Abstract
Purpose
To investigate the impact of primary gross tumor volume (pGTV) and nodal gross tumor volume (nGTV) in oropharyngeal squamous cell carcinoma (OPSCC) and the difference in their role between human papillomavirus (HPV)-positive and HPV-negative patients.
Methods
The patient cohort consists of 91 OPSCC patients treated with definitive radiochemotherapy or radiotherapy using intensity-modulated radiotherapy (IMRT). All patients had a minimum follow-up of 31 months. Volume measurements were made from computer tomography (CT) scans and HPV status was assessed by p16 immunohistochemistry. The end points were as follows: overall survival (OS), disease-free survival (DFS) and locoregional control (LRC).
Results
pGTV was a significant independent prognostic factor for overall survival (OS; p = 0.020) in p16-negative patients. nGTV of p16-negative tumors had significant prognostic value in all end points in multivariate analyses. High-stage (III–IVc) p16-negative tumors were only associated with significantly poorer OS (p = 0.046) but not with poorer LRC or DFS when compared with the low-stage (I–II) tumors. nGTV of p16-positive tumors was an independent prognostic factor for DFS (p = 0.005) and LRC (p = 0.007) in multivariate analyses.
Conclusion
pGTV may serve as an independent prognostic factor in p16-negative patients and nGTV may serve as an independent prognostic factor both in p16-positive and p16-negative patients treated with radiochemotherapy or radiotherapy using IMRT. Tumor volume may have an impact on selecting patients for de-escalation protocols in the future, both in p16-positive and p16-negative patients.
Zusammenfassung
Zielstellung
Ziel war es, die Bedeutung des makroskopischen Tumorvolumens des Primärtumors („primary gross tumor volume“, pGTV) und des makroskopischen Tumorvolumens der Lymphknoten („nodal gross tumor volume“, nGTV) bei Plattenepithelkarzinomen des Oropharynx („oropharyngeal squamous cell carcinoma“, OPSCC) und deren Unterschiede zwischen für humanes Papillomavirus (HPV) positiven und HPV-negativen Patienten zu untersuchten.
Methoden
In die Studie eingeschlossen wurden 91 OPSCC-Patienten, die mit definitiver intensitätsmodulierter Strahlentherapie („intensity-modulated radiotherapy“, IMRT) mit oder ohne gleichzeitige Chemotherapie behandelt wurden. Bei allen Patienten betrug der Nachbeobachtungszeitraum mindestens 31 Monate. Die Volumenmessungen erfolgten anhand von Computertomographie(CT)-Aufnahmen. Der HPV-Status wurde durch die p16-Immunhistochemie ermittelt. Die Endpunkte waren Gesamtüberleben („overall survival“, OS), krankheitsfreies Überleben („disease-free survival“, DFS) und lokoregionale Kontrolle („locoregional control“, LRC).
Ergebnisse
Das pGTV war ein signifikanter unabhängiger prognostischer Faktor für das OS (p = 0,20) bei p16-negativen Patienten. Das nGTV von p16-negativen Tumoren wies einen signifikanten prognostischen Wert für alle Endpunkte in multivariaten Analysen auf. p16-negative Tumoren in hohen Stadien (III-IVc) waren im Vergleich zu den Tumoren in niedrigeren Stadien (I‑II) nur mit einem signifikant schlechteren OS (p = 0,046), nicht aber mit schlechteren Werten für LRC oder DFS assoziiert. Das nGTV von p16-negativen Tumoren erwies sich in multivariaten Analysen als unabhängiger prognostischer Faktor für DFS (p = 0,005) und LRC (p = 0,007).
Schlussfolgerung
Das pGTV kann als unabhängiger prognostischer Faktor bei p16-negativen Patienten und das nGTV als unabhängiger prognostischer Faktor sowohl bei p16-positiven als auch bei p16-negativen Patienten dienen, bei denen eine Radiochemotherapie oder Strahlentherapie unter Verwendung der IMRT erfolgt. Das Tumorvolumen kann bei der Auswahl der Patienten für ein Deeskalationsprotokoll sowohl bei p16-positiven als auch bei p16-negativen Patienten eine Rolle spielen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing evidence exists for the role of primary gross tumor volume (pGTV) and nodal gross tumor volume (nGTV) as prognostic markers in head and neck cancer (HNSCC), especially in oropharyngeal squamous cell carcinoma (OPSCC). Higher pGTV has been shown to correlate with increased risk of recurrence and poorer survival [1,2,3,4,5,6,7,8,9,10]. The suggestion for this phenomenon is that a larger tumor contains a higher number of clonogenic cells, which may impair treatment response to radiotherapy [11,12,13]. Patients with larger tumor volumes may need to be treated with a higher dose of radiotherapy to achieve a favorable treatment response, whereas smaller tumor volumes may need lower doses of radiotherapy and thus avoid toxic side effects of the treatment [10, 14].
