Abstract
Background/Purpose
Distal medium vessel occlusions (DMVOs) account for a large percentage of vessel occlusions resulting in acute ischemic stroke (AIS) with disabling symptoms. We aim to assess whether pretreatment quantitative CTP collateral status (CS) parameters can serve as imaging biomarkers for good clinical outcomes prediction in successfully recanalized middle cerebral artery (MCA) DMVOs.
Methods
We performed a retrospective analysis of consecutive patients with AIS secondary to primary MCA-DMVOs who were successfully recanalized by mechanical thrombectomy (MT) defined as modified thrombolysis in cerebral infarction (mTICI) 2b, 2c, or 3. We evaluated the association between the CBV index and HIR independently with good clinical outcomes (modified Rankin score 0–2) using Spearman rank correlation, logistic regression, and ROC analyses.
Results
From 22 August 2018 to 18 October 2022 8/22/2018 to 10/18/2022, 60 consecutive patients met our inclusion criteria (mean age 71.2 ± 13.9 years old [mean ± SD], 35 female). The CBV index (r = −0.693, p < 0.001) and HIR (0.687, p < 0.001) strongly correlated with 90-day mRS.
A CBV index ≥ 0.7 (odds ratio, OR, 2.27, range 6.94–21.23 [OR] 2.27 [6.94–21.23], p = 0.001)) and lower likelihood of prior stroke (0.13 [0.33–0.86]), p = 0.024)) were independently associated with good outcomes.
The ROC analysis demonstrated good performance of the CBV index in predicting good 90-day mRS (AUC 0.73, p = 0.003) with a threshold of 0.7 for optimal sensitivity (71% [52.0–85.8%]) and specificity (76% [54.9–90.6%]). The HIR also demonstrated adequate performance in predicting good 90-day mRS (AUC 0.77, p = 0.001) with a threshold of 0.3 for optimal sensitivity (64.5% [45.4–80.8%]) and specificity (76.0% [54.9–90.6%]).
Conclusion
A CBV index ≥ 0.7 may be independently associated with good clinical outcomes in our cohort of AIS caused by MCA-DMVOs that were successfully treated with MT. Furthermore, a HIR < 0.3 is also associated with good clinical outcomes. This is the first study of which we are aware to identify a CBV index threshold for MCA-DMVOs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Distal medium vessel occlusions (DMVOs), defined as M2–M4 segments of the middle cerebral artery (MCA), anterior cerebral artery (ACA) segments and vertebrobasilar branches, are thought to represent 25–40% of acute ischemic stroke (AIS) and can result in disabling symptoms [1]. The current standard of care treatment for AIS caused by DMVOs is IV thrombolysis but fails to successfully recanalize DMVOs in up to two thirds of patients [1]. With the recent technological advances, DMVOs are now increasingly being treated with mechanical thrombectomy (MT) despite the current lack of consensus on guidelines [2].
Robust collaterals have been shown to predict good outcomes in large vessel occlusions (LVOs), but the effect of collateral status (CS) on DMVOs is still an area of ongoing research. Although CT angiography-based CS grading can be performed, there is significant variability amongst readers, necessitating automated quantitative pretreatment CT perfusion (CTP) CS assessments [3, 4]. The cerebral blood volume (CBV) index, defined as the mean rCBV obtained by dividing the average of all CBV values from the Tmax > 6 s region within the ischemic hemisphere by the average of all CBV values from all tissues with Tmax ≤ 4 s [5] and the hypoperfusion intensity ratio (HIR), defined as time to maximum (Tmax) greater than 10 s volume divided by the Tmax greater than 6 s volume [6,7,8], have both been previously validated as reliable quantitative CS parameters, particularly for middle cerebral artery (MCA) LVOs. Prior LVO studies have reported thresholds of greater than 0.8 for CBV index [9] and approximately 0.4 for HIR [8], where patients with greater than 0.8 or HIR less than 0.4 have good CS. However, despite being established in LVOs, no studies to our knowledge have assessed the optimal CBV index threshold for MCA-DMVOs.
