Abstract
Purpose
For a large or symptomatic brain metastasis, resection and adjuvant radiotherapy are recommended. Hypofractionated stereotactic radiotherapy (HFSRT) is increasingly applied in patients with a limited number of lesions. Exact target volume definition is critical given the small safety margins. Whilst technical advances have minimized inaccuracy due to patient positioning and radiation targeting, little is known about changes in target volume. This study sought to evaluate potential changes in the resection cavity of a brain metastasis.
Methods
In all, 57 patients treated with HFSRT after surgical resection of one brain metastasis between 2008 and 2015 in our institution were included in this study. Gross tumor volume (GTV) of the initial metastasis and the volume of the resection cavity in the post-operative, planning, and follow-up MRIs were measured and compared.
Results
The mean cavity size decreased after surgery with the greatest change of −23.4% (±41.5%) occurring between post-operative MRI and planning MRI (p < 0.01). During this time period, the cavity volume decreased, remained stable, and increased in 79.1, 3.5, and 17.4%, respectively. A further decrease of −20.7% (±58.1%) was perceived between planning MRI and first follow-up (p < 0.01). No significant difference in pattern of change could be observed depending on the volume of initial GTV, size of the post-operative resection cavity, initial or post-resection FLAIR (fluid-attenuated inversion recovery) hyper-intensity, postsurgical ischemia, or primary tumor. The resection cavities of patients with post-operative ischemia were significantly larger than resection cavities of patients without ischemia.
Conclusion
The resection cavity seems to be very dynamic after surgery. Hence, it remains necessary to use very recent scans for treatment planning.
Zusammenfassung
Hintergrund
Die empfohlene Therapie für große und symptomatische Hirnmetastasen ist die chirurgische Resektion mit nachfolgender Bestrahlung der Resektionshöhle. Bei Patienten mit einer begrenzten Metastasenanzahl werden zunehmend stereotaktisch fraktionierte Konzepte (HFSRT) angewandt. Aufgrund der geringen Sicherheitssäume, die bei der HFSRT verwendet werden, ist die genaue Definition des Zielvolumens entscheidend. Während lagerungsbedingte Ungenauigkeiten durch technische Fortschritte weitgehend minimiert werden konnten, sind bisher wenige Informationen über Veränderungen der Zielvolumina bekannt. Ziel dieser Studie war es, Veränderungen der Resektionshöhlen von Hirnmetastasen zu untersuchen.
Methoden
In die Studie wurden 57 Patienten eingeschlossen, die zwischen 2008 und 2015 an unserer Klinik eine HFSRT der Resektionshöhle einer Hirnmetastase erhalten hatten. Das Tumorvolumen (GTV) der Metastase und die Volumina der Resektionshöhlen in der postoperativen Magnetresonanztomographie (MRT), im Planungs- und Nachsorge-MRT wurden gemessen und verglichen.
Ergebnisse
Die durchschnittliche Größe der Resektionshöhle nahm im Verlauf ab, wobei die größten Veränderungen von −23,4 % (±41,5 %) zwischen dem postoperativen MRT und dem Planungs-MRT auftraten (p < 0,01). In diesem Zeitraum wurde die Resektionshöhle in 79,1 % der Fälle kleiner, blieb in 3,5 % gleich und nahm in 17,4 % zu. Eine weitere signifikante Reduktion des Resektionshöhlenvolumens um −20,7 (±58,1 %) trat zwischen dem Planungs-MRT und der ersten Nachsorge auf. Signifikante Zusammenhänge zwischen der Veränderung der Resektionshöhlenvolumina und dem initialen Volumen der Metastase (GTV), der Größe der postoperativen Resektionskavität, der FLAIR(„fluid-attenuated inversion recovery‟)-Hyperintensität, der postoperativen Ischämie oder des Primärtumors konnten nicht nachgewiesen werden. Patienten mit postoperativer Ischämie hatten eine signifikant größere Resektionshöhle als Patienten ohne postoperative Ischämie.