The tumor, node, metastasis (TNM) classification is a validated standard tool for prognostic evaluation of OPSCC management according to disease stage. It is based on tumor dimensions and extension with metric and anatomic criteria [15, 16]. However, previous studies have indicated that tumor volume as a prognostic factor may be superior to TNM staging among patients treated with radiotherapy [3, 6, 9, 10, 17]. The limitation of TNM classification and staging is that the system may classify tumors with different volumes into the same stage due to one-dimensional determination [3, 6, 10, 18]. The benefit of volume measuring is the information of exact three-dimensional structure of the tumor and estimation of the total tumor mass [1, 3, 6]. Furthermore, the recent 8th edition of the TNM classification divides tumors by p16 status into HPV-positive and HPV-negative OPSCC, and takes into account extracapsular spread of nodal metastases [19, 20].
Previous studies have indicated that pGTV may have prognostic impact on local control in OPSCC [1, 6, 13, 18]. In addition, it has been observed to carry prognostic value also for overall survival and disease-free survival [1, 3, 6]. As is well-known, a clear majority of OPSCCs are HPV positive in the Western countries, with more favorable outcome and response to (chemo)radiotherapy compared to HPV-negative tumors [21,22,23,24,25]. However, there is still an unexplained variation in OPSCC prognosis and treatment outcome irrespective of HPV status. Tumor volume as a prognostic factor has been studied in OPSCC patients [17] in general, and separately only in p16-positive patients [17]; studies comparing p16-negative and p16-positive tumors are still missing. In addition, clear cut-off values for volumes predicting poorer survival remain unknown and the previous studies were done with the 7th edition of TNM classification. Our aim was to study the prognostic value of primary and nodal tumor volume in p16-positive and p16-negative OPSCC tumors by using the 8th edition of the TNM classification.
Materials and methods
Study population
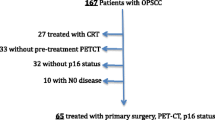
The inclusion criteria for the study were newly diagnosed biopsy-proven OPSCC patients with available tumor p16 status treated with radiochemotherapy or radiotherapy using IMRT. Patients with distant metastases or who underwent neck dissection and primary tumor resection before radiotherapy as well as patients treated with palliative intent were excluded. CONSORT diagram of patient selection is shown in Fig. 1. Altogether, there were 91 consecutive OPSCC patients who met the inclusion criteria and who had been treated at the Departments of Oncology and Otorhinolaryngology—Head and Neck Surgery at the Helsinki University Hospital, Helsinki, Finland during a 3-year time period between January 2012 and December 2014. An institutional study permission was granted for the study design.
Clinical data were collected from electronic medical records. Data included gender, age at the date of OPSCC diagnosis, tobacco smoking status, and the date of radiation treatment termination. History of tobacco smoking was defined as “non-smoker” or ex-smoker/current smoker. Ex-smoker was defined as a user who had more than a 1-year history of tobacco smoking. Tumor spread and stage were evaluated according to the 8th edition of the TNM classification.
Primary and nodal tumor volume evaluation
The pGTV and nGTV were calculated three dimensionally from CT scans by an experienced head and neck radiation oncologist using Varian Eclipse® radiotherapy treatment planning system versions 10.0 and 11.0 (Varian Medical Systems Inc., Palo Alto, CA, USA). The volume results are given in cubic centimeters (cm3). We often use combined MRI and CT imaging for radiotherapy dose planning. MRI was, however, not done for all patients and we therefore we used CT for volume measurements. Different imaging modalities may produce significantly different GTVs [26, 27]. Thus, using MRI measures in the patients for whom it was available and CT-based volumes for the rest of the patients could have resulted in non-comparable GTV volumes. The radiotherapy planning CT was done with contrast enhancement in all patients using Omnipaque™ injection 350 mg I/ml solution. In addition, nodal involvement was defined according to generally accepted criteria, where a minimal axial diameter of 10 mm was the size criterion in the digastric region for the lymph nodes and the size criterion in the subdigastric region was 11 mm [28]. In addition, lymph node necrosis and cystic lymph node metastases were defined according to generally accepted criteria [28, 29].
