Abstract
Background
After lung-sparing radiotherapy for malignant pleural mesothelioma (MPM), local failure at sites of previous gross disease represents the dominant form of failure. Our aim is to investigate if selective irradiation of the gross pleural disease only can allow dose escalation.
Materials and methods
In all, 12 consecutive stage I–IV MPM patients (6 left-sided and 6 right-sided) were retrospectively identified and included. A magnetic resonance imaging-based pleural gross tumor volume (GTV) was contoured. Two sets of planning target volumes (PTV) were generated for each patient: (1) a “selective” PTV (S-PTV), originating from a 5-mm isotropic expansion from the GTV and (2) an “elective” PTV (E-PTV), originating from a 5-mm isotropic expansion from the whole ipsilateral pleural space. Two sets of volumetric modulated arc therapy (VMAT) treatment plans were generated: a “selective” pleural irradiation plan (SPI plan) and an “elective” pleural irradiation plan (EPI plan, planned with a simultaneous integrated boost technique [SIB]).
Results
In the SPI plans, the average median dose to the S‑PTV was 53.6 Gy (range 41–63.6 Gy). In 4 of 12 patients, it was possible to escalate the dose to the S‑PTV to >58 Gy. In the EPI plans, the average median doses to the E‑PTV and to the S‑PTV were 48.6 Gy (range 38.5–58.7) and 49 Gy (range 38.6–59.5 Gy), respectively. No significant dose escalation was achievable.
Conclusion
The omission of the elective irradiation of the whole ipsilateral pleural space allowed dose escalation from 49 Gy to more than 58 Gy in 4 of 12 chemonaive MPM patients. This strategy may form the basis for nonsurgical radical combined modality treatment of MPM.
Zusammenfassung
Hintergrund
Beim malignen Pleuramesotheliom (MPM) ist nach lungenschonender Radiotherapie das lokale Scheitern an Stellen eines früheren, sichtbaren Tumors die dominierende Form des Scheiterns. Unser Ziel ist es, zu untersuchen, ob die selektive Bestrahlung nur des sichtbaren Pleuratumors eine Dosiseskalation ermöglicht.
Material und Methoden
Es wurden 12 konsekutive MPM-Patienten in Phase I–IV (6 links- und 6 rechtshändig) retrospektiv identifiziert und inkludiert. Ein MRT-basiertes makroskopisches Tumorvolumen (GTV) der Pleura wurde umrandet. Für jeden Patienten wurden zwei Typen von Planungszielvolumen (PTV) erzeugt: (1) ein „selektives“ PTV (S-PTV), das einer isotropen Expansion von 5 mm vom GTV entstammt, und (2) ein „elektives“ PTV (E-PTV), das einer isotropen Expansion von 5 mm von der ganzen ipsilateralen Pleurahöhle entstammt. Zwei verschiedene Behandlungspläne mit volumetrisch modulierter Bogentherapie (VMAT) wurden erstellt: ein „selektiver“ pleuraler Bestrahlungsplan (SPI-Plan) und ein „elektiver“ pleuraler Bestrahlungsplan (EPI-Plan; geplant mit simultan integrierter Boost-Technik [SIB]).
Ergebnisse
In den SPI-Plänen betrug die durchschnittliche mittlere Dosis beim S‑PTV 53,6 Gy (Spanne 41–63,6 Gy). Bei 4 von 12 Patienten ließ sich die Dosis beim S‑PTV auf >58 Gy eskalieren. In den EPI-Plänen betrugen die durchschnittlichen mittleren Dosen beim E‑PTV und beim S‑PTV 48,6 Gy (Spanne 38,5–58,7 Gy) bzw. 49 Gy (Spanne 38,6–59,5 Gy). Eine signifikante Dosiseskalation war nicht möglich.
Schlussfolgerung
Das Weglassen der elektiven Bestrahlung der ganzen ipsilateralen Pleurahöhle ermöglichte bei 4 von 12 chemonaiven MPM-Patienten eine Dosiseskalation von 49 Gy bis über 58 Gy. Diese Strategie kann die Basis für eine nichtchirurgische, radikal kombinierte Modalitätsbehandlung von MPM bilden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Malignant pleural mesothelioma (MPM) is a rare asbestos-related tumor. The prognosis is dismal: the median overall survival (OS) times range between 11 and 12 months with 4–6 cycles of cis-/carboplatin and antifolate chemotherapy, which is currently considered the standard of care, with only rare 5‑year survivors [1–4]. Different treatment approaches, which range from best supportive care to multimodality therapy, have been investigated. The survival benefit of very aggressive surgical techniques, such as extrapleural pneumonectomy (EPP), is unclear [5, 6]. Interest towards the use of novel systemic agents, such as targeted agents or immunotherapy together with cis-/carboplatin and antifolate chemotherapy in MPM is growing [7]. Especially the combination of radiotherapy (RT) with immune treatment is appealing, also for MPM [8, 9].