Schlussfolgerung
Nach Operation von Hirnmetastasen treten Änderungen der Resektionshöhlenvolumina auf. Daher ist es notwendig, aktuelle Bildgebungen zur Bestrahlungsplanung zu verwenden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases constitute the most common intracranial malignancy in adults, with an estimated annual incidence of around 26,000 cases in the US [6, 30]. They occur in 5 to 40% of patients with malignant diseases [20]. Most common primary sites are pulmonary cancer, breast cancer, gastrointestinal cancer, and melanoma [10, 24]. Whilst refined systemic treatment options lead to an increased overall survival for many types of primary cancers, prognosis of patients presenting with brain metastasis remains poor [8, 27]. Patients presenting with up to three brain metastases are candidates for local treatment [5, 16, 19, 32]. Recent studies have shown that neurosurgical resection and radiosurgery may be equieffective in the treatment of single brain metastases [23]. Surgical resection is preferred in larger (>2 cm) and symptomatic lesions [19, 21]. Adjuvant radiotherapy (RT) is required even after complete resection, as 2‑year local recurrence rates without adjuvant treatment are approximately 60% [12, 16, 17, 22]. Radiosurgery (SRS) or hypofractionated stereotactic radiotherapy (HFSRT) is increasingly becoming the method of choice in the treatment of resection cavities [1, 4, 11, 15, 15, 28, 31]. Previously, we have shown this approach to combine low toxicity with excellent local control [5, 16, 26]. Recent studies confirmed the advantages of SRS over whole-brain radiotherapy (WBRT) after surgical resection of patients with limited numbers of brain metastases. Local control is comparable and neurotoxicity may be reduced [5, 14]. The advantage of stereotactic treatment techniques is the ability to keep safety margins small and subsequently spare healthy brain tissue. Therefore, precise knowledge about the location of the volume to treat is critical for treatment results. Whilst technical advances have minimized inaccuracy due to patient positioning and radiation targeting, little is known about anatomical changes to the brain that take place after surgery. After the resection of brain metastases, volume changes occur due to removal of tumor mass [2]. Fluctuations in the size of the resection cavity itself have been in the focus of previous studies. However, the results varied from volume increase in around 30% of the cases to other studies reporting a constriction in >90% [2, 5, 25]. The aim of this study was to evaluate volume changes within the resection cavity and to establish a recommendation for re-imaging and radiation start.
Patients and methods
Patients
57 patients treated with HFSRT after surgical resection of a large or symptomatic brain metastasis between 2008 and 2015 in our institution were included in this study. Median age was 62 years (range 33–82 years), most common primaries were non-small cell lung cancer (NSCLC; 16 cases/28%); gastrointestinal cancers (16 cases/28%), and breast cancer (11 cases/19%). General performance status was adequate in most patients with a median Karnofsky index of 90% (range 60–100%). Patients’ characteristics are shown in Table 1.
Out of 57 patients, 49 had an early post-surgical MRI (up to 96 h after surgery). The median time from surgery to the early post-surgical MRI was 1 day (1–4 days). In another 5 patients an MRI was performed within a delayed timeframe after surgery of 26–100 days (median 58 days). In the analysis of the changes in cavity volume, these delayed MRIs were treated as postoperative MRIs since all of those patients also received a planning MRI. A planning MRI was available in 47 patients. Median time between post-surgical MRI and planning MRI was 23 days (7–58 days). Median time from surgery to planning MRI was 31 days (range 8–122 days). Median time from the planning MRI to the initiation of irradiation was 7 days (range 1–25 days). First, second, third, fourth, and fifth follow-up MRI were defined as 4–8 weeks, 3–6 months, 6–12 months, 12–24 months, and 24–36 months after radiotherapy, respectively. At this time, an MRI was available in 45 patients for first, 42 patients for second, 35 patients for third, 27 patients for fourth, and 15 patients for fifth follow-up.
Methods
Delineation of gross tumor volume (GTV) of the initial metastasis, volume of the resection cavity, and of the residual tumor (if present) was carried out on contrast enhancing T1-weighted MRI. Surrounding edema was defined as hyper-intensity in fluid-attenuated inversion recovery (FLAIR) images. Contouring of the structures mentioned above was done in early post-surgical MRI, delayed post-surgical MRI, planning MRI, and follow-up MRI. The post-surgical ischemic area was contoured in the ADC sequence of the early post-surgical MRI. Volumetric evaluations were performed using the Eclipse 13.0 treatment planning software (Varian Medical Systems, Palo Alto, CA, USA). Cavity size was compared between post-operative MRI, planning MRI, planning CT, and follow-up exams. Changes in cavity size, which are stated as percentages of post-operative volume, include only patients with post-surgical MRI. Changes in cavity size of <5% were considered within the fluctuation margin and subsequently regarded as a stable cavity size. The value given for absolute changes in the resection cavity volume (in cm3) includes all patients.
