Abstract
Purpose
Combined high-dose-rate brachytherapy (HDR-BT) and external beam radiation therapy (EBRT) is a favorable treatment option in non-metastatic prostate cancer. However, reports on toxicity and outcome have mainly focused on younger patients. We aimed to determine toxicity and biochemical control rates after combined HDR-BT and EBRT in men ≥75 years.
Methods
From 1999 to 2015, 134 patients aged ≥75 years (median 76 years; 75–82 years) were identified. Patients received 18 Gy of HDR-BT (9 Gy/fraction on days 1 and 8) with an iridium-192 source. After 1 week, supplemental EBRT with a target dose of 50.4 Gy was started (delivered in 1.8 Gy fractions).
Results
Median follow-up time was 25 months (0–127 months). No severe (grade 4) gastrointestinal (GIT) or genitourinary (GUT) toxicities were observed. In 76 patients (56.7%), 3D conformal radiation therapy (CRT) and in 34.3% intensity-modulated radiotherapy (IMRT) was applied. CRT-treated patients were at a 2.17-times higher risk (hazard ratio [HR]: 2.17, 95% confidence interval [CI]: 1.31–3.57, p = 0.002) of experiencing GUT. GIT risks could be reduced by 78% using IMRT (HR: 0.22, 95% CI: 0.07–0.75, p = 0.015). Patients with a higher T stage (T2c–3a/b) were less likely to experience GIT or GUT (HR: 0.49, 95% CI: 0.29–0.85, p = 0.011 and HR: 0.5, 95% CI: 0.3–0.81, p = 0.005, respectively).
Conclusion
HDR-BT/EBRT is a well-tolerated treatment option for elderly men ≥75 years with a limited number of comorbidities and localized intermediate- or high-risk prostate cancer. IMRT should be favored since side effects were significantly reduced in IMRT-treated patients.
Zusammenfassung
Hintergrund
Die Kombination aus HDR-(high-dose-rate)-Brachytherapie (BT) und externe Strahlentherapie (ERBT) ist eine vorteilhafte Behandlungsoption beim nichtmetastasierten Prostatakarzinom. Die Berichte über die Nebenwirkungen und den Therapieausgang beschäftigen sich zumeist mit jüngeren Patientenkollektiven. Ziel der Analyse war die Evaluation der Toxizität und der biochemischen Kontrollrate bei Patienten ≥75 Jahren, die sich einer kombinierten HDR-BT/EBRT unterzogen haben.
Methoden
Von 1999–2015 wurden 134 Patienten ≥75 Jahren (Median 76 Jahre; 75–82 Jahre) identifiziert. Die Patienten erhielten 18 Gy einer HDR-BT (9 Gy/Fraktion an Tag 1 und 8) einer Iridium-192-Quelle. Die EBRT mit einer Zieldosis von 50,4 Gy (Einzeldosis von 1,8 Gy) begann nach einer Woche.
Ergebnisse
Das mediane Follow-up betrug 25 Monate (Spanne 0–127 Monate). Es bestanden keine schwerwiegenden akuten (Grad 4) gastrointestinalen (GIT) oder urogenitalen (GUT) Nebenwirkungen. Bei 76 Patienten (56,7 %) kam die 3‑D-konformale Bestrahlung (CRT) und in 34,3 % die intensitätsmodulierte Radiatio (IMRT) zum Einsatz. Bei CRT-behandelten Patienten bestand ein 2,17-fach erhöhtes Risiko (Hazard-Ratio [HR]: 2,17; 95 %-Konfidenzintervall [KI]: 1,31–3,57; p = 0,002) für GUT. Das GIT-Risiko konnte um 78 % bei der IMRT reduziert werden (HR: 0,22; 95 %-KI: 0,07–0,75; p = 0,015). Bei Patienten mit einem höheren T‑Stadium (T2c–3a/b) bestand eine geringere Wahrscheinlichkeit GIT oder GUT zu erleben (HR: 0,49; 95 %-KI: 0,29–0,85; p = 0,011 bzw. HR: 0,5; 95 %-KI: 0,3–0,81; p = 0,005).
