Abstract
Purpose
The purpose of this work is to report the long-term outcomes of three-dimensional conformal radio(chemo)therapy in the curative management of esophageal squamous cell carcinoma (ESCC).
Patients and methods
A retrospective analysis of patients treated with radio(chemo)therapy between 1988 and 2011 at Klinikum rechts der Isar, Technische Universität München was performed. In all, 168 patients received radio(chemo)therapy for ESCC in curative intention. The median follow-up time was 91 months (range 1–212 months). There were 128 men and 40 women with a median age of 63 years. Selection criteria for radio(chemo)therapy were unfit for surgery and/or unresectable primary tumor (n = 146, 87 %) or patients’ choice (n = 22, 13 %). The majority of the patients received a combination of cisplatin and 5-fluorouracil chemotherapy with 54 Gy in 30 fractions of radiotherapy.
Results
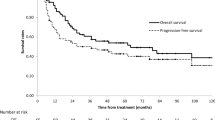
The median overall survival (OS) was 20 months (95 % confidence interval 17–23 months). The OS at 2 and 5 years for the whole cohort was 41 ± 4 % and 22 ± 3 %, respectively. Forty patients (24 %) suffered an in-field recurrence. The most common acute nonhematologic toxicity >grade 2 was dysphagia in 35 % of the patients. Acute hematologic toxicity > grade 2 was recorded in 14 % of the patients. There was no grade 5 toxicity observed during the study. Poor ECOG performance status (0–1 vs. 2–3, HR = 1.70, p = 0.002) and weight loss ≥ 10 % before the start of therapy (HR = 1.99, p = 0.001) were among the factors significantly associated with poor OS in multivariate analysis.
Conclusion
Three-dimensional conformal definitive radio(chemo)therapy is well tolerated and leads to long-term survival in more than 20 % of patients with advanced disease and/or contraindication to surgery. However, 24 % in-field recurrence remains a major concern. Prospective trials are warranted to assess if a well-tailored conformal radiochemotherapy can improve the local control and obviate the need for surgical resection in patients with good general condition and potentially resectable tumors.
Zusammenfassung
Hintergrund
Ziel dieser Arbeit war es, die Langzeitergebnisse von Patienten mit einem Plattenepithelkarzinom des Ösophagus (ESCC), die mit dreidimensional konformaler Radio(chemo)therapie in kurativer Absicht behandelt wurden, zu berichten.
Patienten und Methoden
Durchgeführt wurde eine retrospektive Analyse von Patienten, die zwischen 1988 und 2011 am Klinikum rechts der Isar, Technische Universität München, mit einer Radio(chemo)therapie behandelt wurden. In kurativer Intention erhielten 168 ESCC-Patienten eine Radio(chemo)therapie. Die mediane Nachbeobachtungszeit betrug 91 Monate (Intervall 1–212 Monate). Die Kohorte bestand aus 128 männlichen und 40 weiblichen Patienten mit einem medianen Alter von 63 Jahren. Die Selektionskriterien für eine Radio(chemo)therapie waren "ungeeignet für eine Operation" und/oder nichtresezierbarer Primärtumor (n = 146, 87 %) oder "Entscheidung des Patienten" (n = 22, 13 %). Die Mehrheit der Patienten erhielt eine Kombination aus einer Cisplatin- und 5-Fluorouracil-Chemotherapie mit einer 54-Gy-Bestrahlung in 30 Fraktionen.
Ergebnisse
Das mediane Gesamtüberleben (OS) lag bei 20 Monaten (95 %-Konfidenzintervall 17–23 Monate). Das 2- und 5-Jahres-OS für die gesamte Kohorte betrug jeweils 41 ± 4% und 22 ± 3%. Bei 40 Patienten (24%) trat nach der Behandlung ein In-field-Rezidiv auf. Die häufigste nichthämatologische akute Nebenwirkung der Behandlung >Grad 2 war Dysphagie in 35 % der Patienten. Akute hämatologische Nebenwirkungen >Grad 2 traten in 14 % der Patienten auf. In der Gesamtdauer der Studie wurden keine Grad-5-Nebenwirkungen beobachtet. Ein schlechter ECOG-Performance-Status (0–1 vs. 2–3, HR = 1,70; p = 0,002) und ein Gewichtsverlust ≥ 10 % vor Beginn der Therapie (HR = 1,99; p = 0,001) waren Faktoren, die in der multivariaten Analyse signifikant mit einem schlechterem OS assoziiert waren.
