Abstract
Background
Cochlea sparing can reduce late ototoxicity in head and neck cancer patients treated with cisplatin-based radiochemotherapy. In this situation, a mean cochlear dose (MCD) constraint of 10 Gy has been suggested by others based on the dose–effect relationship of clinical data. We aimed to investigate whether this is feasible for primary and postoperative radiochemotherapy in locoregionally advanced tumors without compromising target coverage.
Patients and methods
Ten patients treated with definitive and ten patients treated with adjuvant intensity-modulated radiotherapy (IMRT) and concurrent chemotherapy were investigated. The cochleae and a planning risk volume (PRV) with a 3 mm margin were newly delineated, whereas target volumes and other organs at risk were not changed. The initial plan was recalculated with a constraint of 10 Gy (MCD) on the low-risk side. The quality of the resulting plan was evaluated using the difference in the equivalent uniform dose (EUD).
Results
A unilateral MCD of below 10 Gy could be achieved in every patient. The mean MCD was 6.8 Gy in the adjuvant cohort and 7.6 Gy in the definitive cohort, while the non-spared side showed a mean MCD of 18.7 and 30.3 Gy, respectively. The mean PRV doses were 7.8 and 8.4 Gy for the spared side and 18.5 and 29.8 Gy for the non-spared side, respectively. The mean EUD values of the initial and recalculated plans were identical. Target volume was not compromised.
Conclusion
Unilateral cochlea sparing with an MCD of less than 10 Gy is feasible without compromising the target volume or dose coverage in locoregionally advanced head and neck cancer patients treated with IMRT. A prospective evaluation of the clinical benefit of this approach as well as further investigation of the dose–response relationship for future treatment modification appears promising.
Zusammenfassung
Zielsetzung
Eine Schonung der Cochlea kann die Toxizität bei Patienten mit Kopf-Hals-Tumoren, die mit einer cisplatinbasierten Radiochemotherapie behandelt werden, senken. Eine prospektive klinische Studie errechnete eine mittlere cochleäre Dosis (MCD) von <10 Gy, die in der kombinierten Therapie maximal appliziert werden darf. Wir untersuchen, ob dies sowohl bei primär radiochemotherapierten als auch bei postoperativ radiochemotherapierten Kopf-Hals-Tumorpatienten möglich ist, ohne dass es zu Unterdosierungen im Zielgebiet kommt.
Patienten und Methoden
Bei 10 Patienten, die eine definitive Radiochemotherapie, und bei 10 Patienten, die eine adjuvante intensitätsmodulierte Radiotherapie (IMRT) und eine gleichzeitige Chemotherapie erhielten, wurden die Bestrahlungspläne unter der Prämisse einer einseitigen MCD <10 Gy neu berechnet. Hierfür wurde die Cochlea an sich sowie mit einem 3‑mm-Saum („planning risk volume“, PRV) neu skizziert; andere Risikoorgane und das Zielvolumen wurden nicht verändert. Die Qualität der resultierenden Bestrahlungspläne wurde nach dem Konzept der „equivalent uniform dose“ (EUD) evaluiert.
Ergebnisse
Eine einseitige MCD <10 Gy wurde in allen Fällen erzielt. Die MCD lag bei 6,8 Gy in der adjuvant therapierten und bei 7,6 Gy in der definitiven Kohorte. Die MCD der nichtgeschonten Cochleae wies 18,7 Gy bzw. 30,3 Gy auf. Die mittlere PVR-Dosis betrug 7,8 Gy bzw. 8,4 Gy der geschonten Cochleae und 18,5 Gy bzw. 29,8 Gy der Gegenseite. Die mittlere EUD der neu berechneten und der ursprünglichen Pläne waren identisch.
