Abstract
Background
In 20 patients with high-grade gliomas, we compared two methods of planning for volumetric-modulated arc therapy (VMAT): simultaneous integrated boost (SIB) vs. sequential boost (SEB). The investigation focused on the analysis of dose distributions in the target volumes and the organs at risk (OARs).
Method
After contouring the target volumes [planning target volumes (PTVs) and boost volumes (BVs)] and OARs, SIB planning and SEB planning were performed. The SEB method consisted of two plans: in the first plan the PTV received 50 Gy in 25 fractions with a 2-Gy dose per fraction. In the second plan the BV received 10 Gy in 5 fractions with a dose per fraction of 2 Gy. The doses of both plans were summed up to show the total doses delivered. In the SIB method the PTV received 54 Gy in 30 fractions with a dose per fraction of 1.8 Gy, while the BV received 60 Gy in the same fraction number but with a dose per fraction of 2 Gy.
Results
All of the OARs showed higher doses (Dmax and Dmean) in the SEB method when compared with the SIB technique. The differences between the two methods were statistically significant in almost all of the OARs. Analysing the total doses of the target volumes we found dose distributions with similar homogeneities and comparable total doses.
Conclusion
Our analysis shows that the SIB method offers advantages over the SEB method in terms of sparing OARs.
Zusammenfassung
Hintergrund
Es wurden 2 Arten der Planung für die volumetrisch modulierte Rotationsbestrahlung (VMAT) bei 20 Patienten mit hochgradigen Gliomen verglichen: simultan integrierter Boost (SIB) und sequenzieller Boost (SEB). Dazu wurde die Dosisverteilung in den Zielvolumina und den Risikoorganen analysiert.
Methoden
Es wurden Planungsvolumina (PTV), Boostvolumina (BV) und Risikoorgane konturiert sowie SIB- und SEB-Pläne erstellt. Der SEB besteht aus 2 Plänen. Im ersten Plan erhält das PTV 50 Gy in 25 Fraktionen. Im zweiten Plan erhält das Boostvolumen 10 Gy in 5 Fraktionen (Einzeldosis jeweils 2 Gy). Die Dosis beider Pläne wurde summiert, um die gesamte verabreichte Dosis zu ermitteln. Beim SIB-Konzept erhält das PTV 54 Gy in 30 Fraktionen (Einzeldosis 1,8 Gy), während das Boostvolumen 60 Gy erhält (30 Fraktionen, Einzeldosis 2,0 Gy).
Ergebnisse
Beim SEB erhielten die Risikoorgane höhere Dosen (Dmax und Dmean) als bei der SIB-Technik. Die Dosisunterschiede zwischen den beiden Techniken waren für nahezu alle Risikoorgane statistisch signifikant. Die Analyse der Dosisverteilungen in den Zielvolumina ergab vergleichbare Gesamtdosen und Dosishomogenitäten.
Schlussfolgerung
Unsere Analyse zeigt eine klare Tendenz, dass die SIB-Technik gegenüber der SEB-Technik einen Vorteil bezüglich der Schonung der Risikoorgane bietet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prognosis of high-grade gliomas is poor, despite the use of combined treatment modalities including surgery, radiotherapy, and chemotherapy. These tumours exhibit an infiltrative growth and it is necessary to control the peripheral infiltrating parts (microscopic tumour, not visible on imaging) as well as the local core tumour (macroscopically visible tumour on imaging). However, the required dose to control the core and the infiltrating volumes of the tumour may be different. At the same time, we must be careful about the tolerance doses of organs at risk (OARs) near the radiation treatment volumes including the optic apparatus and brainstem. Therefore, it seems logical to deliver different doses to a layered (core/periphery) target. In the era of three-dimensional conformal radiotherapy (3D-CRT), different strategies such as altered fractionation, interstitial brachytherapy boost, and stereotactic radiosurgery boost have been tested in several studies. These trials could not show significant survival benefits [1–6].