Schlussfolgerung
Die kombinierte HDR-BT/EBRT ist eine sichere und gut verträgliche Behandlungsoption für ältere Männer ≥75 Jahren, die wenige Komorbiditäten und ein lokal begrenztes, mittel- bis hochgradiges Prostatakarzinom aufweisen. IMRT-Techniken sollten bevorzugt werden, da sich hierdurch die Nebenwirkungen signifikant reduzierten lassen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common tumor entity among males in Europe [9] and accounts for 9.5% of all new cancer cases in the United States [19]. Prostate cancer in the U. S. occurs more often between 65 and 74 years (median age: 66 years) [19]. In contrast, data from the United Kingdom indicate that the incidence peak is found between 75 and 79 years, followed by a subsequent decrease in the 80–94-years age group, before rising again in men ≥90 years [4]. In the future, more men ≥75 years are likely to be affected by prostate cancer due to demographic changes.
The introduction of prostate-specific antigen (PSA) screening has favored the diagnosis of early-stage diseases [7]. However, as comorbidities are often part of an ageing population with a longer life expectancy, choosing treatment options in patients ≥75 years may be controversial. Curative treatment options for localized prostate cancer include radical prostatectomy, monotherapy by means of external beam radiation therapy (EBRT) or interstitial brachytherapy, the combination of both possibilities, as well as active surveillance [15, 20]. In patients ≥75 years, physicians are often confronted with several comorbidities, so that radical prostatectomy is often connected with increased morbidity. Also, according to the current European guidelines on the treatment of patients with intermediate- and high-risk prostate cancer, a life expectancy of at least 10 years is compulsory for radical prostatectomy or radiotherapy [18].
The combination of high-dose-rate brachytherapy (HDR-BT) and EBRT is a favorable treatment option in cases of non-metastatic prostate cancer [11, 12, 14, 24]. There are several reports on the analysis of toxicity and outcome after combined HDR-BT/EBRT. However, these authors focused on younger patients with a mean/median age ranging from 66.4 to 68 years [2, 13]. Data on elderly patient cohorts ≥75 years are still very limited [27]. To our knowledge, this is the largest analysis which evaluates how combined HDR-BT/EBRT is tolerated among patients ≥75 years. The aim of this study was to determine toxicity and biochemical control rates after combined HDR-BT/EBRT in men ≥75 years.
Methods
Patient population
From December 1999 till November 2015, 134 patients aged ≥75 years (median 76 years; range 75–82 years) were identified who underwent combined HDR-BT/EBRT due to localized prostate cancer at the Department of Radiotherapy and Radiooncology of the University Medical Center Hamburg-Eppendorf. All of these patients gave their written informed consent.
Inclusion criteria:
-
Age of ≥75 years at the beginning of treatment
-
Biopsy-confirmed adenocarcinoma of the prostate
-
Prostate volume ≤60 ml and no symptoms of urinary retention
Exclusion criteria:
-
Known involvement of regional lymph nodes and/or the presence of distant metastases
-
Patients where anesthesia not possible
Assessment of clinical parameters
For prostate cancer risk assessment, all patients were classified into three risk categories according to the risk-adapted guidelines of the National Comprehensive Cancer Center (NCCN) [25]: 1) low risk: Gleason score (GS) ≤6 and PSA <10 ng/ml and cT stage T1–2a; 2) intermediate risk: GS 7 or PSA 10–20 ng/ml or cT2b–2c; and 3) high risk GS 8–10 or PSA >20 ng/ml or cT3–4.
Comorbidities were assessed by Charlson comorbidity index (CCI) [5]. Side effects were classified according to the toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) [6].
Follow-up examinations were carried out 6–8 weeks and 8 months after the end of therapy. Subsequent follow-up was performed annually. Acute toxicities were classified as occurring within 3 months or at the first follow up after the completion of radiotherapy. According to Phoenix criteria, biochemical recurrence was defined as PSA increase ≥2 ng/ml above nadir [23].
Radiotherapy
HDR-BT was performed under general or spinal anesthesia. Treatment modalities were previously described by Schiffmann et al. [26]. Patients received 18 Gy of HDR-BT (9 Gy/fraction on days 1 and 8) with an iridium-192 source. After 1 week, supplemental EBRT of the prostate and the seminal vesicles started. The target dose for EBRT was 50.4 Gy (in 1.8 Gy fractions). Initially, a three-dimensional conformal technique (3D-CRT) was used. Since 2012, all patients have been treated with intensity-modulated radiotherapy (IMRT) and, in a few cases, TomoTherapy® (Accuray® Sunnyvale, CA, USA) was employed due to anatomical particularities.