Schlussfolgerung
Eine einseitige Cochleaschonung mit einer MCD <10 Gy ist mit der IMRT möglich, ohne das Zielvolumen in fortgeschrittenen Kopf-Hals-Tumorpatienten zu kompromittieren. Eine prospektive Studie, die die Dosis-Wirkungs-Beziehung und den klinischen Benefit dieses Ansatzes näher untersucht, scheint vielversprechend.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Locoregionally advanced squamous cell carcinoma of the head and neck region (HNSCC) is usually treated with curative radiochemotherapy or surgery and adjuvant radio- or radiochemotherapy. Parallel cisplatin has become the standard chemotherapy [31]. With this approach, a substantial proportion of these patients are cured, making long-term toxicity an increasingly relevant topic. While some late toxicities such as xerostomia and dysphagia have been studied extensively [10, 39], there is a paucity of data concerning late inner ear toxicity [2, 29]. Symptoms associated with inner ear toxicity (hearing loss or deafness, tinnitus, vertigo, and postural problems) are known to significantly reduce quality of life and socioeconomic status of the patients [4, 8, 24, 30, 41]. Both radiotherapy and cisplatin-based chemotherapy can alone cause inner ear toxicity. In general, most authors agree that the combination of radiotherapy and cisplatin is more ototoxic than a single-modality treatment [3, 15,16,17, 20, 25, 32, 38]. To date, there is no generally accepted radiation dose constraint that is known to be safe for application to the inner ear. The QUANTEC analysis [3] found mean cochlear doses (MCD) of 35–45 Gy to be associated with late ototoxicity, with parallel cisplatin reducing the tolerated dose. In this respect, Hitchcock et al. proposed an MCD constraint of 10 Gy when using combined radiochemotherapy based on the results of a clinical study [15]. So far, this dose constraint has not been prospectively validated. The first step towards implementing a clinical trial to investigate the effect of the upper MCD limit of 10 Gy is to demonstrate that target volume coverage is not compromised. The purpose of our planning study was therefore to investigate whether it is feasible to achieve a unilateral MCD <10 Gy in both the definitive and the adjuvant radiochemotherapy setting without compromising the planning target volume or dose coverage, and without applying intolerably high doses to other organs at risk (OAR).
Patients and methods
We investigated 10 consecutive HNSCC patients with adjuvant radiochemotherapy and 10 consecutive patients with definitive radiochemotherapy (for characteristics, see Table 1). Patients with nasopharyngeal or laryngeal tumors were not included. All 20 patients had locoregionally advanced tumors and received bilateral neck irradiation with a simultaneous integrated boost with a Monte Carlo calculated volumetric modulated arc therapy (VMAT) approach (by Hyperion® [Tuebingen University, Tuebingen, Germany, in-house product]) in an individualized thermoplastic mask using a linear accelerator with 6 MV photons.
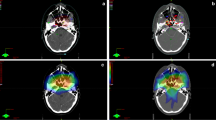
The cochlea was delineated in the 3 mm planning CT scans. Since ototoxicity might also be influenced by damage to other structures of the hearing apparatus, we additionally defined the vestibular organ, the auditory nerves, the middle ear, and the Eustachian tubes (for definition of the structures, see Table 2 and Fig. 1). Together with an otorhinolaryngologist specializing in otology, we reviewed several different contouring and anatomy atlases to develop our anatomical definition of the different structures [7, 13, 28, 33, 35]. For the cochlea, we calculated the dose for two different contours: first, for the fluid signal in the temporal bone only—“cochlea (f)”—and second, for the fluid signal with a 3 mm margin in every direction as a PRV approach—“cochlea (PRV).” The delineation of the initial target volumes and other OAR was not changed. All newly defined structures were delineated by two radiation oncologists together with the otorhinolaryngologist. We aimed to spare the cochlea on the side of the neck with the lower risk for subclinical disease (i.e., contralateral to the primary tumor and/or with less or more distant macroscopic lymph nodes). This was done by recalculating the plan with the identical constraints for target volumes and OAR used in the initial plan, except for the dose to the spared cochlea which was set below 10 Gy MCD. The resulting plan was optimized until clinically applicable and the differences in the resulting equivalent uniform dose (EUD), the dose to 98% (D98), 2% (D2), and 50% of the target volume (D50), and the conformality index (CI) were recorded. An example is depicted in Fig. 2.