In order to investigate appropriately delivered RT in combination with novel agents in the nonsurgical setting, RT should be delivered safely to the hemithoracic pleura without undue lung damage.
A few studies have investigated the feasibility of lung-sparing intensity-modulated radiotherapy (IMRT) alone, after chemotherapy, or after pleurectomy/decortication (P/D) [10–17]. This approach has been proven feasible and safe, but the delivered median RT doses were only 47–48 Gy with grade >3 toxicity rates of 20–30% [10–17]. In all the above mentioned studies, the whole ipsilateral pleura was irradiated (“elective” pleural irradiation), regardless of the radiological extent of the tumor.
Rimner et al. [15] recently published patterns of failure analysis on 67 patients treated with lung-sparing IMRT (the largest cohort published up to now in this setting). The target volume was represented by the whole pleura (involved and uninvolved, that represents the current clinical practice in each lung-sparing IMRT study and can be, thus, defined the standard treatment). The authors showed that 64% of patients experienced local failures, and 74% of these patients experienced a local failure at sites of previous gross disease, most commonly in the pleural space. This could be explained by an insufficient dose to the pleura, since the median delivered dose to the whole pleural space was 46.8 Gy.
Our hypothesis is that a dose escalation to the tumor (with an acceptable dose to the organs-at-risk) is possible in lung-sparing volumetric modulated arc therapy (VMAT) for MPM, and it could be achieved by avoiding the irradiation of the uninvolved ipsilateral pleura (or the “elective” irradiation of the pleura), in favor of a selective irradiation of the gross tumor disease only.
If a reasonable dose such as 60 Gy could be delivered, this could be the basis for a radical nonsurgical treatment of pleural MPM, analogous to unresectable locally advanced nonsmall cell lung cancer. In order to investigate a concurrent approach, we only enrolled patients who had never received chemotherapy in the present study.
Our primary aim was to address the feasibility of a dose escalation to the involved pleura only through the avoidance of the irradiation of the whole pleural space in lung-sparing VMAT.
Materials and methods
Patients and imaging
Patients diagnosed with biopsy-proven and previously untreated stage I–IV MPM between January 2010 and December 2013 at our institution were retrospectively identified from a prospective institutional database. Eligibility criteria were a staging computed tomography (CT) scan with intravenous (IV) contrast and a staging magnetic resonance imaging (MRI), all performed at our institution. Patients with prior pleurodesis were excluded because the inflammation interferes with MRI imaging of the target volume.
Included in the study were the first 6 consecutive right-sided and the first 6 consecutive left-sided patients. No patient enrolled in this study had received prior chemotherapy. Patients were staged using the staging system of the International Mesothelioma Interest Group [18].
Botticella et al. [19, 20] previously showed that the gross tumor volume (GTV) defined on the CT scan with intravenous contrast and MRI had the lowest risk of potential geographical miss, especially in the parietal pleura and in the diaphragm. The GTVs were contoured on a CT scan with intravenous contrast, rigidly coregistered with an MRI.
CT scans with intravenous contrast were acquired in free breathing. Patients were scanned in supine position, with both arms raised above their head. MRI was acquired in breath hold and patients were scanned in supine position, with both arms down positioned.
MRI scans were performed with a 3T whole-body system (Achieva; Philips Healthcare, Best, The Netherlands) with the manufacturer’s 16-channel phased array torso coil (Sense XL Torso; Philips Healthcare) for signal reception. Nonenhanced T2-weighted turbo spin-echo sequence, a T1-weighted fat-suppressed sequence and a contrast-enhanced T1 sequence were performed in the transverse and coronal plane.
Target volume definition
MIM 6.1.7 (MIM Software Inc., Cleveland, OH, USA) was used for the rigid coregistration between the CT and the MRI. The GTV was defined on the CT images, rigidly coregistered to the MRI images (pre- and postcontrast T1 and T2), and the accuracy of the contours was assessed slice by slice. MRI-based GTV has been shown in an earlier study from our group to be the one with the lower rate of potential “geographical missing” [20]. Only the gross tumor volume (GTV) of the primary tumor was taken into account and contoured. Since the standard N‑staging consists of a combination of the functional PET/CT information and, in some cases, from invasive procedures such as mediastinoscopy, and not MRI, the GTV definition of the lymph nodes was excluded and is beyond the scope of this article.