Furthermore, the optimal threshold for HIR in MCA-DMVO still has not been established. A prior study reported that threshold of 0.3 is optimal for predicting infarct growth in 40 successfully recanalized DMVOs [10], which is lower than the previously mentioned 0.4 threshold in LVO (https://onlinelibrary.wiley.com/doi/full/10.1111/ene.14181). Nevertheless, assessing HIR in the setting of DMVO remains underexplored.
We postulate that different CBV index and HIR thresholds apply to DMVOs compared to LVOs because of the smaller area of tissue affected and the longer transit for blood flow to reach the hypoperfused tissue.
Therefore, the primary aim of our study is to a) establish a threshold for CBV index in patients with AIS due to primary MCA-DMVOs who were successfully recanalized by MT for clinical outcomes prediction and compare this threshold to the previously established LVO cut-off and b) determine the predictive value of HIR in the same setting with comparison to CBV index in order to assess the value of each CS parameter in the same cohort. For this aim, we hypothesize that a more restrictive threshold compared to the LVO threshold of 0.4.
Methods
Population and Study Design
In this retrospective study, we identified consecutive patients from two comprehensive stroke centers within the Johns Hopkins Medical Enterprise (Johns Hopkins Hospital—East Baltimore and Bayview Medical Campuses) from 8/22/2018 to 10/18/2022 in a continuously maintained database. This study was approved through the Johns Hopkins School of Medicine institutional review board (JHU-IRB00269637) and follows the STROBE checklist guidelines as an observational study [11].
The inclusion criteria for this study were as follows: a) MT triage within 24 h of symptom onset or last known well, b) diagnostically adequate multimodal pretreatment CT imaging including NCCT, CTA, and CTP, c) AIS due to a CT angiography (CTA) confirmed MCA-DMVO, specifically including M2–M4 segments of the MCA as defined by Saver et al. [1] with additional confirmation on CTP and d) successful recanalization by MT defined as modified thrombolysis in cerebral infarction (mTICI) 2b or 3. M2 occlusions were defined as those distal to the anterior temporal artery origin in order to differentiate these occlusions from distal M1 occlusions, per Menon et al. [12]. Dominant M2 branches equal to or less than 2.1 mm were included based on the definition established by Saver et al. [1].
The study was conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). Informed consent was waived by the institutional review boards given the retrospective study design.
The decisions to administer IV tPA and/or perform MT were made on an individual basis based on consensus stroke team evaluation per our institution protocols and were controlled for in our analyses.
Data Collection
Baseline and clinical data were collected through electronic records and stroke center databases for each patient included demographics, risk factors for AIS (including diabetes mellitus, hypertension, coronary artery disease, atrial fibrillation), admission glucose, admission NIH stroke scale (admission NIHSS), ASPECTS score, site of occlusion, and laterality of occlusion, and IV thrombolysis administration. Additional collected parameters included number of passes, recanalization time, mTICI score; presence of complication such as hemorrhagic transformation (HT) as defined by the ECASS trial [13]. Patients were subsequently grouped into good and poor CS based on the statistically determined optimal CBV threshold.
Imaging Analysis
The ASPECTS scores were calculated on NCCT and baseline CTAs were reviewed for presence and site of DMVO by an experienced neuroradiologist (VSY, 6 years of experience). CTP was also utilized as a confirmatory measure of DMVO in conjunction with CTA. The same neuroradiologist assessed the diagnostic adequacy of the CTPs where only those deemed diagnostically adequate were included in the study.
Imaging Parameters
NCCT:
NCCT is performed in a helical mode at 5 mm slice thickness with 0.75 mm; reconstructions (120 kVp, 365 mA, rotation time 1 s, acquisition time 6–8 s, collimation 128 × 0.6 mm, pitch value 0.55, scan direction craniocaudal).