Statistical analysis
Parameters are given in absolute and relative frequencies. In normally distributed data the mean and standard deviation were determined. Otherwise, the median with interquartile range (IQR) was evaluated. Shapiro-Wilk test was used for the assessment of normal distribution. Two related non-parametrical samples were compared by Wilcoxon signed-rank test and Chi-squared test (χ2 test) was applied in case of categorical variables. The Friedman test was utilized for one-way repeated analyses of variance by ranks in non-parametrical datasets. Survival analysis was performed by Kaplan–Meier survival curves with corresponding logrank test. Cut-off values were determined by calculating the Youden index from receiver operating characteristic curve statistics (ROC). To evaluate risks of gastrointestinal (GIT) and genitourinary (GUT) toxicities, parameters were tested with univariate Cox regression analysis. Only covariates with p < 0.05 were then entered into a multivariate logistic Cox regression model (p = 0.05) to receive the overall prediction model.
Statistical calculation was conducted with SPSS Statistics 24 software (IBM Inc. SPSS Statistics, Armonk, NY, USA).
Results
All treated patients exhibited a good functional status (ECOG 0–1). Most patients presented with intermediate-risk prostate cancer and a median CCI of 3 (range 2–6). Most men suffered from hypertension, while 14 patients (10.4%) had also had a second neoplasm other than prostate cancer. In 76 patients (56.7%, n = 125), external beam percutaneous therapy was performed by means of 3D-CRT, while in 34.3% IMRT was applied. Irradiation of pelvic lymph nodes was reported in 18 patients (16.7%, n = 108). Past transurethral resection of the prostate had occurred in 7 cases (5.6%, n = 125) and androgen deprivation therapy (ADT) in 52 patients (41.3%, n = 126). Patient characteristics are summarized in Table 1.
Clinical parameters and evaluation of acute therapy-related toxicity
In 87 patients (69%, n = 126), no skin toxicity occurred. No grade 3 skin toxicity was observed. Considering GIT, most patients reported mild diarrhea and increased frequency of bowel movement (grade 1 in 53.5%). However, in two cases, grade 3 GIT occurred. During EBRT, in one of these patients, irradiation had to be paused at 36 Gy due to transrectal blood loss. Mild GUT was observed in 71 patients (55.9%, n = 127). Mostly patients reported on nycturia and on increased frequency of urination. In all subgroups, no grade 4 toxicity was reported.
The volume of the prostate gland was measured before each BT session. In our patient cohort, a significant difference between the glandular volume of the first (median: 39.7 cm3) and the second BT fraction (mean: 41.5 cm3; p < 0.001) was found. The average cumulative maximal rectum dose was 5.88 (±1.81) Gy. The rectum dose was significantly lower in the second BT session (p = 0.001). Clinical parameters in brachytherapy are summarized in Table 2 and acute and late therapy-related toxicities are shown in Table 3.
Follow-up
In our patient cohort, median follow-up time was 25 months (range 0–127 months).
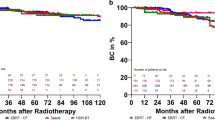
Biochemical response was evaluated by comparing the pre- and post-therapeutic PSA course. Fig. 1 shows significantly reduced post-therapeutic PSA values (p < 0.001). Considering all noted follow-up examinations (up to 16 follow-up examinations in 2 patients), no significant difference in PSA values was observed (p = 0.453). Biochemical relapse was found in 12 patients (10.3%, n = 117) after a median time interval of 22 months (range 1–87 months). Overall biochemical relapse is demonstrated in Fig. 2. Considering different prostate cancer risk categories, no significant differences were found between the three subgroups (p = 0.484; Fig. 3). Fig. 3 also shows patients in whom ADT was started prior to combined HDR-BT/EBRT and those who did not receive ADT before HDR-BT. In our patients, no significant differences were found between the aforementioned groups (p = 0.527).