Two sets of planning target volumes (PTVs) were generated for each patient:
-
A “selective” PTV (S-PTV), originating from a 5-mm isotropic expansion from the GTV. The S‑PTV consists therefore of the involved pleura only.
-
An “elective” PTV (E-PTV), originating from a 5-mm isotropic expansion from the whole pleural space. The whole pleural space represents the “elective” component of the volume and included all pleural surfaces, both involved (GTV) and noninvolved, from the lung apex to insertion of the diaphragm (body L2), excluding the interlobar pleura. Medially, it included the ipsilateral pericardium. The whole pleural space was delineated according to the previously published experiences of lung-sparing IMRT in MPM [12, 17].
The E‑PTV ultimately resulted in a volume consisting of the whole pleura (both involved and uninvolved).
Radiation treatment planning, organs at risk, and dose constraints
Two sets of treatment plans were generated for each patient: (1) a “selective” pleural irradiation plan (SPI plan), where the target was the involved pleura (= GTV) only and (2) an “elective” pleural irradiation plan (EPI plan), where the target is both the involved and uninvolved pleura. Progressive dose escalation to the S‑PTV (with 4‑Gy increments) was attempted and the fraction size of the treatment was 2 Gy. The EPI plan was created using a simultaneous integrated boost (SIB). The contralateral lung (CL), the combined lungs, the spinal cord, the spinal cord planning risk volume (PRV), the heart, the liver, the contralateral kidney, the small bowel, the esophagus, and the stomach were defined as organs at risk (OAR) and delineated on CT.
In the SPI plan, the total lung mean lung dose (MLD) and the V20 (the lung volume receiving a dose equal or superior to 20 Gy) were calculated using the volume of both lungs minus the GTV. In the EPI plan, the total lung mean lung dose (MLD) and the V20 (the lung volume receiving a dose equal or superior to 20 Gy) were calculated using the volume of both lungs minus the whole pleural space and the GTV.
The spinal cord was contoured throughout the whole CT scan and was considered to be at the inner margin of the bony spinal canal. The spinal cord PRV was obtained by adding a 5 mm isotropic margin from the spinal cord. The heart was contoured from the auricles to the tip of the organ. The esophagus was contoured from just below the larynx to the gastro-esophageal junction. The small bowel was contoured as “bowel bag”.
VMAT plans were generated in eclipse for a TrueBeam linac equipped with a Millennium 120 multileaf collimator (Varian Medical Systems, Palo Alto, CA, USA). Each plan consisted of two coplanar complete arcs (beam energy 6 MV) with collimator angles of 10° and 350°. The isocenter was positioned in the center of mass of the PTV. The field sizes encompassed the PTVs in craniocaudal direction and were limited to 14 cm in the X‑jaw direction in order to limit the leaf travel distance.
The dose calculation algorithm was the Analytical Anisotropic Algorithm (AAA) version 10.0.28. The optimizer (DVO 10.0.28) started with fixed weights for the dose–volume constraints (Table 1), which were set according to previously published experiences with lung-sparing IMRT for pleural mesothelioma [12, 17], the hemithoracic irradiation after EPP [21], or the available literature [22]. The weights were adapted interactively during optimization according to the needs for every constraint. Final plan adjustments (e. g., elimination of hotspots) were made during subsequent optimizations until an acceptable plan was achieved after inspection of the dose distribution and the dose–volume histograms. For every attempted dose level, a new plan was generated with the optimization starting from the original weights.
Quantitative evaluation of each plan was performed by means of cumulative dose–volume histograms (DVHs). For the PTV, the homogeneity index was defined by the equation HI = D2 − D98/Dprescription, where D2 and D98 are, respectively, the doses received by the 2% and the 98% of the volume, while the Dprescription is the prescription dose.
For the EPI plans, the median doses to the “selective” PTV and the “elective” PTV were reported and compared.
For the SPI plans, the median dose to the “selective” PTV was reported. Moreover, in the SPI plans, the median dose to the “elective” PTV was reported (and compared to the median dose to the “selective” PTV): this was defined as the “incidental” dose to the uninvolved pleura (since the whole pleural space does not represent the target in the SPI plans).