With the arrival of intensity-modulated radiation treatment (IMRT), it was shown that this technique provided improved target conformity and better sparing of OARs [1, 7]. A special kind of rotational IMRT is volumetric-modulated arc therapy (VMAT) [8]. During rotation of the gantry, the dose rate and shape of the multileaf collimator are dynamically modulated. Compared with “conventional” IMRT, VMAT achieves equal or better coverage of target volumes and improved OAR sparing while using fewer monitor units and requiring less time to treat high-grade gliomas [9, 10]. The faster dose delivery of the VMAT technique can decrease the treatment time at the linac machine and limit the potential for intra-fraction organ and patient motion. The motions can cause unwanted deviations in the dose distribution. However, VMAT may involve more time for plan optimization [11, 12].
One of the advantages of IMRT and VMAT when compared with traditional 3D radiation treatment is that these newer techniques are suitable for the application of the simultaneous integrated boost (SIB) method. SIB offers the possibility to apply a dose to the larger volume, the so-called planning target volume (PTV), and at the same time deliver an escalated dose to the core tumour mass, the so-called gross tumour volume (GTV) [1, 13, 14]. According to ICRU-83 [15], the GTV represents the palpable or visible (on imaging) tumour, whereas the so-called clinical target volume (CTV) is an additional volume with a certain probability of microscopic (subclinical) malignant disease. The irradiated PTV is a geometrical concept. It comprises the CTV plus a safety margin. This safety margin compensates for the effects of organ and patient movement during the radiation fraction and for inaccuracies in patient set-up. Of course, in brain tumours, organ movement is of minor importance.
This study was done performed on the hypothesis that the SIB method is superior to sequential boost (SEB) technique for treating patients with brain tumours. In order to deliver different doses to a layered target, we carried out treatment planning using the SEB and the SIB methods, both by VMAT. The aim of the study was to compare these two methods in terms of coverage of the target volumes and also of the sparing of OARs. To the best of our knowledge, this is the first study comparing SIB vs. SEB in a larger population of patients with brain tumour.
Patients and methods
Our study included 20 patients with pathology-proven high-grade gliomas. All patients underwent surgical resection of their tumour (seven total and 13 subtotal resections) between 2010 and 2013. They received radiation treatment based on the decision of the interdisciplinary board for brain tumours. However, in this planning study we exclusively focused on comparing the dose distributions in the isodose plans of the SIB and SEB techniques.
Patient characteristics are listed in Table 1. Ten patients were men and the other 10 were women; their age range was 23–68 years (median, 51.5 years; average, 49.2 years). According to the World Health Organization (WHO) grading criteria, six patients presented with grade III (anaplastic astrocytoma) and 14 patients with grade IV (glioblastoma) tumours.
We used the iPlan planning system (BrainLab AG, Feldkirchen, Germany) for delineating the target volumes and contouring the OARs. The Eclipse 10 planning system (Varian Medical Systems, Palo Alto, Calif.) was applied for treatment planning and dose distribution calculations.
Beside planning CT, every patient (n = 20) underwent magnetic resonance imaging (MRI; T1 and T2 FLAIR with contrast using 1-mm slice thickness) within a few days before starting the contouring of OARs and delineation of target volumes. MRI was performed because it provides better visualization of the resection cavity, residual tumour, and oedema. We first contoured the OARs, which included the brainstem, chiasm, and the following (always bilateral): optic nerves, retinas, lenses, eyeballs, and lachrymal glands. Contouring was performed on the planning CT scans (application of contrast medium and 2-mm slice thickness).