Kaplan–Meier diagram in a demonstrates biochemical relapse in patients with different prostate cancer risk categories (p = 0.484). Pairwise comparison of subgroups did not show significant differences. Kaplan-Meier survival plot in b distinguishes between patients in whom androgen deprivation therapy (ADT) was started prior to combined high-dose-rate brachytherapy and external beam radiation therapy (HDR-BT/EBRT) and those who did not receive hormone deprivation (p = 0.527)
For evaluating late therapy-related toxicity, data from the first and the last available follow-up were compared. Considering the last available follow-up, significantly more patients presented with no late toxicities in all subgroups (grade 0; p < 0.001). Only in one case, late GUT grade 3 was reported. Table 4 summarizes late toxicities at these two timepoints.
Risk assessment
In univariate and in multivariate logistic regression analysis, both T stage and the used technique (3D-CRT vs. IMRT vs. TomoTherapy [Accuray®, Sunnyvale, Ca, USA]) were identified as being predictors for developing GIT and GUT. Patients who were treated with 3D-CRT were at a 2.17-times higher risk (hazard ratio [HR]: 2.17, 95% confidence interval [CI]: 1.31–3.57, p = 0.002) of experiencing GUT than their counterparts who received IMRT or TomoTherapy. Considering GIT, risks could be reduced by 78% using IMRT (HR: 0.22, 95% CI: 0.07–0.75, p = 0.015). In our population, patients with a higher T stage (T2c–3a/b) were less likely to experience GIT or GUT showing a risk reduction by 50% (HR: 0.49, 95% CI: 0.29–0.85, p = 0.011 and HR: 0.5, 95% CI: 0.3–0.81, p = 0.005, respectively). All other tested covariates did not significantly impact on risk status (see Table 4 and 5). Skin toxicity was very mild. Therefore, no risk stratification occurred.
Discussion
This retrospective study focused on patients ≥75 years who underwent a combined HDR-BT/EBRT due to localized prostate cancer. Other studies have mainly examined younger patients [16]. Between 2004 and 2010, Soumarova et al. [27] examined 20 patients at a median age of 77 years who underwent a combined HDR-BT/EBRT. They reported on acute GUT and GIT in 60 and 25%, respectively. Despite their comparatively limited number of patients, the authors stated that combined HDR-BT/EBRT was a safe and well-tolerated procedure. When dealing with elderly patients, some authors question the benefit of curative treatment options. However, Alibhai et al. concluded that especially older men with moderately or poorly differentiated tumors and a limited number of comorbidities benefit due to prolonged (quality-adjusted) life expectancy [1]. 93.2% of our patients suffered from an intermediate or high-risk tumor. Therefore, it is likely that especially this risk group recorded a therapy-related benefit.
In comparison to Soumarova et al., we observed almost the same percentage of patients who suffered from acute GUT grade 1 (55.9%). Conversely, in our cohort, more patients presented with acute GIT (grade 1 in 53.5%) which later resolved; the long-term course did not show grade 3 GIT on the last available follow-up examination.
Boehm et al. [3] evaluated predictors for biochemical recurrence and overall survival in patients treated with combined HDR-BT/EBRT (group 1) and patients who underwent radical prostatectomy (RP). These authors stated that RP (without additional ADT) offered a comparable response rate to HDR-BT and EBRT. Schiffmann et al. [26] examined 392 patients of whom 56.4% were treated with additional ADT to combined HDR-BT/EBRT. Interestingly, ADT improved biochemical control in high-risk prostate cancer, while in intermediate-risk patients, additional ADT did not reduce the risk of biochemical relapse. We did not notice advantages concerning biochemical control in ADT-treated patients.
We found two predictors of risk for GIT and GUT. The use of 3D-CRT doubled the risk for GUT of any grade. Likewise, the introduction of IMRT led to a risk reduction of 78% for therapy-related GIT. Although only 10 patients were evaluated by Nutting et al. [21], they also stated that IMRT may reduce therapy-related toxicity [10, 28].
Today, the advantages of IMRT over 3D-CRT are obvious. However, data on this specific patient cohort (e. g., elderly males after combined HDR-BT/EBRT) are still lacking. Also, effects may vary due to differences in BT protocols among different radiooncologic centers.