For the organs at risk (OARs), the mean dose and a set of appropriate Vx and Dx values were reported and compared between the SPI plan and the EPI plan.
Statistics
All results are expressed in terms of mean, median, standard deviation (SD), and range. The Wilcoxon matched-paired signed-rank test was used to compare means, the χ2 test to compare proportions. Two-sided p values less than 0.05 were used to determine statistical significance.
For each SPI and EPI plan, a radiation oncologist (AB) and a medical physicist (GD) identified together the dose-limiting OARs (defined as the ones which prevented from a further dose escalation).
Ethics
This was a retrospective contouring study, and patients were diagnosed and treated previously according to standard institutional guidelines. No informed consent was needed according to the policy of the local clinical trial center.
Results
Relevant patients’ characteristics and clinical stages are summarized in Supplementary Table 1. Twelve patients were included (6 left-sided and 6 right-sided; 10 men and 2 women). Table 2 summarizes the target volumes for each planned patient. The mean GTV and whole pleural space volume were 640.92 ml (270 ml, range 291–1150.3 ml) and 784.5 ml (277.4 ml, range 431.9–1322.7 ml), respectively (p = 0.002).
The mean S‑PTV (SD, range) and the mean E‑PTV (SD, range) were respectively 1613.5 ml (430.1 ml, range 1112.1–2567.1 ml) and 2081.2 ml (427.85 ml, range 1583.3–2938.9 ml) (p = 0.002). Table 3 reports the comparisons of dose–volume parameters in the SPI plans and in the EPI plans.
The median dose to the E‑PTV was significantly higher in the SPI plan (52 Gy) compared to the EPI plan (48.6 Gy) (p = 0.005). The median dose to the S‑PTV was higher in the SPI plan (53.6 Gy) compared to the EPI plan (49.1 Gy) (p = 0.003). Table 4 depicts the median doses delivered to the S‑PTV and to the E‑PTV in the SPI plans and in the EPI plans.
In the SPI plans, in 10/12 patients it was possible to achieve a minimal median dose of 50 Gy delivered to the S‑PTV, while in the remaining 2 patients, the maximally achievable median dose was 49.51 Gy and 40.99 Gy. The median dose to the S‑PTV in the SPI plans ranged from 40.99 and 63.62 Gy (mean 53.57 Gy, SD 5.47). In 4/12 patients, the SPI plans allowed to deliver a dose of at least 58 Gy; only one of these patients was administered 62 Gy.
The “incidental” dose to the uninvolved pleura was calculated: the median dose to the E‑PTV ranged from 40.6–61.02 Gy (mean 52 Gy; SD 4.84).
The median doses to the S‑PTV and to the E‑PTV in the SPI plans were significantly different (p = 0.01).
The dose-limiting OAR constraints in the SPI plans were heart maximum dose (Dmax) (11/12 patients), contralateral lung mean lung dose (CL MLD) (8/12 patients), stomach Dmax (6/12 patients), and esophagus Dmax (6/12 patients).
In the EPI plans, the E‑PTV achieved a median dose of at least 50 Gy in 4/12 patients, whereas the S‑PTV achieved a median dose of at least 50 Gy in 5/12 patients. In only 1/12 patients did an E‑PTV plan allow administration of 58 Gy. The median dose to the E‑PTV ranged from 38.5–54.7 Gy (mean 48.6 Gy; SD 5.78 Gy). The median dose to the S‑PTV ranged from 38.64–59.5 Gy (mean 49.1 Gy, SD 6 Gy).
In the EPI plans, the median doses to the S‑PTV and to the E‑PTV were not significantly different (p = 0.67). The dose-limiting OAR constraints in the EPI plans were heart Dmax (10/12 patients), CL MLD (7/12 patients), total lungs V20 (7/12 patients), stomach Dmax (6/12 patients), and esophagus Dmax (6/12 patients).
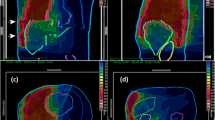
The proportion of patients that could receive 58 Gy or more was significantly in favor of SPI plans (p = 0.003). Fig. 1 depicts an SPI plan where dose escalation was achieved and one patient where it was not (Fig. 1).