In the following step, the PTVs and the boost volumes (BVs) were delineated on the planning CTs and MRIs, based on our institutional protocol. Figures 1a and 1b show the workflow and the details of the delineating process of the target volumes. For delineation of the PTV, first the CTV1 and CTV2 were fused together. Then, parts of this volume that surpassed the bony skeletal borders were deleted.
a Workflow of patient selection and the sequential steps of treatment planning. b Workflow of definition of the target volumes. OAR organ at risk, CT computed tomography, MRI magnetic resonance imaging, GTV gross tumour volume, CTV clinical target volume, PTV planning target volume, SEB sequential boost, SIB simultaneous integrated boost
Delineation of the BVs was based on shrinking the PTV. We mainly excluded those parts of OARs that were located within the PTVs in order to reduce the risk of side effects. In eight patients, ten OARs were invaded by the tumour. These ten OARs were partially located inside the BV. Owing to the anatomical sites and the extension of the tumours, in all the patients (n = 20) at least one of the OARs was included either completely or partially inside the PTV but outside of the BV (Table 2).
All the contoured volumes were transferred from the iPlan to the Eclipse system using DICOM. Treatment planning and calculation of the dose distributions for both methods (SIB and SEB) were carried out for all 20 patients in the Eclipse planning system. In all patients the delineated target volumes (PTV and BV) were the same for the SEB and the SIB methods alike. Figure 2 presents an example of one of the patients showing the delineated target volumes and contoured OARs and also the dose distributions in the SIB and the SEB methods.
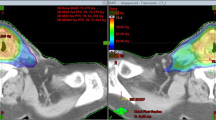
Patient example. a Delineated target volumes—boost volume (BV) and planning target volume (PTV)—and contoured organs at risk. BV: orange; PTV: red; brainstem: blue; chiasm: azure; right optic nerve: purple; right and left retina: yellow; right eyeball: dark blue; left eyeball: green; right lachrymal gland: orange; left lachrymal gland: pink. b Dose distribution for the same patient with the sequential boost (SEB) method. c Dose distribution for the same patient with the simultaneous integrated boost (SIB) method. d Scale of dose distribution. SIB has the advantage that the high-dose region is more distant from the relevant OARs when compared with SEB. This is especially shown for the left eyeball, left retina, and left lachrymal gland in this case
Treatment planning for both methods was performed with a two-arc/VMAT technique. The SEB method consisted of two plans. In the first plan the PTV received 50 Gy in 25 fractions with a dose per fraction of 2 Gy. In the second plan the BV received 10 Gy in 5 fractions with a dose per fraction of 2 Gy. Both dose plans were summed up to show the total doses delivered during treatment. In SIB planning, the PTV received 54 Gy in 30 fractions with a dose per fraction of 1.8 Gy, while the BV received 60 Gy in the same number of 30 fractions but with a dose per fraction of 2 Gy. All plans (SEB and SIB) were normalized to a mean dose of 100 % of the prescription dose to the target volume.
The upper dose constraints for the OARs corresponded to their tolerance doses: 56 Gy for the brainstem; 54 Gy for the optic nerves, chiasm, retinas, and eyeballs; 40 Gy for the lachrymal glands; and 5 Gy for the lenses. In order to achieve better plans in terms of coverage of target volumes and sparing of OARs, planning for both methods was repeated several times. After plan optimization was finished we determined the delivered doses to the target volumes (PTV and BV) and OARs for both methods. In addition we assessed doses to the difference volumes, PTV−BV, which are those parts of PTVs containing some OARs partially or totally. These data included the maximum dose (Dmax) and the mean dose (Dmean). The dose distributions in the SIB and SEB plans were then compared.
Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous measures as means and standard deviations or medians and ranges. We used Bland–Altman plots to show the similarity of the doses received by the target volumes in the two planning methods. The exact Wilcoxon signed-rank test was used to compare the doses of the OARs between the two methods. All p values reported are two-tailed, with a p value of 0.05 indicating statistical significance. Analysis was performed using R software version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
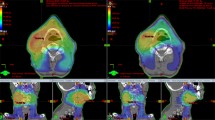
The first important point was to compare the dose inside the target volumes between the two methods. Bland–Altman plots (Fig. 3) show the mean dose against the dose difference between the two methods for each patient. In the plots “Dmax to BV” and “Dmax to PTV” the mean difference is 0.3 Gy (to the benefit of the SIB method). In the plot “Dmean to BV”, again the mean difference is 0.3 Gy, but to the benefit of the SEB method. Except for one patient, all values are within the 95 % confidence interval. In the plot “Dmean to PTV” there is a mean dose difference of 1.3 Gy between the SEB and SIB methods. This indicates that the mean dose to the PTV in the SEB method is higher than in the SIB method, while “Dmean to BV” in both methods is similar. The higher dose in the PTV in the SEB method can be attributed to those parts of the PTV that are not inside the BV (PTV−BV, Table 3). As previously mentioned, PTV−BV consists mainly of OARs that were located in the PTV and were excluded in the process of defining the BV.