Schlussfolgerung
Eine dreidimensional konformale definitive Radio(chemo)therapie wird gut toleriert und führt in über 20 % der Patienten mit fortgeschrittenem Stadium und/oder Kontraindikationen für eine Operation zu einem verbesserten Langzeitüberleben. Eines der größten Probleme bleibt dennoch eine In-field-Rezidivrate von 24 %. Prospektive Studien sind notwendig, um zu beurteilen, ob eine optimal geplante, konformale Radiochemotherapie bei Patienten mit gutem Allgemeinzustand und potentiell resezierbarem Tumor die Lokalrezidivrate verbessern kann und eine Alternative zu einer chirurgischen Resektion darstellt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal squamous cell carcinoma (ESCC) has a poor overall prognosis, unless diagnosed very early. Extent of disease at presentation and performance status have been reported as the most powerful predictors for survival [1]. The best curative treatment for ESCC still remains a highly discussed issue. Historically, surgical resection as a monotherapy was the standard for localized ESCC with cure rates reported in the range of 10–20 %. The use of preoperative radiochemotherapy (N-RCT) has led to better outcomes [2−6].
Three randomized trials have compared definitive (D)-RCT with either surgery alone or N-RCT followed by surgery for patients with ESCC. Although no significant differences in survival were identified, the trials were underpowered to examine the noninferiority of D-RCT compared with surgery [7−9]. However, there are data indicating that a subgroup of patients with ESCC do not have an additional benefit of surgery after RCT, such as patients > 70 years [10]. Other prospective trials excluded patients with a weight loss > 10 %, tumor length > 8 cm, axial tumor dimension > 5 cm, or age > 70 years. At our institution, D-RCT or RT alone is often used for patients with impaired health status and/or comorbidities or patients who refuse surgical resection. Our primary concept for curative treatment is a surgical approach.
In 2012, we reported our institution’s experience with 163 patients treated with RT or RCT from 1988–2006 at the Technische Universität München [11]. In that study a considerable proportion of patients was treated with two-dimensional conventional and/or split course radiotherapy, which were significantly associated with poor outcomes. As these are outdated treatment modalities without impact on current practice, we updated our database and follow-up after excluding these patients, and report the long-term results of patients with ESCC who were treated with three-dimensional (3D) RCT or RT at our institution.
Patients and methods
We reviewed data and medical charts of patients, who were treated with definitive RCT or RT between 1988 and 2011 at Klinikum rechts der Isar, Technische Universität München. Patients with histologically confirmed ESCC (T1-T4, N0-1, cM0), three-dimensional (3D) conformal radiation technique, and no previous history of other malignancies (except nonlife-threatening skin and cervical cancers) were included in this study. Exclusion criteria were the following: distant metastases, split course radiotherapy (SC-RT), and 2D conventional radiation technique or a hybrid technique of conventional/conformal set-up. A total of 168 patients fulfilled the inclusion criteria. The first patient without 2D conventional planning was treated in 1994.
The median follow-up time was 91 months (range 1–212 months). There were 128 men and 40 women with a median age of 63 years (range 40–83 years). Patients’ characteristics are displayed in Table 1.
Selection criteria for RCT were patients deemed unfit for surgery and/or unresectable primary tumor (n = 146, 87 %) or patients’ choice (n = 22, 13 %). The majority of the patients received a combination of cisplatin and 5-fluorouracil (5-FU) chemotherapy with 54 Gy in 30 fractions of radiotherapy.
Staging
The staging evaluation at our department did not considerably vary between patients and generally included the following: complete medical history, physical examination, serum chemistry profile, complete blood count, chest X-ray, barium swallow (in the early 1990s), esophagogastroduodenoscopy with biopsy, endosonography, CT scans of the neck, chest and abdomen, abdominal ultrasound, and cardiopulmonary function tests (to evaluate medical operability). In 70 patients (42 %) a FDG-PET-CT was performed. Bronchoscopy was performed in patients with middle and upper third tumors to exclude tracheobronchial invasion and panendoscopy was performed to rule out simultaneous head and neck cancer.