p16-INK4a immunohistochemistry
We detected the p16-INK4a status by immunohistochemistry on paraffin-embedded formalin-fixed tissue samples. Positive p16 expression was defined when over 70% of tumor cells were positive. Xylene was used for deparaffinization and rehydration was done in graded series of alcohol. “PreTreatment module” (Lab Vision Corp., UK) was used to treat the tissue slides in Tris-HCl buffer (pH 8.5), and endogenous peroxidase was blocked with 0.3% Dako REAL Peroxidase-Blocking Solution. Monoclonal mouse anti-human p16-INK (9517 CINtec Histology Kit, MTM laboratories, Germany) was used as primary antibody.
Radiotherapy and chemotherapy
All tumors were treated with curative intent by radiochemotherapy or radiotherapy using IMRT up to 70 Gray (Gy), in 2 Gy fractions per day with or without platinum-based chemotherapy. In addition, elective nodal areas were treated up to 50 Gy. Chemotherapy consisted of cisplatin 40 mg/m2 given weekly during the radiotherapy.
Follow-up time
Follow-up time was defined as a time in months from the date of treatment termination to the date of last follow up. Follow-up appointments were planned every 3 months in the first year after completion of treatment, every 3 to 4 months in the second year, and in the next 3 years every 6 months.
Statistical analysis
Data were analyzed with a statistical software program (IBM SPSS Statistics 24, IBM, Somers, IL, USA). The primary endpoints were overall survival (OS), disease-free survival (DFS), and locoregional control (LRC) separately for p16-positive and p16-negative patients. Survival time was defined as time in months from the completion of treatment to the date of last follow-up or death. Locoregional failure was defined as tumor residual or a biopsy-proven recurrence locally or regionally during follow-up.
All analyses were conducted separately with pGTV and nGTV in p16-positive and p16-negative patients. Survival curves of dichotomized groups were calculated by the Kaplan–Meier method, and compared using Breslow tests. Cox regression hazard models were used for multivariate analyses evaluating OS, LRC, and DFS. Each of the potential prognostic factors (pGTV, nGTV, T category, N category, and stage) were analyzed in separate Cox regression multivariate models. The models were adjusted for age and gender as they are general clinically relevant factors. The results are presented as mean, median, or number of patients when appropriate. Continuous variables and dichotomized groups’ relationships were assessed by eta correlation coefficient. A two-sided p-value of less than 0.05 was considered statistically significant.
Results
Clinical patient and tumor data of 91 newly diagnosed oropharyngeal cancer cases were evaluated. Majority of the patients were male (72, 79.1%) and the mean age was 62 years (range 41.4–84.7 years). All patients had a minimum follow-up time of 31 months.
Immunohistochemical p16 status was determined for all cases. There were 72 (79.1%) p16-positive and 19 (20.9%) p16-negative cases. All expect 11 (12.1%) patients underwent definitive radiochemotherapy using IMRT. The reasons for the 11 patients receiving IMRT only without chemotherapy were as follows: small primary tumor size without regional metastases for two patients, age over 80 years for three, and high serum creatinine levels or other comorbidities for six. All the patient- and tumor-related demographic parameters are summarized in Table 1.
p16 status and survival
OS (p < 0.001), LRC (p < 0.001), and DFS (p < 0.001) rates were significantly more favorable among p16-positive patients compared with p16-negative patients during the whole follow-up. For p16-positive patients, the estimated 2‑year OS was 93.1% (67/72), DFS 87.5% (63/72), and LRC 93.1% (67/72), whereas for p16-negative patients, OS was 63.2% (12/19), DFS 57.9% (11/19), and LRC 68.4% (13/19). Distant metastases occurred only in two p16-positive patients and in two p16-negative patients during the whole follow-up and therefore the effect of this phenomenon on survival could not be evaluated statistically.