Interestingly, in our cohort, patients with a higher T stage (T2c–3a/b) were less likely to experience GIT or GUT of any grade. Faria and coauthors [8] assessed acute and late toxicity in 105 high-risk prostate cancer patients after hypofractioned irradiation delivered with IMRT and ADT. Within their cohort, acute GIT or GUT grades ≥2 were limited (17% each). Severe side effects occurred in 4 patients, 1 patient with GIT and 3 patients with GUT grade 3). However, statistical results of two different treatment methods, e.g. combined HDR-BT and EBRT vs. hypofractionated EBRT, cannot be transferred to one another due to biophysical and radiobiological differences. Nevertheless, in comparison to their low- and intermediate-risk counterparts, we assume that combined HDR-BT and EBRT in high-risk cancer patients does not result in a higher risk of experiencing severe GIT or GUT.
Pinkawa et al. [22] evaluated dose–volume-related factors in 64 patients who underwent HDR-BT (2 × 9 Gy) as a boost to EBRT. They stated that dose limitations of 15 Gy to the urethra and 6 Gy to the rectal mucosa were recommendable to minimize dose–volume-related side effects in BT. Our measured rectal doses fit these dose constraints. Nevertheless, in our patient cohort, measured rectal doses during BT were not a statistically significant predictor of GIT (HR: 1.06, 95% CI: 0.66–1.69, p = 0.821).
The main limitation of this study is its retrospective design. However, to our knowledge, this is the largest retrospective analysis mainly focusing on this age category. Another limitation relates to the interobserver-related differences, as patients were examined by different investigators during the treatment. Moreover, detailed information on the period of ADT administration was partly missing and not accessible in all cases. Finally, our analysis only included a Kaplan–Meier analysis on biochemical recurrence. Information on metastases-free, cancer-specific, and overall survival is missing. Still, biochemical relapse serves as a screening parameter to indicate tumor progression [17].
Conclusion
Although HDR-BT has been practiced over several decades, data on elderly patients ≥75 years are still very limited. In summary, we consider combined HDR-BT/EBRT to be a well-tolerated treatment option for older males with a modest number of comorbidities and localized intermediate- or high-risk prostate cancer. IMRT technique should be favored.
References
Alibhai SMH, Naglie G, Nam R, Trachtenberg J, Krahn MD (2003) Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol 17:3318–3327
Aoki M, Miki K, Kido M, Sasaki H, Nakamura W, Kijima Y, Kobayashi M et al (2014) Analysis of prognostic factors in localized high-risk prostate cancer patients treated with HDR brachytherapy, hypofractionated 3D-CRT and neoadjuvant/adjuvant androgen deprivation therapy (trimodality therapy). J Radiat Res 3:527–532
Boehm K, Schiffmann J, Tian Z, Lesmana H, Larcher A, Mandel P, Karakiewicz PI et al (2016) Five-year biochemical recurrence-free and overall survival following high-dose-rate brachytherapy with additional external beam or radical prostatectomy in patients with clinically localized prostate cancer. Urol Oncol 3(119):e11–e18
Cancer Research UK (2016) Prostate cancer incidence statistics. Prostate cancer incidence by age. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence#ref-1. Accessed 14 June 2018
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies. Development and validation. J Chron Dis 5:373–383
Cox J, Stetz J, Pajak T (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 5:1341–1346
Etzioni R, Penson DF, Legler JM, Di Tommaso D, Boer R, Gann PH, Feuer EJ (2002) Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 13:981–990
Faria S, Ruo R, Cury F, Duclos M, Souhami L (2017) Acute and late toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated pelvic radiation therapy. Pract Radiat Oncol 4:264–269
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, Forman D et al (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 6:1374–1403
Finazzi T, Guckenberger M (2017) Bildgeführte intensitätsmodulierte Strahlentherapie vermindert die gastrointestinalen Spätfolgen nach Radiotherapie des Prostatakarzinoms (Image-guided intensity-modulated radiation therapy decreases late gastrointestinal side effects after radiation therapy for prostate cancer). Strahlenther Onkol 2:162–164
