of semiquantitative PET features with pathologic response of esophageal cancer to Neoadjuvant Chemoradiotherapy. Int J Radiat Oncol Biol Phys 102(4):1002–1007. https://doi.org/10.1016/j.ijrobp.2018.07.187
Hatt M, Lee JA, Schmidtlein CR, Naqa IE, Caldwell C, De Bernardi E, Lu W, Das S, Geets X, Gregoire V, Jeraj R, MacManus MP, Mawlawi OR, Nestle U, Pugachev AB, Schoder H, Shepherd T, Spezi E, Visvikis D, Zaidi H, Kirov AS (2017) Classification and evaluation strategies of auto-segmentation approaches for PET: report of AAPM task group no. 211. Med Phys 44(6):e1–e42. https://doi.org/10.1002/mp.12124
Hofheinz F, Langner J, Petr J, Beuthien-Baumann B, Steinbach J, Kotzerke J, van den Hoff J (2013) An automatic method for accurate volume delineation of heterogeneous tumors in PET. Med Phys 40(8):82503. https://doi.org/10.1118/1.4812892
Yu W, Cai XW, Liu Q, Zhu ZF, Feng W, Zhang Q, Zhang YJ, Yao ZF, Fu XL (2015) Safety of dose escalation by simultaneous integrated boosting radiation dose within the primary tumor guided by (18)FDG-PET/CT for esophageal cancer. Radiother Oncol 114(2):195–200. https://doi.org/10.1016/j.radonc.2014.12.007
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by F.E.M. Voncken, E. Vegt, S.L. Takken, J.J. Sonke, J.B. van de Kamer and B.M.P. Aleman. The first draft of the manuscript was written by F.E.M. Voncken and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge Tineke Vijlbrief, Mark Kroon, Lyandra Rooze en Linda de Wit for their contribution to image acquisition and logistics.
Funding
This study was funded by the Netherlands Cancer Institute—Antoni van Leeuwenhoek.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F.E.M. Voncken, E. Vegt, J.W. van Sandick, J.M. van Dieren, C. Grootscholten, A. Bartels-Rutten, S.L. Takken, J.J. Sonke, J.B. van de Kamer and B.M.P. Aleman declare: There are no conflicts of interest of any co-authors regarding this manuscript. J.J. Sonke reports license fees from Elekta AB, outside the submitted work; In addition, J.J. Sonke has a patent Computed Tomography Scanning with royalties paid by Elekta AB, a patent Motion Artefact Reduction in CT Scanning, a patent Methods and System for Protecting Critical Structures During Radiation Treatment with royalties paid by Elekta AB, and a patent Radiotherapy and Imaging Methods and Apparatus with royalties paid by Elekta AB.
Ethical standards
This study was approved by the medical ethical committee of the Netherlands Cancer Institute in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. Consent to participation and publication: written informed consent was obtained from all participants included in the study.
Additional information
Availability of data and material
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary Information
66_2021_1761_MOESM2_ESM.docx
Supplementary Fig. 1 Scatter plots of 1A SUVmax of the tumour, 1B SUVmax of the lymph node, 1C MTV2.5 and 1D MTV50% on 3D PET/CT versus motion-compensated (4D) PET/CT. The solid lines represent y = x. The dashed lines represent the linear fit. (3D three-dimensional, MC motion-compensated, 4D four-dimensional, PET positron-emission tomography, CT computed tomography, SUVmax maximal standardized uptake value, MTV2.5 metabolic tumour volume with SUV threshold of 2.5, MTV50% metabolic tumour volume with threshold 50% of SUVmax)
Rights and permissions
About this article
Cite this article
Voncken, F.E.M., Vegt, E., van Sandick, J.W. et al. Motion-compensated FDG PET/CT for oesophageal cancer. Strahlenther Onkol 197, 791–801 (2021). https://doi.org/10.1007/s00066-021-01761-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-021-01761-w