The general performance status of the patients was determined according to the ECOG Performance Status scale. Toxicities were retrospectively assessed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0.
Treatment
Conformal external-beam radiotherapy with 6–15 MeV photons was delivered using 3- and 4-field techniques with a median dose of 54 Gy (range 49–70 Gy). The clinical target volume (CTV) comprised the primary tumor (based on CT, endo-sono or PET criteria) with a margin of 4 cm in the craniocaudal direction and regional lymph nodes. The margin of the planning target volume (PTV1) to the CTV was 8–10 mm in all directions, taking internal organ movements as well as setup errors into account. PTV1 was treated with 45 Gy (1.8–2 Gy per fraction). PTV2 comprised the primary tumor with a margin of 4 cm in the craniocaudal and 1 cm in the anterior–posterior and lateral direction plus involved nodes with a safety margin of 1 cm in all directions. PTV2 was treated with a median dose of 9 Gy (range 4–30 Gy). In all, 28 patients were treated with radiotherapy alone, due to poor performance status or refusal of chemotherapy.
A total of 140 patients (83 %) were treated with concomitant chemotherapy to radiotherapy, consisting of 5-FU (300 mg/m2/day; n = 44), cisplatin (20 mg/m2/day on days 1–5 and 29–33; n = 12) or both (n = 73). Nine patients with worse renal function received carboplatin (AUC2/week) with (n = 6) or without (n = 3) 5-FU (300 mg/m2/day). Two patients received oxaliplatin (40–50 mg/m2/week) in combination with 5-FU (300 mg/m2/day).
Follow-up
During the first year after treatment, patients were seen every 3 months. In the second year, follow-up took place every 6 months, and then annually. During follow-up, diagnostic investigations including CT and endoscopy were performed up to 5 years, and thereafter only when recurrence was suspected.
Statistical methods
Overall survival (OS) was defined as death from any cause, disease-free survival (DFS) was defined as the time to a new locoregional failure, metastasis or death of any cause, and recurrence-free survival (RFS) was defined as the time to a new locoregional failure or death. All time estimates began with the first day after start of radiotherapy. Locoregional recurrences were defined by radiological/clinical signs of recurrent or progressive disease in the esophagus or regional lymph nodes. OS, DFS, and RFS were analyzed according to the Kaplan–Meier method. Survival curves were compared between subgroups by the log-rank test. To adjust for potentially confounding factors, multivariable Cox proportional hazard regression models were employed. Comparison of cumulative incidence functions was performed using Gray’s test. A two-sided level of significance of α = 5 % was used for all statistical tests.
Results
The median overall survival (OS) was 20 months [95 % confidence interval (CI) 17–23 months; Fig 1]. The survival rates at 2 and 5 years were 41 ± 4 % and 22 ± 3 %, respectively. DFS at 2 and 5 years was 31 ± 4 % and 20 ± 3 %, respectively. RFS at 2 and 5 years was 36 ± 4 % and 20 ± 3 %, respectively. Locoregional recurrence occurred in a total of 45 patients (27 %) after a median time of 13 months (range 3–74 months); in 40 patients (24 %) recurrence was within the treated volume (in-field recurrence). In 54 patients (32 %), distant metastases were observed after completion of the treatment. The most common site of distant metastasis was the lung (n = 25) followed by nonregional lymph nodes (n = 22), bone (n = 12), and liver (n = 10). Some of the patients had two or more metastases locations.
Uni- and multivariate analyses
Results of the univariate analyses for OS, DFS, and RFS are displayed in Table 2. To identify independent prognostic factors for OS, we performed a multivariate analysis including all significant factors from the initial univariate tests, i.e., ECOG-PS, weight loss ≥ 10 %, UICC stage, paresis of recurrent laryngeal nerve, age, and length of the tumor. Poor ECOG-PS (0–1 vs. 2–3, HR = 1.63, p = 0.003), weight loss ≥ 10 % (HR = 1.77, p = 0.003), and paresis of recurrent laryngeal nerve at the time of diagnosis (HR = 1.61, p = 0.047) were significantly associated with poor OS in the multivariate analysis (Fig. 2).
Toxicity
There was no grade 5 toxicity observed during the study. The list of observed acute toxicity > grade 2 is displayed in Table 3. The most common acute non-hematologic toxicity > grade 2 was dysphagia in 35 % of the patients. In 30 patients it was not possible to differentiate retrospectively between dysphagia caused by the primary disease and dysphagia induced by treatment. Acute hematologic toxicities > grade 2 was recorded in 14 % of the patients.