Tumor volume and prognosis in p16-negative patients
The mean pGTV for p16-negative tumors was 38 cm3 (standard deviation, SD, 45.87) and median 19 cm3 (range 1–147). pGTV as a continuous variable in a multivariate model in p16-negative patients showed an independent statistically significant impact on OS (p = 0.020; hazard ratio, HR, 1.02; 95% confidence interval, CI95%, 1.00–1.04) but not in LRC or DFS when age and gender were adjusted (Table 2). Furthermore, for different analyses, T categories of p16-negative tumors were distributed to low (T1–T2) and high (T3–T4b) T category groups. Small and large T categories groups were analyzed and compared in a similar multivariate model as above, without showing any significant prognostic value in any of the endpoints (Table 2). Due to high reciprocal correlation (eta correlation coefficient 0.978), it was not possible to present different T categories and pGTV in the same multivariate Cox regression model.
For the survival analyses and in order to assess clear survival cut-off values, pGTV of p16-negative tumors was also dichotomized by its mean value to small (≤38 cm3) and large volumes (>38 cm3) and by its median value to small (≤19 cm3) and large volumes (>19 cm3). The group of large pGTV (>38 cm3) had statistically significantly poorer OS (p = 0.005) and DFS (p = 0.028), and closely but not significantly poorer LRC (p = 0.062) compared with the small pGTV group. Furthermore, when dichotomized and compared by its median value, large pGTV (>19 cm3) was associated with significantly poorer DFS (p = 0.049) and LRC (p = 0.029), and closely but not significantly poorer OS (p = 0.064) when compared with the small pGTV (≤19 cm3) group. As with the p16-positive patients, we also dichotomized T categories of p16-negative patients to small (T1–T2) and large (T3–T4b) T category groups. We found no significant differences in OS (p = 0.263), DFS (p = 0.458), or LRC (p = 0.371) between these two groups by Breslow test in Kaplan–Meier curves (Fig. 2) or in multivariate models (Table 2).
The mean nGTV for p16-negative tumors was 13 cm3 (SD 34.22) and median 0 cm3 (range 0–142). As a continuous variable, nGTV in p16-negative patients showed an independent statistically significant impact on OS (p = 0.027, HR 1.02, CI95% 1.01–1.04), LRC (p = 0.017, HR 1.02, CI95% 1.00–1.04), and DFS (p = 0.022, HR 1.02, CI95% 1.00–1.04) in a multivariate model (Table 2). nGTV dichotomized by its mean value to small and large volume groups did not show any differences in any of the endpoints. nGTV could not be dichotomized by its median value (0 cm3).
For further analyses, N categories were dichotomized to low (N0–N2a) and high (N2b–N3b) N category groups. We found no significant differences in OS (p = 0.548), DFS (p = 0.685), or LRC (p = 0.284) between these two groups by using Breslow test or in multivariate models. Clinical stage of p16-negative tumors where dichotomized to low (I–II) and high (III–IVc) stages. High-stage group (III–IVc) was associated with statistically significantly poorer OS (p = 0.046, HR 9.86, CI95% 1.05–93.03) but not with poorer LRC or DFS in multivariate models (Table 2). In addition, high-stage (III–IVc) group had no significant difference in OS (p = 0.169), DFS (p = 0.362), or LRC (p = 0.234) when compared with the low-stage (I–II) group. All the results of multivariate analyses are shown in Table 2. Survival differences between different groups of pGTV, T category, N category, and stage on OS in p16-negative patients are shown as Kaplan–Meier curves in Fig. 2 and on LRC in Fig. 3.
Tumor volume and prognosis in p16-positive patients
The mean pGTV for p16-positive tumors was 23 cm3 (SD 29.03) and median 14 cm3 (range 1–190). As a continuous variable pGTV showed no independent statistically significant impact on any of the endpoints (OS, LRC, or DFS) in p16-positive patients in a Cox regression multivariate model when age and gender were adjusted (Table 2). Furthermore, for different analyses, T categories of p16-positive tumors were distributed to low (T1–T2) and high (T3–T4) T category groups. Dichotomized T categories were analyzed in a similar multivariate model as above and a significant prognostic value was not found in any of the endpoints (Table 2). Due to high reciprocal correlation (eta correlation coefficient 1.000), it was not possible to present different T categories and pGTV in the same multivariate Cox regression model.