CTA:
The CTA of the head and neck is performed with non-ionic iodinated contrast with 50–70 ml injected at 5–6 ml/s from the aortic arch through the vertex using a bolus triggered method at 3 mm slice thickness with 0.75 mm reconstructions. The CTA parameters are as follows: 90/150 kVp with an Sn filter, Quality Reference mA 180, Rotation Time 0.25 s, Average Acquisition Time 3–5 s, Collimation 128 × 0.6 mm, Pitch Value 0.7, Scan Direction Craniocaudal.
CTP:
CTP is then performed with injection of 50 ml non-ionic iodinated contrast with a 30 ml saline flush at 5–6 ml/s with anatomic coverage of 70–100 mm at 5 mm slice thickness. Parameters as follows: 70 kVP, 200 Effective mA, Rotation Time 0.25 s, Average Acquisition Time 60 s, Collimation 48 × 1.2 mm, Pitch Value 0.7, 4D Range 114 mm × 1.5 s, Scan Direction Craniocaudal. CTP images are then postprocessed using RAPID commercial software (IschemiaView, Menlo Park, CA, USA) for generating Tmax maps, from which the HIR and CBV index are calculated.
HIR was calculated as the ratio of the Tmax > 10 s volume divided by the Tmax > 6 s volume [6, 8].
The CBV index is calculated as the mean rCBV obtained by dividing the average of all CBV values from the Tmax > 6 s region within the ischemic hemisphere by the average of all CBV values from all tissue with Tmax ≤ 4 s [5].
Angiographic Assessment
The pre-MT DSA collateral assessment was performed by two experienced neuroradiologists (MH and VSY, 3 and 6 years of experience, respectively) using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) criteria [14]. Any discrepancies were assessed with a final score based on consensus evaluation. Grades 3 and 4 were categorized as good CS. Although grade 2 is considered moderate CS, it was included in the poor CS group for dichotomized analysis.
The mTICI score was determined by the performing neurointerventionalist at the time of the procedure.
Clinical Outcomes Assessment
Modified Rankin scores at discharge and 90 days (90-day mRS) in addition to discharge NIHSS were determined by a stroke neurologist or certified nurse practitioner by either telephone interviews or during the patients’ follow-up visits.
Outcome Measures
The primary outcomes were good clinical outcomes defined as 90-day mRS 0–2. The secondary outcomes included excellent outcomes (90-day mRS 0–1), discharge mRS, discharge NIHSS, and NIHSS shift (defined as the difference between discharge and admission NIHSS).
Statistical Analysis
The collected data were coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences, software version 28.0, IBM, Armonk, MY, USA). Quantitative data were tested for normality using Shapiro-Wilk test, then if normally distributed described as mean ± SD (standard deviation) as well as minimum and maximum of the range. If data were not normally distributed, they were described as median (1st–3rd interquartiles) as well as minimum and maximum then compared using Mann-Whitney test. Correlations between HIR and CBV index as well as CBV index with DSA CS and 90-day mRS were assessed by Spearman rank correlation. Receiver operating characteristics (ROC) curve was used to evaluate the performance of HIR and CBV index where the optimal thresholds to predict mRS at 90 days of 0–2 based on highest sensitivity and specificity were determined. Multivariate logistic regression analyses were also performed to assess the association of CBV index with 90-day mRS. Patients were then grouped based on the determined CBV optimal threshold. The level of significance taken at P value ≤ 0.050 was significant, otherwise was non-significant.
Results
From 8/22/2018 to 10/18/2022, we identified 147 consecutive patients with AIS due to an MCA-DMVO. Of these 147 patients, 60 patients (mean age 71.2 ± 13.9 years old, mean ± SD, 35 female) met the inclusion criteria and were included in this study. In total, 56 out of the 60 patients had available 90-day mRS. All patients had discharge mRS and discharge NIHSS available. Please see Table 1 for demographic information.
Of the 60 patients 51 had M2 occlusions (51/60, 85%), 6 had M3s (6/60, 10%), and 3 had M4s (3/60, 5%). See Table 2.