Chronic toxicity was assessed by RTOG/EORTC Late Radiation Morbidity Scoring Scheme. Grade 1–3 esophageal strictures were observed in 75 patients (45 %). In 7 patients an endoscopic dilation was performed (grade 3). Airway fistulas (grade 4) were observed in 8 patients (5 %).
Discussion
It has been half a century since Myhre et al. [12] reported the results of radiation and infusional 5-FU in 8 patients with esophageal cancer. Subsequent studies in the 1980s using primary radiation and concurrent chemotherapy were performed with encouraging results [13−16]. In the early 1990s, the landmark trial RTOG 8501 demonstrated the advantage of combining radiation and chemotherapy compared to radiotherapy alone [17]. In RTOG 8501, patients with locoregional confined (T1–3, N0–1, M0) esophageal cancer were randomly assigned to RCT (two cycles of infusional 5-FU plus cisplatin during weeks 1 and 5, plus RT with 50 Gy in 25 fractions, or RT without chemotherapy (total dose 64 Gy). The combination of RT with chemotherapy was associated with a significantly better median survival (14 vs. 9 months) and 5-year survival (27 versus 0 %).
We recently reported our experience with radio(chemo)therapy for ESCC between 1988 and 2006, and here we report updated data through December 2011. What have we learned from this 23-year experience with concurrent radiation and chemotherapy for ESCC? First, long-term survival is achievable with radio(chemo)therapy. If the disease has not spread to distant organs, a well-tailored conservative approach can achieve long-term survival for more than 30 % of patients with favorable prognostic factors such as good performance status and limited weight loss. Second, if a patient is not chemotherapy eligible, it does not mean that there is no chance for long-term survival. We demonstrated that long-term survival is possible in patients in whom chemotherapy could not be performed due to different contraindications or patients’ refusal. Our median survival data were markedly better than those of RTOG 8501, which might result from different staging procedures, patient selection or improved treatment techniques including supportive care. The risk of locoregional failure after radio(chemo)therapy was of concern (27 %). Research towards more efficacious conservative regimes is warranted. Previous studies, e.g., the one published by Brunner et al. [18], suggested that higher radiation doses are not sufficient to improve survival (50 patients, 90 % with ESCC, median survival 16 months after D-RCT with 64.8 Gy plus brachytherapy boost). Likely, identification of resistance pathways is necessary to design better treatments. Effective salvage is rarely feasible in this patient population, except in patients who are still fit for surgery, after they initially preferred nonsurgical management or in patients who can be re-irradiated [19].
The vast majority of previous studies included Asian patients with ESCC. The study by Delcambre et al. [20] is comparable to our own cohort of Caucasian patients (n = 63). Five-year survival was 26 %, median survival 24 months. These figures are in line with the present study and also those reported by Jeremic et al. [21]. Their study included 28 patients, and median survival was 26 months (5-year rate: 29 %). Direct comparison with surgical series from our institution is difficult since definitive RCT is retained for patients with poor health status and/or comorbidities or patients who refuse a surgical resection, which leads to an unfavorable patient cohort in the RCT arm. Even multivariate analysis cannot adjust for all potential sources of bias. Convincing evidence requires prospective randomized studies. Despite its inherent limitations, the present retrospective analysis adds important data to our knowledge base because it is one of the largest studies of ESCC in Caucasian patients treated with radio(chemo)therapy and includes long-term follow-up data.
Conclusion
Three-dimensional conformal definitive radio(chemo)therapy is well tolerated and leads to long-term survival in more than 20 % of patients with advanced disease and/or contraindication to surgery. However, 24 % in-field recurrence remains a major concern. Prospective trials are warranted to assess if well-tailored conformal radiochemotherapy can improve the local control and obviate the need for surgical resection in patients with good general condition and potentially resectable tumors.