Treatment planning was carried out according to standard of care procedure. Clinical target volume (CTV) was defined as the resection cavity (encompassing residual tumor, if present) and a safety margin of 2–3 mm. Planning target volume (PTV) was generated with an additional margin of 1–2 mm to the CTV. Treatment planning was carried out using iPlan RT Dose treatment planning software (BrainLAB AG, Munich, Germany) or Eclipse software.
35 Gy was applied in 7 fractions (daily dose of 5 Gy). Dose prescription was to the 95–100% isodose line. Dmean (PTV), Dmax (PTV), and Dmin (PTV) were 35.24 Gy (±0.52), 36.7 Gy (±0.74), and 33.34 Gy (±0.51), respectively. Irradiation was performed with a Clinac Trilogy linear accelerator equipped with a 120 HD multileaf collimator (Varian Medical Systems, Palo Alto, CA, USA) and 6 MV photons. A high-precision treatment set-up was applied using a frameless thermoplastic mask system (BrainLAB AG, Munich, Germany). Daily image-guided radiotherapy (IGRT) was performed with the ExacTrac stereoscopic X‑ray imaging system.
Local recurrence and loco-regional recurrence were calculated from the first day of radiation therapy until the date of tumor recurrence. For the evaluation of OS, the time interval between the start of radiation therapy to the date of death or the last contact was calculated. Recurrence was documented if stated as such in the MRI report.
All patients were treated in accordance with the Declaration of Helsinki. A written informed consent for the use of scientific data was obtained from all patients. This study was approved by the Ethics Committee of the Technical University Munich, Faculty of Medicine.
Statistical evaluation
Continuous data were expressed as means ± standard deviation (SD) or median and range, categorical data as frequency counts or percentages.
Statistical analysis was performed by two-sided students’ t‑test. A p-value of 0.05 was defined as the threshold for statistical significance within a confidence interval of 95%. All calculations and figures were done with the software packages Graphpad Prism 7 (Graphpad Software Inc, USA).
Results
Outcome
At time of analysis, 36 patients had died and 21 patients were still alive. Median follow-up time was 54.0 months. The median OS of the complete cohort was 24.1 months (range 2.2–102.6 months). The OS of patients that had received prior radiation to the brain was 7.9 months (range 7.7–9.2 months). Patients receiving additional radiotherapy for asymptomatic brain metastasis had a median OS of 40.5 months (range 17.6–60.2 months). With a median OS of 35.5 months, breast cancer was the entity with the best prognosis (range 4.7–73.2 months).
In 32 patients (56%) the metastasis was resected completely. In 17 patients (30%) residual tumor was suspected based on early postoperative MRI. Resection status was unknown in 8 (14%) patients. Mean volume of residual tumor was 0.4 ± 0.4 cm3. Mean residual tumor volume in planning MRI was 0.7 ± 1.0 cm3. Comparing the early post-operative MRI to planning MRI, 5 patients had a local progression and local recurrence was suspected in 4 patients. The median time between imaging in patients with progressive disease or recurrence was 28 days (range 11–56 days), in patients without progression or recurrence 21 days (range 12–50 days). This difference in time between patients with and without recurrence was not statistically significant (p = 0.12).
Of the 57 analyzed patients, 34 (59.5%) experienced a cranial progression. In one patient (1.7%) the recurrences was isolated within the field of HFSRT, in 11 patients (19.3%) the recurrence was in- and out of field, and in 22 patients (38.5%) only distant recurrences occurred. The 1‑year local recurrence rate was 16.9%. The 1‑year loco-regional recurrence rate was 48.8%. No difference in local recurrence between patients with or without suspicion of residual tumor was observed (p = 0.91). During the course of observation, 19 patients received salvage treatment in the form of whole-brain radiotherapy with or without radiosurgery for further recurrences, 9 patients in the form of radiosurgery only, and one patient in the form of HFSRT.