Bland–Altman plots for maximum dose (Dmax) and mean dose (Dmean) to boost volume and planning target volume (PTV) show the mean dose against the difference in dose of both methods for each patient. The mean difference between the sequential boost (SEB) and simultaneous integrated boost (SIB) methods and the 95 % confidence interval (± 2 SD) are depicted as dashed lines
Table 2 shows the involvement of any of the OARs inside the target volumes. For example, in seven patients the brainstem was involved in the BV and in 13 patients in the PTV (not in the BV). The retinas were not involved in the BVs but were involved in the PTVs of two patients. Table 2 also shows that ten OARs (brainstem, chiasm, and right optic nerve) were inside the BV. The details concerning the doses in these ten OARs are shown in supplementary material 1.
Only in very few cases did the Dmax and Dmean exceed the tolerance doses of the OARs in any of the methods. The only exceptions were the lenses. In both methods, the Dmax and Dmean values exceeded the tolerance dose of the lenses in a considerable number of treatment plans. The reason for this exception is due to the low tolerance dose of the lenses, which is only 5 Gy (supplementary material 2).
Table 3 shows the difference between the doses received by OARs and the PTV−BV volumes in the two methods. All OARs received more doses (Dmax and Dmean) in the SEB method than in the SIB procedure. These differences between the two methods are significant both for Dmaxand Dmean in all OARs, except for Dmax in three OARs (right retina, left lachrymal gland, and right eyeball). Higher Dmax and Dmean values were also found in the SEB method for PTV−BV.
Discussion
IMRT is an innovative technique in radiation oncology [1, 7, 16]. During the last decade it was increasingly applied in clinical radiation treatment of tumours. For a number of entities, such as prostate cancer, head and neck tumours, and brain tumours, it could be shown that IMRT is advantageous owing to its better dose distribution and better sparing of normal tissues when compared with traditional 3D radiation treatment [17–20].
The aim of our study was to find out whether in high-grade gliomas treated with the IMRT technique, different modes of radiation application to the BV (SEB vs. SIB) offer the potential of further improvements. We tested this question by using VMAT (see introductory section). The most important result of our study is that the SIB method within the VMAT technique can achieve high conformity in dose delivery to the irradiated target volumes (PTV and BV) and in addition the SIB method is obviously linked to an improved chance of sparing the OARs. This latter very important advantage of SIB has been shown in several other cancers such as prostate cancer, head and neck malignancies, lung cancer, etc. [21–25].
As mentioned, very few comparative SIB/SEB studies in brain tumours have been conducted [26–28]. These studies have shown better sparing of normal brain tissues and OARs and also equal or better coverage of target volumes. However, it is important to mention that these studies were done on hypothetical lesions [26] or on a small number of patients [27] or on phantoms [28]. In contrast to these three investigations, our study has focused only on patients (not phantom) and on real tumours. In addition, our study was performed with a comparatively larger sample volume (Table 4). Our study was of patients with high-grade gliomas, but the potential benefit of better sparing of OARs can be expected for other brain tumours too.
When comparing both methods, it is important to note that the comparatively higher Dmean to PTV in SEB (Dmean to BV is similar in SEB and SIB) is related to the delivered doses to PTV−BV. These volumes consist mainly of parts of OARs that were inside the PTV and were deleted in the process of defining and contouring the BV. Thus, in our study, while the SIB seems to be able to spare OARs more efficiently, some parts of the PTV that consist of OARs receive lower doses.