References
Tanisada K, Teshima T, Ikeda H et al (1998) Prognostic factors for patients with esophageal cancer treated with radiation therapy in PCS: a preliminary study. Radiat Med 16:461–468
Van Hagen P, Hulshof MC, van Lanschot JJ et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084
Kranzfelder M, Schuster T, Geinitz H et al (2011) Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg 98:768–783
Fakhrian K, Ordu AD, Lordick F et al (2014) Long-term outcomes of trimodality treatment of squamous cell carcinoma of esophagus with cisplatin and/or 5-FU. More than 20 years experience at a single institution. Strahlenther Onkol [Epub ahead of print]
Fakhrian K, Ordu AD, Haller B et al (2014) Cisplatin- vs. oxaliplatin-based radiosensitizing chemotherapy for squamous cell carcinoma of the esophagus: a comparison of two preoperative radiochemotherapy regimens. Strahlenther Onkol [Epub ahead of print]
Fakhrian K, Oechsner M, Kampfer S (2013) Advanced techniques in neoadjuvant radiotherapy allow dose escalation without increased dose to the organs at risk. Strahlenther Onkol 189:293–300
Stahl M, Stuschke M, Lehmann N (2005) Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23:2310–2317
Chiu PW, Chan AC, Leung SF et al (2005) Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE). J Gastrointest Surg 9:794–802
Bedenne L, Michel P, Bouché O et al (2007) Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25:1160–1168
Abunasra H, Lewis S, Beggs L et al (2005) Predictors of operative death after oesophagectomy for carcinoma. Br J Surg 92:1029–1033
Fakhrian K, Heilmann J, Schuster T et al (2012) Primary radiotherapy with or without chemotherapy in non-metastatic esophageal squamous cell carcinoma: a retrospective study. Dis Esophagus 25:256–262
Myhre K, Fjaerli J (1964) Treatment of malignant tumours with 5-Fluorouracil in 80 patients. Acta Radiol Ther Phys Biol 2:129–138
Byfield JE, Barone R, Mendelsohn J, Frankel S, Quinol L, Sharp T, Seagren S (1980) Infusional 5-fluorouracil and X-ray therapy for non-resectable esophageal cancer. Cancer 45:703–708
Chan A, Wong A, Arthur K (1989) Concomitant 5-fluorouracil infusion, mitomycin C and radical radiation therapy in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 16:59–65
Coia L, Engstrom P, Paul A (1987) Nonsurgical management of esophageal cancer: report of a study of combined radiotherapy and chemotherapy. J Clin Oncol 5:1783–1790
Coia L, Paul A, Engstrom P (1988) Combined radiation and chemotherapy as primary management of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer 61:643–649
Herskovic A, Martz K, Al-Sarraf M et al (1992) Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 326:1593–1598
Brunner TB, Rupp A, Melzner W, Grabenbauer GG, Sauer R (2008) Esophageal cancer. A prospective phase II study of concomitant-boost external-beam chemoradiation with a top-up endoluminal boost. Strahlenther Onkol 184:15–22
Fakhrian K, Gamisch N, Schuster T et al (2012) Salvage radiotherapy in patients with recurrent esophageal carcinoma. Strahlenther Onkol 188:136–42
Delcambre C, Jacob JH, Pottier D, Gignoux M, Ollivier JM, Vie B, Roussel A, Segol P (2001) Localized squamous-cell cancer of the esophagus: retrospective analysis of three treatment schedules. Radiother Oncol 59:195–201
Jeremic B, Shibamoto Y, Acimovic L, Matovic Z, Milicic B, Milisavljevic S, Nikolic N (1998) Accelerated hyperfractionated radiation therapy and concurrent 5-fluorouracil/cisplatin chemotherapy for locoregional squamous cell carcinoma of the thoracic esophagus: a phase II study. Int J Radiat Oncol Biol Phys 40:1061–1066
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.D. Ordu, C. Nieder, H. Geinitz, P.G. Kup, L.F. Deymann, V. Scherer, S.E. Combs, and K. Fakhrian state that there are no conflicts of interest.
The accompanying manuscript does not include studies on humans or animals.
Rights and permissions
About this article
Cite this article
Ordu, A., Nieder, C., Geinitz, H. et al. Radio(chemo)therapy for locally advanced squamous cell carcinoma of the esophagus. Strahlenther Onkol 191, 153–160 (2015). https://doi.org/10.1007/s00066-014-0779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0779-x
Keywords
- Definitive radiochemotherapy
- Squamous cell carcinoma of the esophagus
- Esophageal neoplasms
- Survival
- Prognosis