For the survival analyses and in order to assess clear survival cut-off values, pGTV was dichotomized into two different volume groups by its mean value to small (≤23 cm3) and large volumes (>23 cm3) and by its median value to small (≤14 cm3) and large volumes (>14 cm3). We found no significant differences in OS (p = 0.676), DFS (p = 0.662), or LRC (p = 0.601) between small and large pGTV when dichotomized by its mean value or for OS (p = 0.507), DFS (p = 0.752), or LRC (p = 0.887) when dichotomized by its median value in p16-positive patients. Furthermore, T categories were distributed to small (T1–T2) and large (T3–T4) tumors and compared in a multivariate model without showing any statistically significant impact on any of the endpoints (Table 2). Small and large T categories were also analyzed with Breslow test as Kaplan–Meier curves, but no significant prognostic differences were found for OS (p = 0.895), DFS (p = 0.901), or LRC (p = 0.603).
The mean nGTV for p16-positive tumors was 26 cm3 (SD 34.12) and median 15 cm3 (range 0–200). As a continuous variable, nGTV showed an independent statistically significant impact on DFS (p = 0.005, HR 1.02, CI95% 1.01–1.03) in p16-positive patients in a Cox regression multivariate model when age and gender were adjusted (Table 2). Furthermore, large nGTV was associated with statistically significant poorer DFS (p = 0.046) when dichotomized by its mean value to small (≤26 cm3) and large (>26 cm3) volumes. Statistically significant differences were not found in OS (p = 0.508) or LRC (p = 0.159) between small and large nGTV when dichotomized by its mean value. nGTV was also dichotomized and analyzed by its median value, but significant prognostic differences in OS (p = 0.625), DFS (p = 0.627), or LRC (p = 0.673) were not found between small and large volumes. For comparison, we also dichotomized N categories to low (N0–N1) and high (N2–N3) N categories and clinical stages to low (I–II) and high (III–IV) stages. Different N categories or clinical stages showed no prognostic differences in any of the endpoints in multivariate models when age and gender were adjusted (Table 2). In addition, comparison of low and high N categories showed no significant prognostic differences in OS (p = 0.124), DFS (p = 0.328), or LRC (p = 0.806), whereas the high clinical stage (III–IV) group had significantly poorer DFS (p = 0.041) but not OS (p = 0.056) or LRC (p = 0.123) when compared with the low-stage (I–II) group. Multivariate analyses are shown in Table 2. Survival differences in DFS between different groups of nGTV, T categories, N categories, and stages in p16-positive patients are shown as Kaplan–Meier curves in Fig. 4. Finally, an example of a treatment planning CT scan of a p16-positive tumor is illustrated in Fig. 5.
P16-positive tumor (T2N2) of the base of tongue: primary gross tumor volume (pGTV) yellow contour, nodal gross tumor volume (nGTV; left) brown contour; clinical target volume (CTV) light blue contour; planning target volume (PTV) red contour; right submandibular gland blue contour; left submandibular gland dark blue contour; spinal canal purple contour. Metastasis at the right side of neck is not visualized at this level
Discussion
This single-institution study on 91 OPSCC patients treated with definitive radiochemotherapy or radiotherapy using IMRT provides new evidence that pGTV and nGTV may have specific roles as independent prognostic factors for the subgroups of p16-negative and p16-positive cancers.
pGTV was found to be an independent prognostic factor in p16-negative patients for OS in multivariate analysis where age and gender were adjusted. Large p16-negative tumors with the mean pGTV (38 cm3) as the cut-off value were associated with significantly poorer OS and DFS, and the trend was similar with LRC but not significant when compared with smaller tumors. p16-negative tumors larger than a median pGTV (>19 cm3) were associated with significantly poorer DFS and LRC, and the trend was similar for OS but not statistically significant. For comparison, T categories, N category, and stage of p16-negative patients were dichotomized into low and high groups but none had differences in any of the endpoints. In multivariate analyses of T category, N category, and stage, only stage had prognostic impact on OS but the confidence interval was relatively high. pGTV, T category, N category, or clinical stage showed no significant impact on any of the endpoints in p16-positive patients in multivariate analyses. Our findings of the prognostic value of pGTV in OPSCC are in line with previous studies [1, 3, 6, 17, 18] but we are the first to report these findings separately in p16-positive and p16-negative tumors and according to the newest TNM classification [19].