Examples of two SPI plans: axial (a) and coronal (b) images of the planning CT of a patient where dose escalation was possible beyond 58 Gy in the SPI plan (mean dose to the “selective” PTV: 58 Gy); axial (c) and coronal (d) images of the planning CT of a patient where dose escalation was not achievable (mean dose to the “selective” PTV: 40 Gy)
Discussion
Malignant pleural mesothelioma is still a devastating disease with only a very limited proportion of patients experiencing long-term survival. As the prognosis remains poor even after a very extensive surgery such as EPP and given the lack of survival benefit of P/D, there is growing interest in combining RT with novel interventions, such as systemic immune treatment [8, 9]. In the latter case, a therapeutic RT dose to the tumor, together with immune interventions, may lead to a systemic cancer effect. However, RT should be delivered to an adequately high dose, without harming the underlying lung and the others organs at risk.
In all previously published studies on lung sparing IMRT in MPM, the target volume was encompassed the whole pleura (both involved and uninvolved), and the mean doses delivered to the “elective” PTV were below 50 Gy. Rimner et al. [15] analyzed the pattern of failure of 67 patients treated with lung-sparing IMRT as either definitive or adjuvant therapy, showing that after hemithoracic pleural IMRT local failure is the dominant form of failure pattern. One-year actuarial in-field local failure rates were 43% in patients who underwent P/D versus 66% in those who received a partial pleurectomy or were deemed unresectable. The mean dose to the whole pleural space was only 46.8 Gy. For patients who undergo incomplete or no surgery, these radiation doses are clearly insufficient to provide long-term control of MPM.
Our primary aim was to address the feasibility of a dose escalation to the involved pleura only through the avoidance of the irradiation of the whole pleural space in lung-sparing VMAT.
Using selective pleural irradiation, it was possible to deliver radiation of 58 Gy or more in 4/12 patients, whereas this was achievable in only 1/12 patients with elective pleural irradiation. Among those 4 patients, in only one was it possible to achieve a median dose of 62 Gy. The median dose to the elective PTV and to the selective PTV was significantly higher in the selective pleural irradiation plans, but this difference was rather small in absolute terms (3.5 Gy).
The heart was the most frequent dose-limiting organ, impeding further dose escalation in 11/12 patients in the SPI plans. The constraints to the heart were derived from the QUANTEC papers [21] for pericarditis. As the heart was the most critical OAR, new validated constraints for heart substructures (heart chambers, coronary arteries, pericardium) could in the future lead in some patients to a slight increase in achievable tumor doses compared to our results. As the heart Dmax constraint specifically was mostly critical, we used the overlapping volume between PTV and heart during plan optimization, which resulted in lower doses in that overlap region.
As for the influence of the tumor side, among the 4/12 patients who received an escalated dose, 3 were left-sided and 1 was right-sided; therefore, in our experience, the side does not play a role.
Limitations
The main caveats of our study include the small sample size, that it is a planning study (that prevents from having any information on patterns of failure), and the rigid coregistration between different imaging modalities in the contouring phase (suboptimal fusion). Concerning the latter point, we used a rigid registration method: the use of deformable image registration (DIR) in multimodal registration is far from being without uncertainties, especially in a setting like MPM, where large differences in breathing cycles may increase inaccuracies in image registration [23]. The inclusion of more than two arcs during VMAT optimization could result in some improvement in plan dose conformity, but at the expense of a longer treatment time. The free-breathing nature of the planning CT scans might also have influenced the planning dose-limitation of the normal lung OAR. However, to the best of our knowledge, this is the first planning study on the feasibility of dose escalation on the gross tumor volume in lung-sparing RT in pleural mesothelioma.
Conclusion
Selective pleural RT allows for dose escalation in a proportion of MPM patients that were chemotherapy naïve and had no lymph node involvement. For the other patients, other strategies are needed such as delivering RT after chemotherapy or the use of proton therapy [24].