Of the patients nineteen received IV thrombolysis (19/60, 31.7%) prior to MT. 46 (46/60, 76.7%) had pretreatment DSA that was adequate for CS assessment and 43 patients achieved mTICI 3 recanalization (43/60, 71.7%). Hemorrhagic transformation of any subtype was found in 15 patients (15/60, 25%). A higher percentage of moderate and good CS based on DSA were found in the CBV ≥ 0.7 group (4/6 vs. 2/6, grade 2 and 14/20 vs. 6/20, grade 3; p = 0.004) compared to the CBV < 0.7 group. Moreover, a higher percentage of poor CS based on DSA was found in the CBV < 0.7 group (4/4 vs. 0.4 grade 0; 6/6 vs. 0/6 grade 1; p = 0.004) versus the CBV ≥ 0.7 group. Based on dichotomized DSA CS assessment, a higher percentage of good CS patients (19/30 vs. 11/30) and a lower percentage of poor CS patients (4/16 vs. 12/16) were also seen in the CBV > = 0.7 group (p = 0.013). HIR was also lower in the CBV > = 0.7 group median 0.3, IQR 0.0–5.0 vs. median 0.5, IQR 0.3–0.6; p = 0.001[median, IQR] [0.0–5.0] vs 0.5 [0.3–0.6; p = 0.001]]. See Table 3.
Outcomes analysis revealed significantly lower 90-day mRS ([median, IQR] 1.0 [0.0–2.0] vs 4.5 [3.0–6.0], p < 0.001), discharge mRS (2.0 [2.0–3.0] vs 4.0 [3.0–5.0], p < 0.001), and discharge NIHSS (2.0 [1.0–5.0] vs 6.0 [2.0–12.0], p = 0.001) in the CBV > = 0.7 group. A larger favorable NIHSS shift was also found in the CBV > = 0.7 group (−10.5 [−16.0–−4.0] vs −3.0 [−6.0–3.0], p < 0.001). Please see Table 4 for details.
Correlation Analysis
The CBV index demonstrated a strong inverse correlation with 90-day mRS (−0.693, p < 0.001) and moderate inverse correlation with HIR (−0.494, p < 0.001). The HIR also demonstrated a strong positive correlation with 90-day mRS (0.687, p < 0.001). Neither the CBV index nor HIR were significantly correlated with DSA CS assessment. Please see Table 5.
ROC Analysis
The ROC analysis demonstrated adequate performance of CBV index in predicting good 90-day mRS (AUC 0.73, p = 0.003) with a threshold of 0.7 for optimal sensitivity (71% [52.0–85.8%]) and specificity (76% [54.9–90.6%]). CBV index also predicted excellent 90-day mRS (AUC 0.73, p = 0.003).
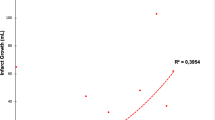
HIR also demonstrated good performance in predicting good 90-day mRS (AUC 0.77, p = 0.001) with a threshold of 0.3 for optimal sensitivity (64.5% [45.4–80.8%]) and specificity (76.0% [54.9–90.6%]). HIR also predicted excellent 90-day mRS (AUC 0.741, p = 0.002). Please see Fig. 1.
No significant differences were noted between the diagnostic performances of the CBV index and HIR thresholds with respect to predicting good 90-day mRS (p = 0.558). Please see.
Multivariate Regression Analysis
A CBV index of > = 0.7 (OR 2.27 [6.94–21.23], p = 0.001) was significantly associated with good outcomes. Furthermore, prior stroke (OR 0.13 [0.33–0.86]), p = 0.024) was inversely associated with good outcomes.
Discussion
In this study, we identified CBV index and HIR thresholds that predict good outcomes after thrombectomy treatment of MCA-DMVOs. We also demonstrate that a CBV index > = 0.7 is an independent predictor of good outcomes in this group of patients. This is the first study to our knowledge to determine a CBV index threshold for successfully recanalized AIS patients with DMVOs.