nGTV of p16-negative tumors had significant prognostic value in all endpoints in multivariate analyses but significant differences were not found between large and small nGTV groups when dichotomized by its mean or median value. As a conclusion, nGTV may serve as a prognostic factor for p16-negative patients but clear cut-off values remain unknown. nGTV of p16-positive tumors was an independent prognostic factor for DFS and LRC in multivariate analyses and DFS was significantly poorer among large tumors when dichotomized by mean nGTV (26 cm3) to large and small tumors. Differences in survival were not found between large and small tumors when the cut-off value was assessed by nGTV median value (15 cm3). Davis et al. 2016 had similar results of nGTV for DFS in p16-positive tumors in multivariate analysis but they did not analyze impact of nGTV on LRC. To our knowledge, this is the first report to study the prognostic value of pGTV and nGTV both in multivariate analyses and with different volume cut-off values in p16-positive tumors.
As shown in various studies [21, 25, 30], p16-positive primary tumors are in general smaller compared with p16-negative tumors by T category but have a tendency to spread more often to regional lymph nodes. Despite regional spreading, their response to (chemo)radiotherapy, and thus also locoregional control, are more favorable [21, 23, 25]. Our findings are in line with previous studies showing p16-negative tumors to be larger by T category, whereas p16-positive tumors have a tendency to spread regionally more often. In addition, the prognosis of p16-positive patients was significantly more favorable compared with p16-negative tumors. pGTV of p16-negative tumors was clearly larger and nGTV smaller when compared with p16-positive tumors, which correlates with their T and N categories defined with the new 8th TNM classification. However, a few cases had different T categories or N categories despite similar pGTV and nGTV values (data not shown). The definition of T category and N category is based on diametric and anatomic criteria [15, 16], whereas volume is measured three-dimensionally, which seems to give a more accurate dimension for the tumor and thus explain our findings. Larger tumor mass (volume) may contain a larger number of malignant tumor cells [11,12,13], which in certain cut-off values may impair treatment response to concurrent radiochemotherapy or radiotherapy using IMRT in OPSCC. In addition, the differences in treatment response may be explained by the differences in biological and genetic background of p16-positive and p16-negative tumors according to the viral status [24, 31]. We found a significant impact of pGTV on all endpoints in p16-negative patients depending on mean or median cut-off values as shown above.
There are clear etiological, biological, clinical, and treatment planning differences between p16-positive and p16-negative tumors. Furthermore, the majority of newly diagnosed OPSCCs are currently p16-positive in Western countries [23]. Therefore, the recently launched UICC (8th edition) TNM classification includes specific criteria for p16-positive patients [20]. The new classification also includes extracapsular spread as a parameter for nodal extension estimation and thus further aids in treatment planning and prognostication [19, 32]. In our study, TNM classification of the tumors was made with the newest 8th edition, which was a clear strength of the study design compared with previous OPSCC studies. Another study strength was a homogenous study group with a long follow-up time without dropouts. The clear study limitations were lack of HPV detection with PCR or in situ hybridization, and a relatively small number of p16-negative patients. In addition, there was a rather small number of events in survival analyses, which is the consequence of successful treatment planning and favorable response to (chemo)radiotherapy.
In previous studies of HNSCC [6, 9, 10, 13, 33], pGTV has shown higher prognostic value compared to TNM classification as well as in the present study in p16-negative patients. Additionally, our results on nGTV showed higher prognostic value in multivariate analyses of p16-negative patients and p16-positive patients. Whether this result is partly due to the fact that all the patients in the present series were treated with definitive radiochemotherapy or radiotherapy using IMRT and not with a combination of surgery and postoperative radiotherapy remains to be elucidated.
In the present study, mean pGTV as a cut-off value was closer to the point which clearly distributes tumors to groups with either favorable or non-favorable prognosis, as compared to the median volume. In previous studies, pGTV cut-off values have been assessed with a large range from 6 cm3 to 70 cm3 by median or mean volume without a clear consensus [2,3,4, 6, 9, 18]. In the present study, larger p16-negative tumors than mean pGTV (>38 cm3) were associated with poorer OS and DFS, whereas p16-negative tumors larger than median pGTV (>19 cm3) were associated with poorer LRC. To our knowledge, this is the first study to assess and compare cut-off value with mean and median values in the same study cohort.