References
Baas P, Fennell D, Kerr KM, Van Schil PE, Haas RL, Peters S, Guidelines Committee ESMO (2015) Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(S5):v31–v39
Van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, European Organisation for Research and Treatment of Cancer Lung Cancer Group, National Cancer Institute et al (2005) Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 23:6881–6889
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, European Respiratory Society, European Society of Thoracic Surgeons Task Force et al (2010) Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 35:479–495
Cao C, Tian D, Park J, Allan J, Pataky KA, Yan TD et al (2014) A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 83:240–245
Bovolato P, Casadio C, Billè A, Ardissone F, Santambrogio L, Ratto GB et al (2014) Does surgery improve survival of patients with malignant pleural mesothelioma? A multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 9:390–396
Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D (2015) The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 41:503–510 (Jun)
Marcq E, Pauwels P, van Meerbeeck JP, Smits EL (2015) Targeting immune checkpoints: New opportunity for mesothelioma treatment? Cancer Treat Rev 41:914–924
Haas AR, Sterman DH (2013) Malignant pleural mesothelioma: Update on treatment options with a focus on novel therapies. Clin Chest Med 34:99–111
Minatel E, Trovo M, Polesel J, Rumeileh IA, Baresic T, Bearz A et al (2012) Tomotherapy after pleurectomy/decortication or biopsy for malignant pleural mesothelioma allows the delivery of high dose of radiation in patients with intact lung. J Thorac Oncol 7:1862–1866
Minatel E, Trovo M, Polesel J, Baresic T, Bearz A, Franchin G et al (2014) Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 83:78–82
Rosenzweig KE, Zauderer MG, Laser B, Krug LM, Yorke E, Sima CS et al (2012) Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 83:1278–1283
Fodor A, Fiorino C, Dell’Oca I, Broggi S, Pasetti M, Cattaneo GM et al (2011) PET-guided dose escalation tomotherapy in malignant pleural mesothelioma. Strahlenther Onkol 187:736–743
Feigen M, Lee ST, Lawford C, Churcher K, Zupan E, Scott AM et al (2011) Establishing locoregional control of malignant pleural mesothelioma using high-dose radiotherapy and (18) F‑FDG PET/CT scan correlation. J Med Imaging Radiat Oncol 55:320–332
Rimner A, Spratt DE, Zauderer MG, Rosenzweig KE, Wu AJ, Foster A et al (2014) Failure patterns after hemithoracic pleural intensity modulated radiation therapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 90:394–401
Chance WW, Rice DC, Allen PK, Tsao AS, Fontanilla HP, Liao Z et al (2015) Hemithoracic intensity modulated radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma: toxicity, patterns of failure, and a matched survival analysis. Int J Radiat Oncol Biol Phys 91:149–156
Minatel E, Trovo M, Bearz A, Di Maso M, Baresic T, Drigo A et al (2015) Radical radiation therapy after lung-sparing surgery for malignant pleural Mesothelioma: Survival, pattern of failure, and prognostic factors. Int J Radiat Oncol Biol Phys 93:606–613
Rusch VW (1995) A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 108:1122–1128
Botticella A, Defraene G, Nackaerts K, Deroose CM, Coolen J, Nafteux P, Peeters S, De Ruysscher D (2015) Optimization of gross tumor volume definition and treatment planning in lung-sparing volumetric modulated arc therapy for pleural mesothelioma. Ann Oncol 26(Supplement 1):i48–i50
Botticella A, Defraene G, Nackaerts K, Deroose CM, Coolen J, Nafteux P et al (2016) Optimal gross tumor volume definition in lung-sparing intensity modulated radiotherapy for pleural mesothelioma: An in silico study. Acta Oncol 55:1450–1455
Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM et al (2010) Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 76:77–85
Allen AM, Czerminska M, Jänne PA, Sugarbaker DJ, Bueno R, Harris JR et al (2006) Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 65:640–645
Brock KK, Dawson LA (2014) Point: Principles of magnetic resonance imaging integration in a computed tomography-based radiotherapy workflow. Semin Radiat Oncol 24:169–174
Krayenbuehl J, Hartmann M, Lomax AJ, Kloeck S, Hug EB, Ciernik IF (2010) Proton therapy for malignant pleural mesothelioma after extrapleural pleuropneumonectomy. Int J Radiat Oncol Biol Phys 78:628–634
Acknowledgements
A. B. is supported by a grant from the Stichting tegen kanker/Fondation contre le cancer (CA/2014/354) and by Kom op Tegen Kanker (Stand up to Cancer, The Flemish Cancer Society).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Botticella, G. Defraene, K. Nackaerts, C. Deroose, J. Coolen, P. Nafteux, B. Vanstraelen, S. Joosten, L.A.W. Michiels, S. Peeters, and D. DeRuysscher declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Botticella, A., Defraene, G., Nackaerts, K. et al. Does selective pleural irradiation of malignant pleural mesothelioma allow radiation dose escalation?. Strahlenther Onkol 193, 285–294 (2017). https://doi.org/10.1007/s00066-017-1108-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1108-y
Keywords
- Malignant pleural mesothelioma
- Lung-sparing VMAT
- Nonsurgical treatments
- Selective pleural irradiation
- Elective pleural irradiation