Although the current standard of care for DMVOs is IV thrombolysis, actual practice is increasingly using MT since IV thrombolysis fails to achieve recanalization in up to half of patients [15]. The advancements in endovascular technology have enabled better navigation of these smaller caliber and often tortuous vessels. Several studies have demonstrated feasibility and safety of attempting MT in DMVOs [1, 2, 15, 16], leading to an increased number of these patients being treated with MT.
In light of these advances in interventions, the effect of CS in DMVOs has become more relevant yet requires further investigation. As an established biomarker of infarct growth and outcomes in LVOs [7], it stands to reason that CS similarly influences outcomes in DMVOs as well. Pretreatment CTP quantitative assessments with CBV index [5, 9, 17] and HIR [6,7,8] have been established as imaging biomarkers of CS in LVOs. However, due to the smaller volume of affected tissue and the longer transit to reach these regions, the same LVO thresholds may not apply to DMVOs, which is the main purpose of our investigation.
As an indicator of the relative blood volume within critically hypoperfused tissue, CBV index is thought to represent an indirect compensatory response to the acute occlusion through collateralization. Our results indicate that an optimal threshold of 0.7 where a pretreatment CBV index of 0.7 or greater best predicts good clinical outcomes. This threshold is lower than the established LVO threshold of greater than 0.8 used for 24 h infarct volume prediction [9]. In direct comparison with respect to DMVOs, the 0.7 threshold is more accurate (73.2% versus 60.7%) and more sensitive (71.0% versus 35.5%) than 0.8, although less specific (76% versus 92%) in predicting good clinical outcomes. We postulate that the lower CBV index for MCA-DMVOs determined in our study is due to the smaller area of affected tissue, where compared to LVOs a less robust compensatory response may suffice to maintain tissue viability.
Interestingly, we did not find a correlation between DSA and both CBV index and HIR; however, we found a strong inverse correlation between CBV index and 90-day mRS in addition to a strong direct correlation between HIR and 90-day mRS. This discordance between both CS parameter correlations with 90-day mRS and DSA may be due to a smaller sample of patients with DSA CS evaluations. In our cohort, 46 patients (46/60, 76.7%) had DSAs that were imaged long enough to perform adequate CS assessments. Furthermore, despite DSA being considered the reference standard for CS assessment, prior studies are mixed on the robustness of DSA CS in predicting functional outcomes [3, 18, 19]. It is possible that both CS parameters capture a compensatory component of CS that may not translate completely to DSA but is reflected with subsequent clinical outcomes.
We also assessed the value of HIR as a predictive outcome measure in successfully recanalized DMVOs. Physiologically, HIR is based on the hypothesis that comparatively less perfused tissue will have prolonged transit and is thought to represent tissue microvascular perfusion [8]. Patients with lower HIR have more robust CS, as reflected by slower infarct growth rate based on the time-based volumetric assessments [6, 7]. HIR is a well-established CS imaging biomarker in LVOs where a 0.4 threshold has been optimal [6,7,8]. LVOs patients with an HIR of less than 0.4, thought to represent good CS, has been correlated with CTA collaterals [20], good DSA collaterals in M1 occlusions [6], validated for transferring patients for MT [8], predictive of infarct growth rate and clinical outcomes [7] as well as post MT HT [21]; however, the role HIR plays in DMVOs is still being investigated.
Guenego et al. most recently concluded that an HIR of less than 0.3 was associated with good CS and predicted less infarct growth in successfully recanalized DMVO cohort of 40 patients [10]. They also found that patients with an HIR equal to or greater than 0.3 had unfavorable outcomes on univariate analysis; however, this did not persist on multivariate analysis [10]. Our results are concordant with Guenego et al. where we further validated the threshold of 0.3. In our cohort, patients with an HIR less than 0.3 predicted good and excellent outcomes, even doing so slightly superior to CBV index in diagnostic performance, although the difference was not significant (p = 0.558). Our study also has some notable differences compared to Guenego et al. First, our study has a larger sample size of 60 patients. Our analysis also focused on directly predicting clinical outcomes as opposed to infarct growth as a clinical outcome surrogate. In comparison to the LVO threshold of 0.4, the 0.3 threshold was slightly more accurate (69.6% versus 67.9%) and more specific (76.0% versus 68.0%), although less sensitive (64.5% versus 67.7%) in predicting good clinical outcomes. We hypothesize that this lower threshold for HIR compared to the LVO threshold of 0.4 is likely due to longer transit time to reach the affected region, necessitating a more restrictive threshold.