Results
The delineation of organs at risk (OAR) for the inner ear, middle ear, and Eustachian tubes by two radiation oncologists was very consistent, with median differences ≤0.1 cm3, except for the tubes with a median difference of 0.7 cm3. In all patients, the cochlea could be spared as intended with an MCD of 6.8 Gy (cochlea (f)) and 7.8 Gy (cochlea (PRV)) for the adjuvant radiochemotherapy patient cohort. For the adjuvant patient cohort, the non-spared side received a mean MCD of 18.7 Gy (cochlea (f)) and 18.5 Gy (cochlea (PRV)). For the definitive radiochemotherapy cohort, a mean MCD of 7.6 Gy (cochlea (f)) and 8.4 Gy (cochlea (PRV)) was achieved while the contralateral side received 30.3 Gy (cochlea (f)) and 29.8 Gy (cochlea (PRV)), respectively (Table 3). Table 4 outlines the difference in MCD of the spared cochlea between the initial and the recalculated plan.
The absolute difference in EUD between the original and the recalculated plan was 0.15 Gy (range −1.2/0.6 Gy) for the adjuvant planning target volume and 0.05 Gy (range −0.5/0.8 Gy) for the boost volume (Table 5). All plans (initial and recalculated) were in accordance with the International Commission on Radiation Units (ICRU 83) report [9].
Discussion
For advanced tumors of the head and neck region, radiochemotherapy as a definitive therapy or adjuvant to surgery is an essential part of treatment. A better understanding of the biology of the disease together with more sophisticated radiation techniques such as IMRT and the combination with systemic treatment have led to an improvement in overall survival rates [6, 31]. As the long-term survivor rates are rising, long-term toxicities are becoming more important. Unfortunately, the combination of irradiation with platinum-based chemotherapy (e. g., cisplatin) leads to higher acute and potentially long-term toxicity in normal tissues [6, 31]. However, with IMRT, it is possible to reduce the radiation dose to vulnerable organs at risk such as the parotid glands, in order to avoid long-term toxicities without compromising tumor control as has already been shown for quality of life-impairing xerostomia [10, 14, 40].
An organ known to be particularly vulnerable to cisplatin is the inner ear, particularly the outer hair cells [19, 20]. Therefore, in recent years there has been growing interest in defining a maximum radiation dose that can be safely applied to the inner ear and retrocochlear structures, particularly in combination with cisplatin. For radiation only, a dose around (35–) 45 Gy is believed to be safe to prescribe to the inner ear structures in adult patients [3]. However, there is evidence that doses lower than 45 Gy MCD can cause clinically relevant ototoxicity, especially in patients who receive a combination of radiotherapy and platinum-based chemotherapy. In this regard, Theunissen et al. found 90.7% of patients receiving combined radiochemotherapy with a median cochlear dose of 13.6 Gy to experience hearing loss according to American Speech–Language–Hearing Association (ASHA) criteria [43]. This appears to be a very high rate of hearing deterioration; however, the patients received a high-dose cisplatin regimen (3 × 100 mg/m2) and the ASHA criteria are not as sophisticated as the Common Terminology Criteria for Adverse Events (CTCAEv4) scoring system for ototoxicity.