One point that should be taken into consideration is that the SEB method was mostly used before the arrival of IMRT, within the 3D conformal irradiation technique [1]. However, in our study we used VMAT not only for the SIB method but also for the SEB method. Therefore, it could be expected that the homogeneity and conformity of doses to the target volumes would be similar in the two methods. However, concerning the OARs, this study shows that the SIB method is significantly superior to the SEB procedure. The superiority is true for almost all of the OARs (right and left optic nerves, chiasm, brainstem, left retina, right lachrymal gland, right and left lenses, and left eyeball) that we investigated.
Finally, we would like to mention a radiobiological aspect that might be of interest in patients with brain tumours with longer life expectancies. It is a well-known fact that higher single doses are potentially more dangerous regarding the development of late radiation side effects such as necrosis of central nervous system tissues and other injuries [29–31]. In our study with the SIB method, the single dose per fraction to the PTV (not BV) was lower when compared with the SEB. This is of advantage when considering that in the PTV there is a comparatively high probability of irradiation of sub-volumes of relatively sensitive OARs. Thus, in addition to “physical sparing”, the SIB method can also offer the possibility of a “biological sparing”. The biological effective doses (BED) to the OARS also proved to be superior in the SIB method compared with the SEB method (supplementary material 3), but the true significance of this “theoretical” advantage of SIB should be evaluated in clinical trials.
Conclusion
Our results from 20 patients with high-grade gliomas suggest a greater potential of sparing of OARs when using the SIB technique. Dose coverage of the BV with the SIB method is similar to that with the SEB method, while the delivered dose to the PTV is higher in the SEB method. This difference is related to the higher delivered dose to the PTV−BV. This volume mainly contains sub-volumes of OARs that were located in the PTV but not in the BV. Because to date it is unclear whether SIB offers a better outcome of patients (including radiation-treated brain tumour patients with a longer survival) more large-scale studies are needed. On the basis of our planning study observations, we believe the clinical advantages and disadvantages of these two methods in clinical radiation treatment should be investigated in more detail.
References
Cho KH, Kim J-Y, Lee SH et al (2010) Simultaneous integrated boost intensity-modulated radiotherapy in patients with high-grade gliomas. Int J Radiat Oncol Biol Phys 78:390–397
Iuchi T, Hatano K, Narita Y et al (2006) Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys 64:1317–1324
Prados MD, Wara WM, Sneed PK et al (2001) Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 49:71–77
Nieder C, Andratschke N, Wiedenmann N et al (2004) Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther Onkol 180:401–407
Selker RG, Shapiro WR, Burger P et al (2002) The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery 51:343–355
Souhami L, Seiferheld W, Brachman D et al (2004) Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 60:853–860
Nakamatsu K, Suzuki M, Nishimura Y et al (2008) Treatment outcomes and dose-volume histogram analysis of simultaneous integrated boost method for malignant gliomas using intensity-modulated radiotherapy. Int J Clin Oncol 13:48–53
Otto K (2008) Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 35:310–317
Lu SH, Cheng JC, Kuo SH et al (2012) Volumetric modulated arc therapy for nasopharyngeal carcinoma: a dosimetric comparison with TomoTherapy and step-and-shoot IMRT. Radiother Oncol 104:324–330
Shaffer R, Nichol AM, Vollans E et al (2010) A comparison of volumetric modulated arc therapy and conventional intensity-modulated radiotherapy for frontal and temporal high-grade gliomas. Int J Radiat Oncol Biol Phys 76:1177–1184