Changes in cavity volume
The mean volumes of the initial GTV and post-operative resection cavity were 15.1 cm3 ± 14.4 and 8.7 ± 6.2 cm3, respectively. The size of the initial GTV was on average 76.5% ± 116.7% larger than the post-operative resection cavity (p < 0.01). In very small metastases with a volume below 3 cm3 (mean GTV 2.2 cm3 ± 0.56), the size of the initial GTV was smaller than the initial metastases and made up 85.4% ± 48.6% of the postoperative resection cavity. In metastasis with a volume of more than 3 cm3 (mean GTV 17.56 cm3 ± 17.25), the initial GTV was on average twice the size of the postoperative resection cavity (200.5% ± 117.9%; p < 0.01).
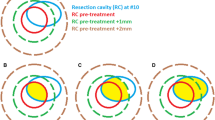
The mean cavity size decreased over time from post-operative MRI to follow-up MRIs as can be seen in Fig. 1. MRI images of one patient at different time points is demonstrated in Fig. 2. The greatest reduction in cavity volume with a mean percentage change of −23.4% ± 41.5% occurred between postoperative MRI and planning MRI (p < 0.01). The mean absolute reduction of cavity volume was 1.4 cm3 ± 2.7 cm3. In 79.1% of the cases the volume of the cavity size decreased and in 3.5% cavity size was stable. An increase in volume was perceived in 17.4%. All patients with an increased cavity volume in planning MRI showed a reduction in cavity volume in the first follow-up MRI. There was no significant difference in cavity shrinkage between patients with early or delayed postoperative MRI (p = 0.29).
The reduction in cavity size was significant for the time between planning MRI to first follow-up (20.8% ± 58.2%, p < 0.01). Median time from planning MRI to first follow up was 41 days (range 36–77 days).
The size of the initial resection cavity was negatively correlated with proportional size reduction of the cavities between planning and post-surgical MRI, as small cavities tended to experience greater shrinkage (p = 0.03; Fig. 3), but was not correlated with reduction in size between post-op MRI and any other point during follow-up (p = 0.34). The mean percentage change and mean absolute volume reduction were −28.5% (±43.9%)/−1.3 cm3 (±1.7 cm3) and −13.3% (±23.0%)/−1.5 cm3 (±3.7 cm3) for cavities with a volume <10 cm3 and ≥10 cm3 in the post-operative MRI, respectively.
No significant difference in pattern of change in cavity size could be observed depending on volume of initial GTV (p = 0.58). Neither initial FLAIR hyper-intensity nor post-resection FLAIR hyper-intensity correlated significantly with cavity size reduction (p = 0.22 and 0.26, respectively). Patterns in volume change did not significantly differ for different primaries (p = 0.35) as can be seen in Fig. 4. The presence of residual tumor mass was not significantly associated with the change in cavity volume (p = 0.12).
A post-surgical ischemia was observed in 12 patients with early post-surgical MRI. No significant difference in volume of initial GTV of patients experiencing ischemia and patients without post-surgical ischemia was observed (18.1 cm3 ± 21.5 cm3 vs. 13.5 cm3 ± 15.7 cm3, p = 0.5). The resection cavities of patients with postoperative ischemia were significantly larger than resection cavities of patients without ischemia (12.8 cm3 ± 10.4 cm3 vs. 7.7 cm3 ± 5.9 cm3, p = 0.05). Reduction in cavity size from post-operative MRI to planning MRI did not differ significantly between these two groups (7.4% ± 26.4% for patients with ischemia and 25.5% ± 39.1% for patients without ischemia, p = 0.17). Volume of ischemic area was not correlated with initial GTV (p = 0.92), size of post-surgical resection cavity (p = 0.86), or change in cavity size between early post-surgical and planning MRI (p = 0.09).
Toxicities
Acute toxicity included skin toxicity CTC I in two patients, hair loss CTC I in four patients, headache and dizziness CTC I in two, and CTC II in one patient. No patient experienced seizures or nausea during radiotherapy.
Asymptomatic radionecrosis was suspected in 5 patients based on MRI in the location of HFSRT: 3 patients had undergone solely postoperative HFSRT; 2 patients had received whole-brain irradiation as well as two further stereotactic treatments to other cerebral lesions. In 4 of these patients, radionecrosis was confirmed histologically. One patient did not undergo surgery.