To define a clear dose–effect relationship between a distinct radiation dose combined with cisplatin and the resulting ototoxicity is challenging, due to varying fractionation schemes and total doses of radiotherapy, different cisplatin regimes, heterogeneous patient collectives, different timepoints of measuring hearing levels, and diverse grading scales for the evaluation of ototoxicity [36, 37]. In a prospective study by Hitchcock et al., the relative effects of radiation and cisplatin dose on the posttherapeutic hearing levels of 62 patients were evaluated. Based on the clinical data, a mathematical model was developed to estimate the probability of clinically relevant damage to the cochlea. Following their model, in combination with weekly low dose cisplatin (40 mg/m2), an MCD of 10 Gy or below is predicted to be well tolerated by the inner ear structures. Another essential result was the need to put a dose constraint on the cochlea. When using an inverse radiation planning approach, structures without a dose constraint may receive even higher total radiation doses than the target region, as was the case for the cochlea. Their conclusion is firstly, to put a dose constraint on the cochlea to avoid overdosing and secondly, to try to reach an MCD of 10 Gy in combination with cisplatin as long as tumor control is not compromised [15]. This might be lower than other suggested thresholds, but as an MCD of 10 Gy or below without compromising plan quality is achievable, the dose to the cochlea should be kept as low as possible, particularly with a combined radiochemotherapy approach.
The aim of our planning study was to investigate whether this MCD constraint of 10 Gy is achievable in a clinical setting with target volumes that were not designed for cochlear sparing in locoregionally advanced oro- to hypopharyngeal tumor patients. A premise for this approach was to realize a clinically applicable plan (i. e., to avoid underdosage in the target volume and overdosage in other organs at risk). Therefore, we did not include epipharyngeal tumors, since in most of these patients, there is no option to spare the cochlea to an MCD of 10 Gy or below as the GTV or at least the high-risk PTV is directly adjacent to the middle and inner ear structures. Furthermore, the incidence of these tumors is far lower, rendering a prospective investigation with adequate numbers challenging.
We achieved an MCD of 10 Gy or less in all recalculated plans for this locally advanced head and neck cancer patient collective. All plans were in accordance with the ICRU 83 report. The resulting mean EUDs of the PTC were identical between the initial and the recalculated plans, indicating that the dose coverage of the PTVs was not compromised. Regarding target volume coverage in terms of D2, D98, D50, and conformality index, there were no relevant differences between the plans (Table 6). Furthermore, the median difference in dose to the investigated OAR is almost unchanged (Table 7).
We could demonstrate that it is feasible to attain a consistent delineation of the cochlea in a 3 mm planning CT scan. Nevertheless, for small structures like the cochlea, a slice thickness of 1 or 2 mm would be more suitable. However, the evaluation of the MCD for the cochlea and the cochlea PRV showed only small differences, indicating that OAR variations of a few millimeters do not change the results significantly.
Potential clinical benefits of unilateral sparing of the cochlea for the patient may be the following: Firstly, if serviceable hearing on at least one side can be preserved, this has an enormous impact on the patient’s social participation, as it facilitates communication, e.g., on the telephone. Secondly, sparing the vestibular organ as well as the acoustic nerve can be crucial to avoid posttherapeutic vertigo and postural problems [5, 12], as well as to permit hearing rehabilitation by cochlea implantation, if necessary [1, 21,22,23]. Thirdly, besides the vestibulocochlear organ, the temporal bone itself will be spared excessive radiation doses. Therefore, the risk for temporal osteoradionecrosis, ageusia caused by lesions of the chorda tympani, and temporomandibular joint dysfunction may be reduced [18, 34].
There have already been attempts to perform cochlea-sparing radiotherapy in head and neck cancer patients. In a prospective study by Zuur et al. including 101 patients treated with radiation therapy only, a dose constraint was put on the cochlea according to the “as low as reasonably achievable” (ALARA) principle. The median dose to the cochlea was 17.8 Gy and the short-term hearing deterioration was negligible [38, 42]. In long-term follow-up of the remaining 36 patients, hearing loss due to radiation therapy remained subclinical [38]. Use of the ALARA principle for the cochlea represents a valuable tool for reducing radiation-associated ototoxicity, but unfortunately, no conclusion for the combined therapy can be drawn.
A study by Nguyen et al. demonstrated that tomotherapy can reduce MCD in comparison to IMRT in head and neck cancer patients [26, 27]. However, tomotherapy is not the standard radiation technique for head and neck tumors, and it is not widely available. Nevertheless, for a certain patient collective (e.g., locally advanced nasopharyngeal tumors), it might be beneficial for cochlea sparing due to its steeper dose gradient [11, 26].