Roa DE, Schiffner DC, Zhang J et al (2012) The use of RapidArc volumetric-modulated arc therapy to deliver stereotactic radiosurgery and stereotactic body radiotherapy to intracranial and extracranial targets. Med Dosim 37:257–264
Panet-Raymond V, Ansbacher W, Zavgorodni S et al (2012) Coplanar versus noncoplanar intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) treatment planning for fronto-temporal high-grade glioma. J Appl Clin Med Phys 13:3826
Suzuki M, Nakamatso K, Kanamori S et al (2003) Feasibility study of the simultaneous integrated boost (SIB) method for malignant gliomas using intensity-modulated radiotherapy (IMRT). Jpn J Clin Oncol 33:271–277
Thilmann C, Zabel A, Grosser KH et al (2001) Intensity-modulated radiotherapy with an integrated boost to the macroscopic tumor volume in the treatment of high-grade gliomas. Int J Cancer 96:341–349
n a (2010) The international commission on radiation units and measurements. J ICRU 10(1). doi:10.1093/jicru/ndq001. (Report 83. Oxford University Press)
Bortfeld T (2006) IMRT: a review and preview. Phys Med Biol 51:R363–R379
Sveistrup J, af Rosenschöld PM, Deasy JO et al (2014) Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol 9:44
Mok G, Gauthier I, Jiang H et al (2015) Outcomes of intensity-modulated radiotherapy versus conventional radiotherapy for hypopharyngeal cancer. Head Neck. doi:10.1002/hed.23649
Matthiesen C, Herman TD, Singh H et al (2015) Dosimetric and radiobiologic comparison of 3D conformal, IMRT, VMAT and proton therapy for the treatment of early-stage glottic cancer. J Med Imaging Radiat Oncol. doi:10.1111/1754-9485.12227
Burnet NG, Jena R, Burton KE et al (2014) Clinical and practical considerations for the use of intensity-modulated radiotherapy and image guidance in neuro-oncology. Clin Oncol (R Coll Radiol) 26:395–406
Bansal A, Kapoor R, Singh SK et al (2012) Dosimetric comparison of standard three-dimensional conformal radiotherapy followed by intensity-modulated radiotherapy boost schedule (sequential IMRT plan) with simultaneous integrated boost-IMRT (SIB IMRT) treatment plan in patients with localized carcinoma prostate. Indian J Urol 28:300–306
Al-Mamgani A, Heemsbergen WD, Peeters ST, Lebesque JV (2009) Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys 73:685–691
Li XA, Wang JZ, Jursinic PA et al (2005) Dosimetric advantages of IMRT simultaneous integrated boost for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 61:1251–1257
Dogan N, King S, Emami B et al (2003) Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. Int J Radiat Oncol Biol Phy 57:1480–1491
Chen SW, Yang SN, Liang JA et al (2005) Comparative dosimetric study of two strategies of intensity-modulated radiotherapy in nasopharyngeal cancer. Med Dosim 30:219–227
Baisden JM, Sheehan J, Reish AG et al (2011) Helical tomotherapy simultaneous integrated boost provides a dosimetric advantage in the treatment of primary intracranial tumors. Neurol Res 33:820–824
Prokic V, Wiedenmann N, Fels F et al (2013) Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: a planning study on treatment concepts. Int J Radiat Oncol Biol Phys 85:264–270
Yang W, Jones R, Read P et al (2011) Standardized evaluation of simultaneous integrated boost plans on volumetric modulated arc therapy. Phys Med Biol 56:327–339
Lee AW, Kwong DL, Leung SF et al (2002) Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys 53:75–85
Marks JE, Baglan RJ, Prassad SC, Blank WF (1981) Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys 7:243–252
Ruben JD, Dally M, Bailey M et al (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65:499–508
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Farzin, M. Molls, S. Astner, I-C. Rondak, and M. Oechsner state that there are no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Farzin, M., Molls, M., Astner, S. et al. Simultaneous integrated vs. sequential boost in VMAT radiotherapy of high-grade gliomas. Strahlenther Onkol 191, 945–952 (2015). https://doi.org/10.1007/s00066-015-0888-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-015-0888-1
Keywords
- Simultaneous integrated boost
- Sequential boost
- Volumetric-modulated arc therapy
- High-grade glioma
- Organs at risk