Discussion
Stereotactic radiation techniques enable precise delivery of relatively high daily doses to the target volume. Therefore, only small safety margins are required and healthy tissue can be spared. A recent randomized controlled trial showed a significant reduction in cognitive deterioration for stereotactic radiation to the resection cavity compared to whole-brain irradiation without compromising overall survival [5]. At the same time, the risk of radionecrosis is higher than in whole-brain radiotherapy as a result of the high daily doses applied [14]. This risk is elevated by prior irradiation, higher doses, and larger irradiation volume [13]. Fractionated radiotherapy is used to reduce the threat of radionecrosis in large lesions. HFSRT with 35 Gy in daily fractions of 5 Gy was effective and well tolerated with a 1-year local control rate of 83.1%, a 10.5% rate of radionecrosis, and few acute toxicities, as previously demonstrated ([26]; Table 2). The biologically effective dose (BED) that is equivalent to 35 Gy à 5 Gy depends on the alpha/beta ratio of the irradiated tissue. For breast cancer, lung cancer, and GI cancer cells, with estimated alpha/beta ratios of 4–8, this corresponds to a BED of 65.6 to 96.3 Gy [29]. When assuming an alpha/beta of 2 for healthy brain tissue, the equivalent BED is 122.5 Gy.
In order to fully exploit the advantages stereotactic radiation provides, exact knowledge of the resection cavity’s anatomy at the time of treatment is critical. In this study, anatomical changes before and after radiation therapy were monitored, revealing significant alterations in cavity size over time.
Dimensions of initial metastasis tended to be larger than the corresponding resection cavities. This is not surprising, as metastatic mass effect is reduced by surgery, allowing healthy brain tissue to return to its initial extent. This is particularly visible in large metastases. Small metastases, on the other hand, are frequently resected supra-marginally. Hence, there is a statistically significant proportional change in volume size between initial metastasis and resection cavity when comparing smaller with larger lesions. Similarly, Atalar et al. reported an alteration in volume of up to minus 29% when comparing pre-resection tumor and post-resection cavity. In the following time period of up to 33 days after resection, they observed no statistically significant size changes [2]. In our cohort, in contrast, a statistically relevant size reduction occurred from post-operative MRI to planning MRI. Changes in cavity size have already been demonstrated by prior publications. While Jarvis et al. observed a stable size in 47% of the cases, expansion in 30%, and reduction in 23% [9], Shah et al. reported cavity constriction in >90% resulting in a mean volume reduction of 45%. They observed a significant reduction in cavity size after more than 30 days after surgery [25]. We observed a significant reduction in cavity volume after a median time of 31 days. Even though time between planning and post-resection MRI was not significantly associated with a decrease in cavity size, we can confirm the findings of Shah et al. taking into account the further reduction on cavity size taking place after irradiation. Other than that, no significant factor to predict cavity dynamics was identified in the prior studies conducted. Similarly to previous results, neither initial tumor size, the size of vasogenic edema, nor tumor histology was prognostic for the pattern of volume change. Initial cavity size, on the other hand, correlated negatively with the proportional cavity shrinkage between post-resection and planning MRI. In the further follow-up there was no difference, suggesting a faster response in small cavities. The theory behind the analysis of the resection cavities’ dynamics, depending on whether an ischemia was present or not, was the assumption that an ischemia would result in a necrosis and subsequently larger volumes of the resection cavity. Post-surgical ischemia was associated with significantly larger post-surgical resection cavities compared to patients without ischemia. However, the difference in cavity volume was neither significant for the planning MRI nor the MRI at any other time. If necrosis was the relevant factor for a larger cavity volume in the post-surgical MRI, one would expect the difference to increase over time. On the other hand, initial GTV in patients that later experienced necrosis tended to be larger than in patients without necrosis, even though this was not statistically significant. Therefore, it is not clear whether the larger volume of patients with ischemia really is associated with biological processes following ischemia or rather with the fact that ischemia occurs more often in patients with more extensive surgery.