Conclusion
The primary goal in cancer patients is to achieve long-lasting tumor control. With an increasing number of long-term survivors, quality of life is essential. Posttherapeutic xerostomia, trismus, fibrosis, and dysarthria are common in head and neck cancer patients. Additional hearing impairment potentiates the difficulties in communication. We believe that the impact of hearing loss on the socioeconomic status of these patients is still underestimated. Defining the cochlea as an avoidance structure is not routinely recommended in treatment protocols and contouring atlases, possibly leading to overdosage in the cochlea [15]. Our study demonstrates that a unilateral MCD of 10 Gy appears feasible without compromising target coverage in a locally advanced patient cohort. The clinical effects of this strategy should be prospectively investigated.
References
Adunka OF, Buchman CA (2007) Cochlear implantation in the irradiated temporal bone. J Laryngol Otol 121(01):83–86. https://doi.org/10.1017/S0022215106002180
Bhandare N, Antonelli P, Morris C, Malayapa R, Mendenhall W (2007) Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys 67(2):469–479. https://doi.org/10.1016/J.IJROBP.2006.09.017
Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P, Mendenhall WM (2010) Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys 76(3 Suppl). https://doi.org/10.1016/j.ijrobp.2009.04.096
Bhandare N, Mendenhall WM, Antonelli PJ (2009a) Radiation effects on the auditory and vestibular systems. Otolaryngol Clin North Am. https://doi.org/10.1016/j.otc.2009.04.002
Bhandare N, Mendenhall WM, Antonelli PJ (2009b) Radiation effects on the auditory and vestibular systems. Otolaryngol Clin North Am. https://doi.org/10.1016/j.otc.2009.04.002
Blanchard P, Baujat P, Holostenco V, Bourredjem A, Baey C, Bourhis J, Pignon J (2011) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother Oncol 100(1):33–40. https://doi.org/10.1016/J.RADONC.2011.05.036
Brouwer CL, Steenbakkers RJHM, Bourhis J, Budach W, Grau C, Grégoire V et al (2015) CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol 117:83–90. https://doi.org/10.1016/j.radonc.2015.07.041
Cohen SM, Turley R (2009) Coprevalence and impact of dysphonia and hearing loss in the elderly. Laryngoscope 119(9):1870–1873. https://doi.org/10.1002/lary.20590
Das IJ, Andersen A, Chen ZJ, Dimofte A, Glatstein E, Hoisak J et al (2017) State of dose prescription and compliance to international standard (ICRU-83) in intensity modulated radiation therapy among academic institutions. Pract Radiat Oncol 7(2):e145–e155. https://doi.org/10.1016/J.PRRO.2016.11.003
Eisbruch A (2009) Radiotherapy: IMRT reduces xerostomia and potentially improves QoL. Nat Rev Clin Oncol 6(10):567–568. https://doi.org/10.1038/nrclinonc.2009.143
Fiorino C, Dell’Oca I, Pierelli A, Broggi S, Cattaneo GM, Chiara A et al (2007) Simultaneous Integrated Boost (SIB) for nasopharynx cancer with helical tomotherapy. Strahlenther Onkol 183(9):497–505. https://doi.org/10.1007/s00066-007-1698-x
Gabriele P, Orecchia R, Magnano M, Albera R, Sannazzari G (1992) Vestibular apparatus disorders after external radiation therapy for head and neck cancers. Radiother Oncol 25(1):25–30. https://doi.org/10.1016/0167-8140(92)90191-V
Genovesi D, Perrotti F, Trignani M, Di Pilla A, Vinciguerra A, Augurio A et al (2015) Delineating brachial plexus, cochlea, pharyngeal constrictor muscles and optic chiasm in head and neck radiotherapy: a CT-based model atlas. Radiol Med 120:352–360. https://doi.org/10.1007/s11547-014-0448-2