Conclusion
pGTV may serve as an independent prognostic factor in p16-negative patients in order to predict poor treatment response to radiochemotherapy or radiotherapy using IMRT. nGTV may serve as an independent prognostic factor both in p16-positive and p16-negative OPSCC treated with radiochemotherapy or radiotherapy using IMRT. There are many ongoing trials where the aim is to establish specific patient selection criteria for de-escalation protocols. Our findings on tumor-related factors might have an impact in this field in the future. For this reason, future studies are warranted with larger patient cohorts to confirm these results and in order to assess clear cut-off values for favorable and non-favorable treatment response.
References
Chao KS, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, Spector GJ, Sessions DG (2004) Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys 59(1):43–50. https://doi.org/10.1016/j.ijrobp.2003.08.004
Chen SW, Yang SN, Liang JA, Lin FJ, Tsai MH (2009) Prognostic impact of tumor volume in patients with stage III–IVA hypopharyngeal cancer without bulky lymph nodes treated with definitive concurrent chemoradiotherapy. Head Neck 31(6):709–716. https://doi.org/10.1002/hed.21011
Lok BH, Setton J, Caria N, Romanyshyn J, Wolden SL, Zelefsky MJ, Park J, Rowan N, Sherman EJ, Fury MG, Ho A, Pfister DG, Wong RJ, Shah JP, Kraus DH, Zhang Z, Schupak KD, Gelblum DY, Rao SD, Lee NY (2012) Intensity-modulated radiation therapy in oropharyngeal carcinoma: effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys 82(5):1851–1857. https://doi.org/10.1016/j.ijrobp.2011.03.029
He YX, Wang Y, Cao PF, Shen L, Zhao YJ, Zhang ZJ, Chen DM, Yang TB, Huang XQ, Qin Z, Dai YY, Shen LF (2016) Prognostic value and predictive threshold of tumor volume for patients with locally advanced nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. Chin J Cancer 35(1):96. https://doi.org/10.1186/s40880-016-0159-2
Linge A, Lohaus F, Lock S, Nowak A, Gudziol V, Valentini C, von Neubeck C, Jutz M, Tinhofer I, Budach V, Sak A, Stuschke M, Balermpas P, Rodel C, Grosu AL, Abdollahi A, Debus J, Ganswindt U, Belka C, Pigorsch S, Combs SE, Monnich D, Zips D, Buchholz F, Aust DE, Baretton GB, Thames HD, Dubrovska A, Alsner J, Overgaard J, Krause M, Baumann M, DKTK-ROG (2016) HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother Oncol 121(3):364–373. https://doi.org/10.1016/j.radonc.2016.11.008
Studer G, Lutolf UM, El-Bassiouni M, Rousson V, Glanzmann C (2007) Volumetric staging (VS) is superior to TNM and AJCC staging in predicting outcome of head and neck cancer treated with IMRT. Acta Oncol 46(3):386–394. https://doi.org/10.1080/02841860600815407
Doweck I, Denys D, Robbins KT (2002) Tumor volume predicts outcome for advanced head and neck cancer treated with targeted chemoradiotherapy. Laryngoscope 112(10):1742–1749. https://doi.org/10.1097/00005537-200210000-00006
Rutkowski T (2014) The role of tumor volume in radiotherapy of patients with head and neck cancer. Radiat Oncol 9:23. https://doi.org/10.1186/1748-717x-9-23
Strongin A, Yovino S, Taylor R, Wolf J, Cullen K, Zimrin A, Strome S, Regine W, Suntharalingam M (2012) Primary tumor volume is an important predictor of clinical outcomes among patients with locally advanced squamous cell cancer of the head and neck treated with definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys 82(5):1823–1830. https://doi.org/10.1016/j.ijrobp.2010.10.053
Knegjens JL, Hauptmann M, Pameijer FA, Balm AJ, Hoebers FJ, de Bois JA, Kaanders JH, van Herpen CM, Verhoef CG, Wijers OB, Wiggenraad RG, Buter J, Rasch CR (2011) Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck 33(3):375–382. https://doi.org/10.1002/hed.21459
Brenner DJ (1993) Dose, volume, and tumor-control predictions in radiotherapy. Int J Radiat Oncol Biol Phys 26(1):171–179
Dubben HH, Thames HD, Beck-Bornholdt HP (1998) Tumor volume: a basic and specific response predictor in radiotherapy. Radiother Oncol 47(2):167–174
Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK (1995) The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys 33(2):281–287
Yang SN, Chiou YR, Zhang GG, Chou KT, Huang TC (2017) The clinical outcome correlations between radiation dose and pretreatment metabolic tumor volume for radiotherapy in head and neck cancer: a retrospective analysis. Medicine (Baltimore) 96(26):e7186. https://doi.org/10.1097/MD.0000000000007186
Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. https://doi.org/10.1245/s10434-010-0985-4
Davis KS, Lim CM, Clump DA, Heron DE, Ohr JP, Kim S, Duvvuri U, Johnson JT, Ferris RL (2016) Tumor volume as a predictor of survival in human papillomavirus-positive oropharyngeal cancer. Head Neck 38(Suppl 1):E1613–1617. https://doi.org/10.1002/hed.24287
Hermans R, Op de beeck K, Van den Bogaert W, Rijnders A, Staelens L, Feron M, Bellon E (2001) The relation of CT-determined tumor parameters and local and regional outcome of tonsillar cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys 50(1):37–45
Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP (2017) Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 67(2):122–137. https://doi.org/10.3322/caac.21389
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (2017) AJCC cancer staging manual, 8th edn. Springer, New York
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363(1):24–35. https://doi.org/10.1056/NEJMoa0912217
Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R (2008) Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 100(6):407–420. https://doi.org/10.1093/jnci/djn025
Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S (2013) Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck 35(5):747–755. https://doi.org/10.1002/hed.22015
D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356(19):1944–1956. https://doi.org/10.1056/NEJMoa065497
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100(4):261–269. https://doi.org/10.1093/jnci/djn011
Bird D, Scarsbrook AF, Sykes J, Ramasamy S, Subesinghe M, Carey B, Wilson DJ, Roberts N, McDermott G, Karakaya E, Bayman E, Sen M, Speight R, Prestwich RJ (2015) Multimodality imaging with CT, MR and FDG-PET for radiotherapy target volume delineation in oropharyngeal squamous cell carcinoma. BMC Cancer 15:844. https://doi.org/10.1186/s12885-015-1867-8
Emami B, Sethi A, Petruzzelli GJ (2003) Influence of MRI on target volume delineation and IMRT planning in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 57(2):481–488. https://doi.org/10.1016/s0360-3016(03)00570-4
van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, Meyer CJ, Snow GB (1990) Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 177(2):379–384. https://doi.org/10.1148/radiology.177.2.2217772
Cantrell SC, Peck BW, Li G, Wei Q, Sturgis EM, Ginsberg LE (2013) Differences in imaging characteristics of HPV-positive and HPV-Negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol 34(10):2005–2009. https://doi.org/10.3174/ajnr.A3524
McIlwain WR, Sood AJ, Nguyen SA, Day TA (2014) Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg 140(5):441–447. https://doi.org/10.1001/jamaoto.2014.141
Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA (2007) Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer 43(2):415–432. https://doi.org/10.1016/j.ejca .2006.09.001
Huang SH, O’Sullivan B (2017) Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol 18(7):40. https://doi.org/10.1007/s11864-017-0484-y
van den Broek GB, Rasch CR, Pameijer FA, Peter E, van den Brekel MW, Tan IB, Schornagel JH, de Bois JA, Zijp LJ, Balm AJ (2004) Pretreatment probability model for predicting outcome after intraarterial chemoradiation for advanced head and neck carcinoma. Cancer 101(8):1809–1817. https://doi.org/10.1002/cncr.20556
Acknowledgements
This study was supported by grants from the Helsinki University Hospital Research Fund and the Finska Läkaresällskapet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Carpén, K. Saarilahti, C. Haglund, A. Markkola, J. Tarkkanen, J. Hagström, P. Mattila, and A. Mäkitie declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors. Consent was obtained from all patients identifiable from images or other information within the manuscript. In the case of underage patients, consent was obtained from a parent or legal guardian.
Rights and permissions
About this article
Cite this article
Carpén, T., Saarilahti, K., Haglund, C. et al. Tumor volume as a prognostic marker in p16-positive and p16-negative oropharyngeal cancer patients treated with definitive intensity-modulated radiotherapy. Strahlenther Onkol 194, 759–770 (2018). https://doi.org/10.1007/s00066-018-1309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1309-z