Despite the fact that the reasons for cavity dynamics are not yet clear, implications for RT may be drawn from these findings. A direct comparison of WBRT with stereotactic radiation to the surgical bed revealed higher incidences of local failure in stereotactic radiation [4]. In the study of Brown et al., baseline MRI was performed before randomization, planning MRI was not specifically required, and the local recurrence rate was high compared to other studies [5, 16]. Differences in local control may certainly result from differences in study population or definition of local control. Nevertheless, an increase in cavity volume in some of the patients cannot be fully excluded as a reason for local failure. Moreover, very small or no safety margins were used, which might be the most relevant factor for the lower local control.
Shah et al. recommended a longer interval between surgery and stereotactic radiation to take advantage of the volume reduction that may occur during this period [25]. The influence of the irradiated volume on the risk of radionecrosis in stereotactic radiosurgery is well established. Particularly the volumes that receive 10 Gy (V10 Gy) and 12 Gy (V12 Gy) have been shown to be predictive for the risk of radionecrosis [3, 18]. Therefore, fractionated therapy is preferred for V10 Gy >10.5 cm3 or V12 Gy >7.9 cm3 [3]. In resection cavities planned for treatment with radiosurgery, a reduction of the risk for radionecrosis through a decrease in target volume can be assumed. However, the vast majority of resection cavities in this study exceeded sizes that can safely be treated with radiosurgery and were therefore treated with hypofractionated stereotactic radiotherapy. Only limited data exist on the effect of an increased radiation volume in hypofractionated stereotactic radiotherapy. The publications available retrospectively analyzed the risk of radionecrosis in a cohort of patients that received 3 × 7.7 Gy (prescribed to the 70%-isodose line) to the postoperative resection cavity of brain metastases. While the analysis with 95 patients demonstrated a predictive value of the V21 Gy, an analysis of 189 patients did not confirm these results, showing no association of the V8 Gy–V22 Gy with the appearance of radionecrosis [7, 11]. Another factor that should be taken into account, when debating on longer waiting periods before adjuvant radiotherapy of the resection cavity is the risk of recurrence or progression. We observed 5 progressions in patients with residual tumor after surgery and 4 recurrences after complete resection. Even though no significant difference in time between surgery and radiation start was observed for patients with and without recurrence/progression, a connection is likely and may not be discernible due to small patient numbers. Therefore, in our experience, HFSRT should begin as soon as wound healing is complete based on most recent MRI.
Limitations to this study include the fact that early postoperative MRI was available in only 70% of the patients and 5 patients received their baseline MRI with a longer interval after surgery. Moreover, neither ischemia nor histology can be omitted as an influence in cavity change due to small patient numbers.
Conclusion
We confirmed a volume change in the resection cavity after surgery of brain metastases. Smaller initial resection cavities experienced a faster proportional decrease in size. Other than that, no predictive factor for the pattern of volume change could be identified. Even if the mechanisms of decrease in cavity size are unclear, it remains necessary to adapt the process of stereotactic radiation of resection cavities to these findings. As a practical approach, target volume delineation should be based on a planning MRI performed shortly before start of radiation therapy. Older MRIs may result in increased local failure in case of cavity volume increase or unnecessary irradiation of healthy tissue in case of volume reduction. We do not recommend postponing radiation therapy in order to achieve a reduction in cavity size, as recurrence or progression of disease may result from the delay.