Hawkins P, Lee J, Mao Y, Li P, Green M, Worden F et al (2017) Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother Oncol. https://doi.org/10.1016/J.RADONC.2017.08.002
Hitchcock YJ, Tward JD, Szabo A, Bentz BG, Shrieve DC (2009) Relative contributions of radiation and cisplatin-based chemotherapy to sensorineural hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 73(3):779–788. https://doi.org/10.1016/j.ijrobp.2008.05.040
Huang E, Teh BS, Strother DR, Davis QG, Chiu JK, Lu HH, Carpenter LS, Mai WY, Chintagumpala MM, South M, Grant WH 3rd, Butler EB, Woo SY (2002) Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 52(3):599-605
Jereczek-Fossa BA, Zarowski A, Milani F, Orecchia R (2003) Radiotherapy-induced ear toxicity. Cancer Treat Rev. https://doi.org/10.1016/S0305-7372(03)00066-5
Johannesen TB, Rasmussen K, Winther FØ, Halvorsen U, Lote K (2002) Late radiation effects on hearing, vestibular function, and taste in brain tumor patients. Int J Radiat Oncol Biol Phys 53:86–90
Karasawa T, Steyger PS (2016) An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett 237(3):219–227. https://doi.org/10.1016/j.toxlet.2015.06.012.An
Landier W (2016) Ototoxicity and cancer therapy. Cancer. https://doi.org/10.1002/cncr.29779
Low W‑K, Gopal K, Goh LK, Fong KW (2006) Cochlear implantation in postirradiated ears: outcomes and challenges. Laryngoscope 116(7):1258–1262. https://doi.org/10.1097/01.mlg.0000225935.80559.11
Low W‑K, Tan MG, Chua AW, Sun L, Wang D‑Y (2009) 12th Yahya cohen memorial lecture—the cellular and molecular basis of radiation-induced sensori-neural hearing loss. Ann Acad Med Singapore 38(1):91–94
Low WK, Burgess R, Fong KW, Wang DY (2005) Effect of radiotherapy on retro-cochlear auditory pathways. Laryngoscope 115(10):1823–1826. https://doi.org/10.1097/01.mlg.0000175061.59315.58
Monzani D, Galeazzi GM, Genovese E, Marrara A, Martini A (2008) Psychological profile and social behaviour of working adults with mild or moderate hearing loss. Acta Otorhinolaryngol Italica 28(2):61–66 (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18669069)
Mujica-Mota M, Waissbluth S, Daniel SJ (2014) Characteristics of radiation-induced sensorineural hearing loss in head and neck cancer: a systematic review. Head Neck 36(10):1391. https://doi.org/10.1002/HED
Nguyen NP, Ceizyk M, Vinh-Hung V, Sroka T, Jang S, Khan R et al (2012) Feasibility of tomotherapy to reduce cochlea radiation dose in patients with locally advanced nasopharyngeal cancer. Tumori J 98(6):709–714. https://doi.org/10.1700/1217.13493
Nguyen N, Smith-Raymond L, Vinh-Hun V, Sloan D, Davis R, Vosc P et al (2011) Feasibility of tomotherapy to spare the cochlea from excessive radiation in head and neck cancer. Oral Oncol 47(5):414–419. https://doi.org/10.1016/J.ORALONCOLOGY.2011.03.011
Pacholke HD, Amdur RJ, Schmalfuss IM, Louis D, Mendenhall WM (2005) Contouring the middle and inner ear on radiotherapy planning scans. Am J Clin Oncol 28:143–147. https://doi.org/10.1097/01.coc.0000143847.57027.16
Pan CC, Eisbruch A, Lee JS, Snorrason RM, Ten Haken RK, Kileny PR (2005) Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2004.08.019
Peng G, Wang T, Yang K, Zhang S, Zhang T, Li Q et al (2012) A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 104(3):286–293. https://doi.org/10.1016/J.RADONC.2012.08.013
Pignon J‑P, le Maître A, Maillard E, Bourhis J (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1):4–14. https://doi.org/10.1016/J.RADONC.2009.04.014