In addition to our CS parameter assessment, we also report that a history of prior stroke decreases the likelihood of good outcomes in these patients. Prior stroke as a predictive biomarker is underexplored within this patient population. In a study assessing medium vessel occlusions with discrepant infarct patterns, Ospel et al. found a history of prior stroke in 16.4% (43/262) of patients in their cohort [22]. Our cohort had a substantially higher percentage of patients with prior stroke (58.3%, 35/60). The difference in sample size may be the reason for this discrepancy with Ospel et al. Nevertheless, prior stroke is a well-established risk factor for stroke recurrence of all types [23,24,25] and, for that reason it is understandable that a similar trend may also apply to the MCA-DMVO population. This may be an area of future research with larger studies.
Our study has several limitations. Firstly, it is inherently limited by its retrospective design. Secondly, CTP has some limitations as a technique because it provides a snapshot in time where the time parameters acquired only correspond to the patient’s status at the time of imaging [26]. Thirdly, we focused on only MCA-DMVOs with a predominance of M2 occlusions, which may introduce a bias. The M2 occlusion predominance is most likely due to the relative proximity of the vessel, making these occlusions more amenable to MT. Lastly, our analysis is restricted to use of one commercial software platform, which may limit generalizability. Nevertheless, our study is strengthened by an adequate sample size of 60, given the stringent inclusion criteria of successfully recanalized MCA-DMVOs with MT. Our cohort consists of two comprehensive stroke centers serving different demographics, therefore improving generalizability.
In conclusion, the use of automated pretreatment CTP CS measures may have promise in everyday clinical practice. Currently, to our knowledge, no threshold for CBV index in the setting of DMVOs exists. Moreover, the utility of HIR in DMVOs still requires additional exploration. Our study demonstrates that in comparison to LVO thresholds, a lower CBV index and a more restrictive HIR are associated with improved clinical outcomes in successfully recanalized MCA-DMVO patients. The more restrictive HIR corroborates prior hypotheses that there are differences in tissue level collaterals between LVOs and DMVOs [10, 27]. With respect to CBV, our comparatively lower CBV index threshold may be due to the CBV index capturing a venous outflow component, where a less robust compensatory response can suffice to maintain tissue viability in DMVOs compared to LVOs. Given the prevalence of DMVOs, these thresholds have potential utility in everyday clinical practice as additional predictive imaging biomarkers in this group. Nevertheless, our findings should be interpreted with some degree of caution as larger scale studies must be performed to further assess the strength of our results.
Conclusion
A CBV index ≥ 0.7 may be independently associated with good clinical outcomes in our cohort of patients presenting with AIS caused by MCA-DMVOs who were successfully treated with MT. Furthermore, a HIR < 0.3 is also associated with good clinical outcomes. This is the first study of which we are aware to identify a CBV index threshold for MCA-DMVOs. Additional studies are warranted to further assess the strength of our findings.
References
Saver JL, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke. 2020;51:2872–84.
Guenego A, et al. Thrombectomy for distal medium vessel occlusion with a new generation of Stentretriever (Tigertriever 13). Interv Neuroradiol. 2021;15910199211039926.
Kauw F, et al. Collateral status in Ischemic stroke: a comparison of computed tomography angiography, computed tomography perfusion, and digital subtraction Angiography. J Comput Assist Tomogr. 2020;44:984–92.
Wolff L, et al. Inter-rater reliability for assessing intracranial collaterals in patients with acute ischemic stroke: comparing 29 raters and an artificial intelligence-based software. Neuroradiology. 2022;64:2277–84.