References
Al-Omair A, Soliman H, Xu W et al (2013) Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technol Cancer Res Treat 12(6):493–499
Atalar B, Choi CYH, Harsh GR et al (2013) Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery 72(2):180–185 (discussion 185)
Blonigen BJ, Steinmetz RD, Levin L et al (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77(4):996–1001
Broemme J, Abu-Isa J, Kottke R et al (2013) Adjuvant therapy after resection of brain metastases. Frameless image-guided LINAC-based radiosurgery and stereotactic hypofractionated radiotherapy. Strahlenther Onkol 189(9):765–770
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3). Lancet Oncol 18(8):1049–1060
Pérez-Larraya JG, Hildebrand J (2014) Chapter 77 – Brain metastases. In: Biller J, Ferro JM (Hrsg) Neurologic Aspects of Systemic Disease. Part III. Elsevier, Amsterdam, S 1143–1157
Doré M, Martin S, Delpon G et al (2017) Stereotactic radiotherapy following surgery for brain metastasis. Cancer Radiother 21(1):4–9
Frisk G, Svensson T, Bäcklund LM et al (2012) Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer 106(11):1850–1853
Jarvis LA, Simmons NE, Bellerive M et al (2012) Tumor bed dynamics after surgical resection of brain metastases: Implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys 84(4):943–948
Johnson JD, Young B (1996) Demographics of brain metastasis. Neurosurg Clin N Am 7(3):337–344
Keller A, Doré M, Cebula H et al (2017) Hypofractionated Stereotactic radiation therapy to the resection bed for Intracranial metastases. Int J Radiat Oncol Biol Phys 99(5):1179–1189. https://doi.org/10.1016/j.ijrobp.2017.08.014
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol 29(2):134–141
Kohutek ZA, Yamada Y, Chan TA et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125(1):149–156
Lamba N, Muskens IS, DiRisio AC et al (2017) Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: A systematic review and meta-analysis. Radiat Oncol 12(1):106
Lima LCS, Sharim J, Levin-Epstein R et al (2017) Hypofractionated Stereotactic Radiosurgery and radiotherapy to large resection cavity of metastatic brain tumors. World Neurosurg 97:571–579
Mahajan A, Ahmed S, McAleer MF et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases. Lancet Oncol 18(8):1040–1048
Mehta MP, Tsao MN, Whelan TJ et al (2005) The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 63(1):37–46
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases. Radiat Oncol 6:48
NCCN (2017) NCCN clinical practice guidelines in oncology (NCCN guidelines ® ) central nervous system cancers. https://education.nccn.org/node/81831
Nussbaum ES, Djalilian HR, Cho KH et al (1996) Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78(8):1781–1788
O’Neill BP, Iturria NJ, Link MJ et al (2003) A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys 55(5):1169–1176
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322(8):494–500
Qin H, Wang C, Jiang Y et al (2015) Patients with single brain metastasis from non-small cell lung cancer equally benefit from stereotactic radiosurgery and surgery. Med Sci Monit 21:144–152
Schouten LJ, Rutten J, Huveneers HAM et al (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10):2698–2705
Shah JK, Potts MB, Sneed PK et al (2016) Surgical cavity constriction and local progression between resection and Adjuvant Radiosurgery for brain metastases. Cureus 8(4):e575
Specht HM, Kessel KA, Oechsner M et al (2016) HFSRT der Resektionshöhle bei Patienten mit Hirnmetastasen. Strahlenther Onkol 192(6):368–376
Spencer K, Hall A, Jain P (2014) Brain metastases. Clin Med (lond) 14(5):535–537
Steinmann D, Maertens B, Janssen S et al (2012) Hypofractionated stereotactic radiotherapy (hfSRT) after tumour resection of a single brain metastasis: Report of a single-centre individualized treatment approach. J Cancer Res Clin Oncol 138(9):1523–1529
van Leeuwen CM, Oei AL, Crezee J et al (2018) The alfa and beta of tumours. Radiat Oncol 13(1):96
Walker AE, Robins M, Weinfeld FD (1985) Epidemiology of brain tumors: The national survey of intracranial neoplasms. Baillieres Clin Neurol 35(2):219–226
Wang C‑C, Floyd SR, Chang C‑H et al (2012) Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: Efficacy and safety of an 800 cGy × 3 daily fractions regimen. J Neurooncol 106(3):601–610
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901). Lancet Oncol 15(4):387–395
Acknowledgements
The authors thank our team of technicians for excellent patient care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Scharl, A. Kirstein, K.A. Kessel, M.-N. Duma, M. Oechsner, C. Straube, and S.E. Combs declare that they have no competing interests.
Ethical standards
All patients were treated in accordance with the Declaration of Helsinki. A written informed consent for the use of scientific data was obtained from all patients. This study was approved by the Ethics Committee of the Technical University Munich, Faculty of Medicine.
Rights and permissions
About this article
Cite this article
Scharl, S., Kirstein, A., Kessel, K.A. et al. Cavity volume changes after surgery of a brain metastasis—consequences for stereotactic radiation therapy. Strahlenther Onkol 195, 207–217 (2019). https://doi.org/10.1007/s00066-018-1387-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1387-y
Keywords
- Resection cavity dynamics
- Hypofractionated stereotactic irradiation
- Neuro-oncology
- Adjuvant radiotherapy
- Constriction of the surgical bed