Scobioala S, Parfitt R, Ebrahimi F, Matulat P, Zehnhoff-Dinnesen A, Eich H (2017) Impact of radiation technique, fraction dose and total cisplatin dose on hearing: Retrospective analysis of 29 medulloblastoma patients. Strahlenther Onkol 193(1):96–S97. https://doi.org/10.1007/s00066-017-1137-6
Scoccianti S, Detti B, Gadda D, Greto D, Furfaro I, Meacci F et al (2015) Organs at risk in the brain and their dose-constraints in adults and in children: A radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol 114:230–238. https://doi.org/10.1016/j.radonc.2015.01.016
Strojan P, Hutcheson KA, Eisbruch A, Beitler JJ, Langendijk JA, Lee AWM et al (2017) Complications of treatment treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev 59:79–92. https://doi.org/10.1016/j.ctrv.2017.07.003
Sun Y, Yu XL, Luo W, Lee AWM, Wee JTS, Lee N et al (2014) Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother Oncol 110:390–397. https://doi.org/10.1016/j.radonc.2013.10.035
Theunissen EAR, Bosma SCJ, Zuur CL, Spijker R, van der Baan S, Dreschler WA et al (2014) Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: A systematic review of the literature. Head Neck 36(10):1391. https://doi.org/10.1002/HED
Theunissen EAR, Dreschler WA, Latenstein MN, Rasch CRN, van der Baan S, de Boer JP et al (2014) A new grading system for ototoxicity in adults. Ann Otol Rhinol Laryngol 123(10):711–718. https://doi.org/10.1177/0003489414534010
Theunissen E, Zuur CL, Lopez Yurda M, Van Der BS, Kornman AF, De Boer PJ et al (2014) Cochlea sparing effects of intensity modulated radiation therapy in head and neck cancers patients: a long-term follow-up study. J Otolaryngol Head Neck Surg 43. https://doi.org/10.1186/s40463-014-0030-x
Van Der Laan HP, Bijl HP, Steenbakkers RJHM, Van Der Schaaf A, Chouvalova O, Vemer-Van Den Hoek JGM et al (2015) Prediction of dysphagia acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiother Oncol 115:56–62. https://doi.org/10.1016/j.radonc.2015.01.019
Vergeer MR, Doornaert PAH, Rietveld DHF, Leemans CR, Slotman BJ, Langendijk JA (2009) Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 74(1):1–8. https://doi.org/10.1016/j.ijrobp.2008.07.059
Wu Y, Hu W‑H, Xia Y‑F, Ma J, Liu M‑Z, Cui N‑J (2007) Quality of life of nasopharyngeal carcinoma survivors in Mainland China. Qual Life Res 16(1):65–74. https://doi.org/10.1007/s11136-006-9113-0
Zuur CL, Simis YJ, Lamers EA, Hart AA, Dreschler WA, Balm AJ, Rasch CR (2009) Risk factors for hearing loss in patients treated with intensity-modulated radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys 74(2):490–496. https://doi.org/10.1016/j.ijrobp.2008.08.011
Theunissen EA, Zuur CL, Jozwiak K, Lopez-Yurda M, Hauptmann M, Rasch CR, van der Baan S, de Boer JP, Dreschler WA, Balm AJ (2015) Prediction of hearing loss due to cisplatin chemoradiotherapy. JAMA Otolaryngol Head Neck Surg 141(9):810–815
Lange J (2013) Klinische Anatomie des Ohres. Springer, Wien
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.H. Braun, K. Braun, B. Frey, S.M. Wolpert, H. Löwenheim, D. Zips, and S. Welz declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Braun, L.H., Braun, K., Frey, B. et al. Unilateral cochlea sparing in locoregionally advanced head and neck cancer: a planning study. Strahlenther Onkol 194, 1124–1131 (2018). https://doi.org/10.1007/s00066-018-1344-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1344-9