Galalae RM, Martinez A, Nuernberg N, Edmundson G, Gustafson G, Gonzalez J, Kimming B (2006) Hypofractionated conformal HDR brachytherapy in hormone naive men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther Onkol 3:135–141
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M et al (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 1:124–137
Hjalm-Eriksson M, Ullen A, Johansson H, Levitt S, Nilsson S, Kalkner K‑M (2017) Comorbidity as a predictor of overall survival in prostate cancer patients treated with external beam radiotherapy combined with HDR brachytherapy boosts. Acta Oncol 1:21–26
Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L (2012) Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. J Natl Cancer Inst 2:217–222
Kim Y‑J, Cho KH, Pyo HR, Lee KH, Moon SH, Kim TH, Shin KH et al (2015) Radical prostatectomy versus external beam radiotherapy for localized prostate cancer. Comparison of treatment outcomes. Strahlenther Onkol 4:321–329
Kotecha R, Yamada Y, Pei X, Kollmeier MA, Cox B, Cohen G’aN, Zaider M et al (2013) Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy 1:44–49
López Torrecilla J, Hervás A, Zapatero A, Gómez Caamaño A, Macías V, Herruzo I, Maldonado X et al (2015) Uroncor consensus statement. Management of biochemical recurrence after radical radiotherapy for prostate cancer: from biochemical failure to castration resistance. Rep Pract Oncol Radiother 4:259–272
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, de Santis M, Fossati N et al (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1. Screening, diagnosis, and local treatment with curative intent. Eur Urol 4:618–629
National Cancer Institute (2018) Surveillance, Epidemiology, and End Results Program Cancer Stat Facts: Prostate Cancer. How Common Is This Cancer? https://seer.cancer.gov/statfacts/html/prost.html. Assessed: 14 June 2018
National Comprehensive Cancer Center Clinical Practice Guidelines in Oncology (2013) Prostate Cancer version 3. http://www.cus.cz/wp-content/uploads/2012/10/NCCN-C61-2014.pdf. Assessed: 14 June 2018
Nutting CM, Convery DJ, Cosgrove VP, Rowbottom C, Padhani AR, Webb S, Dearnaley DP (2000) Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys 3:649–656
Pinkawa M, Fischedick K, Treusacher P, Asadpour B, Gagel B, Piroth MD, Borchers H et al (2006) Dose-volume impact in high-dose-rate Iridium-192 brachytherapy as a boost to external beam radiotherapy for localized prostate cancer—a phase II study. Radiother Oncol 1:41–46
Roach M, Hanks G, Jr Thames H, Schellhammer P, Shipley WU, Sokol GH, Sandler H (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 4:965–974
Sathya JR, Davis IR, Julian JA, Guo Q, Daya D, Dayes IS, Lukka HR et al (2005) Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 6:1192–1199
Scherr D, Swindle PW, Scardino PT (2003) National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology 2:14–24
Schiffmann J, Lesmana H, Tennstedt P, Beyer B, Boehm K, Platz V, Tilki D et al (2015) Additional androgen deprivation makes the difference. Biochemical recurrence-free survival in prostate cancer patients after HDR brachytherapy and external beam radiotherapy. Strahlenther Onkol 4:330–337
Soumarova R, Homola L, Perkova H (2012) Long term results of HDR brachytherapy in men older than 75 with localized carcinoma of the prostate. Rep Pract Oncol Radiother 1:11–15
Waldstein C, Dörr W, Pötter R, Widder J, Goldner G (2018) Postoperative Strahlentherapie beim Prostatakarzinom. Morbidität nach lokaler Radiatio vs. lokaler Radiatio und Beckenbestrahlung (Postoperative radiotherapy for prostate cancer: Morbidity of local-only or local-plus-pelvic radiotherapy). Strahlenther Onkol 1:23–30
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Löser, B. Beyer, C.O. Carl, B. Löser, Y. Nagaraj, T. Frenzel, C. Petersen, A. Krüll, M. Graefen, and R. Schwarz declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Löser, A., Beyer, B., Carl, C.O. et al. Toxicity and risk factors after combined high-dose-rate brachytherapy and external beam radiation therapy in men ≥75 years with localized prostate cancer. Strahlenther Onkol 195, 374–382 (2019). https://doi.org/10.1007/s00066-018-1380-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1380-5