Arenillas JF, et al. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab. 2018;38:1839–47.
Guenego A, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020;27:864–70.
Olivot JM, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45:1018–23.
Guenego A, et al. Hypoperfusion intensity ratio is correlated with patient eligibility for thrombectomy. Stroke. 2019;50:917–22.
Rao VL, et al. Collateral status contributes to differences between observed and predicted 24‑h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab. 2020;40:1966–74.
Guenego A, et al. Hypoperfusion intensity ratio predicts infarct growth after successful thrombectomy for distal medium vessel occlusion. Clin Neuroradiol. 2022;32:849–56.
Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53.
Menon BK, et al. Efficacy of endovascular thrombectomy in patients with M2 segment middle cerebral artery occlusions: meta-analysis of data from the HERMES Collaboration. J Neurointerv Surg. 2019;11:1065–9.
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second european-australasian acute stroke study investigators. Lancet. 1998;352:1245–1251.
Liu L, et al. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol. 2018;3:117–30.
Saber H, et al. Endovascular therapy vs medical management for patients with acute stroke with medium vessel occlusion in the anterior circulation. JAMA Netw Open. 2022;5:e2238154.
Farouki Y, et al. First-pass effect predicts clinical outcome and infarct growth after thrombectomy for distal medium vessel occlusions. Neurosurgery. 2022;91:913–9.
MacLellan A, et al. Perfusion imaging collateral scores predict infarct growth in non-Reperfused DEFUSE 3 patients. J Stroke Cerebrovasc Dis. 2022;31:106208.
Lu W‑Z, Lin H‑A, Hou S‑K, Bai C‑H, Lin S‑F. Diagnostic test accuracy of pretreatment collateral score in predicting stroke outcomes after intra-arterial endovascular thrombectomy: a meta-analysis in DSA and CTA. Eur Radiol. 2022;32:6097–107.
van den Wijngaard IR, et al. Impact of collateral status evaluated by dynamic computed tomographic angiography on clinical outcome in patients with Ischemic stroke. Stroke. 2015;46:3398–404.
Lyndon D, et al. Hypoperfusion intensity ratio correlates with CTA collateral status in large-vessel occlusion acute ischemic stroke. AJNR Am J Neuroradiol. 2021;42:1380–6.
Winkelmeier L, et al. Hypoperfusion intensity ratio is correlated with the risk of parenchymal hematoma after endovascular stroke treatment. Stroke. 2023;54:135–43.
Ospel JM, et al. Prevalence and outcomes of medium vessel occlusions with discrepant infarct patterns. Stroke. 2020;51:2817–24.
Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke recurrence: a population-base cohort study. Stroke. 2020;51:2435–44.
Burn J, et al. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire community stroke project. Stroke. 1994;25:333–7.
Kolmos M, Christoffersen L, Kruuse C. Recurrent Ischemic stroke—A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2021;30:105935.
Kargiotis O, et al. Computed tomography perfusion imaging in acute ischemic stroke: accurate interpretation matters. Stroke. 2023;54:e104–8.
Faizy TD, et al. Distinct intra-arterial clot localization affects tissue-level collaterals and venous outflow profiles. Eur J Neurol. 2021;28:4109–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V. Yedavalli, M. Koneru, O. Hamam, M. Hoseinyazdi, E.B. Marsh, R. Llinas, V. Urrutia, R. Leigh, F. Gonzalez, R. Xu, J. Caplan, J. Huang, H. Lu, M. Wintermark, J. Heit, A. Guenego, G. Albers, K. Nael and A. Hillis declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yedavalli, V., Koneru, M., Hamam, O. et al. Pretreatment CTP Collateral Parameters Predict Good Outcomes in Successfully Recanalized Middle Cerebral Artery Distal Medium Vessel Occlusions. Clin Neuroradiol 34, 341–349 (2024). https://doi.org/10.1007/s00062-023-01371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-023-01371-2