Abstract
Purpose
To develop an imaging prediction model for patients with embolic stroke of undetermined source (ESUS), we investigated the association of topographic diffusion-weighted imaging (DWI) patterns with potential embolic sources (PES) identified by transesophageal echocardiography.
Methods
From a total of 992 consecutive patients with embolic stroke, 366 patients with the ESUS group were selected. ESUS was defined as no atrial fibrillation (Af) within 24h from admission and no PES after general examination. Clinical variables include age (> 80years, 70–80 years), sex, vascular risk factors and left atrial diameter > 4 cm. Age, sex and vascular risk factors adjusted odds ratio of each DWI for the different PESs were calculated. DWI was determined based on the arterial territories. Middle cerebral arteries were divided into 4 segments, i.e., M1–M4. Moreover, M2 segments were subdivided into superior and inferior branches.
Results
The 366 patients consisted of 168 with paroxysmal Af (pAf), 77 with paradoxical embolism, 71 with aortic embolism and 50 with undetermined embolism after transesophageal echocardiography. The variables adjusted odds ratio (OR) of internal carotid artery (OR: 12.1, p = 0.037), M1 (4.2, p = 0.001), inferior M2 (7.5, p = 0.0041) and multiple cortical branches (12.6, p < 0.0001) were significantly higher in patients with pAf. Striatocapsular infarction (12.5, p < 0.0001) and posterior inferior cerebellar artery infarcts (3.6, p = 0.018) were significantly associated with paradoxical embolism. Clinical variables adjusted OR of multiple small scattered infarcts (8.3, p < 0.0001) were significantly higher in patients with aortic embolism.
Conclusion
The associations of DWI with different PES have their distinctive characteristics and DWI along with clinical variables may help predict PES in patients with ESUS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Embolic strokes that cannot be clearly categorized to any established etiology are referred to as embolic stroke of undetermined source (ESUS), which range from 9% to 25% of all ischemic patients [1,2,3]. Although ESUS may be caused by various underlying potential sources of embolism (PSE), [5,6,7,8] currently proposed criteria for the definition of ESUS do not include transesophageal echocardiography (TEE) as the mandatory diagnostic work-up [4]. Thus, the exact constituent of PSE in ESUS has not been well refined [9,10,11]. We have conducted the prospective registration for consecutive patients with acute ischemic stroke in a single tertiary medical center since January 2010. For patients with embolic stroke in whom underlying PSE was not detected, TEE was applied as exhaustively as possible to identify the aortocardiac source of embolism.
More importantly, patients with ESUS have a considerable rate of annual stroke recurrence of 4–5% [3]. Thus, early detection of underlying potential embolic sources (PES), particularly atrial fibrillation (Af), is crucial for acute treatment and/or secondary prevention. If acute topographic diffusion-weighted imaging patterns (DWI) would be associated with a specific PES, it may help to identify PES in patients with ESUS in addition to various clinical scores. We conducted the present study to identify aortocardiac sources of embolisms and investigated how DWI patterns may be associated with the different PSEs in the consecutive patients with embolic stroke.
Material and Methods
Stroke Registry
We studied patients from the prospective ischemic stroke registry that included all consecutive patients with first ever acute ischemic stroke within 5 days admitted in the tertiary medical institution Kyoto Second Red Cross Hospital between January 2010 and December 2018. Data on admission were prospectively recorded including demographics, cardiovascular risk factors, the NIHSS score, brain MRI and MRA, carotid ultrasonography, transthoracic echocardiography and left atrial diameter. During hospitalization, the stroke mechanism or etiology was investigated as vigorously as possible applying TEE. The use of human subjects for this study has been approved by the institutional review board of Kyoto Second Red Cross Hospital (reference no. Sp2022-15). Because this is a retrospective analysis of a prospectively collected data where the de-identified clinical records were reviewed without any interventions to participants, our institutional review board waived the need for written informed patient consent in this study. Oral informed consent was obtained from all patients on the occasion of registration.
From 2915 patients with acute ischemic stroke, non-embolic stroke patients who were categorized as having large artery atherosclerosis (n = 871) and small vessel occlusion including branch atheromatous disease (n = 954) classified based on Trial of Org 10172 in Acute Stroke Treatment (TOAST) were exclude [12]. Large artery atherosclerosis was defined as infarctions caused by extracranial or intracranial atherosclerosis defined as > 50% luminal stenosis or occlusion detected by MRA, carotid echography or CT angiography. In the present study, artery to artery embolism is classified as large artery atherosclerosis. Various vasculopathies such as arterial dissection involving proximal vessels (n = 32) or patients in whom TEE was not performed (n = 66) were also excluded leaving 992 patients.
Among 992 patients, various PES were identified after general examination including TTE and specific blood tests. When particular causes were not found by general examination, extensive work-up, such as long-term ECG monitoring and TEE were performed. Patients in whom PES was identified after general examination were categorized as the general group and those in whom PES was identified after extensive work-up were categorized as the ESUS group. Of the patients 66 who could not receive TEE were excluded.
Extensive examinations for underlying Prolonged ECG monitoring and echocardiography (PES)
-
1.
Long term ECG monitoring
Repetitive ECG, inpatient cardiac telemetry monitoring or 24‑h Holter monitoring were applied as frequently as possible during hospitalization. Patients in whom Af was found within 24h from admission were diagnosed as continuous Af (cAf) and those found after 24h from admission were diagnosed as paroxysmal Af (pAf).
-
2.
Left atrial diameter
In the extensive group, left atrial diameter was assessed using two-dimensional echocardiography by TTE [13]. The anteroposterior diameter of the left atrium was measured at the end of left ventricular systole in the parasternal long axis view perpendicular to the aortic root long axis, and measured at the level of the aortic sinuses by using the inner edge to inner edge convention.
-
3.
Transesophageal echocardiography (TEE)
A commercially available TEE machine equipped with a multiplane 5‑MZ transducer (iE33xMATRIX system, Philips Andover, MA, USA) was used. Paradoxical embolism was defined as embolus carried from the venous side of circulation to the arterial side predominantly via a patent foramen ovale (PFO) detected by TEE. Aortogenic embolism was defined as embolus carried from a vulnerable aortic atheroma detected by TEE [8].
-
a.
Assessment of aortic atherosclerosis
The ascending aorta and the aortic arch including the outlet of the left subclavian artery were imaged in short axis and long axis views. Vulnerable aortic arch atheroma was defined as aortic plaques in the ascending aorta or aortic arch that met ≥ 1 of the following criteria: (1) ≥ 4 mm of intima media thickness or (2) ulcerated plaque or (3) mobile plaque [7].
-
b.
Assessment of PFO
Using TEE, PFO and atrial septal aneurysm were assessed by an agitated saline contrast study at rest and after Valsalva maneuver. Paradoxical embolism was diagnosed when the passage of > 3 microbubbles to the left atrium within 3 cardiac cycles after complete opacification of the right atrium [8]. Even if the number of bubbles was 1–3, paradoxical embolism was diagnosed when an atrial septal aneurysm defined as ≥ 10 mm of phasic septal excursion into the atrium or venous source of thrombosis were identified [7].
-
a.
When there were simultaneously two or more possible underlying PES, we arbitrarily prioritized a PES as the following ranking: first pAf, second paradoxical embolism and then aortogenic embolism. Patients in whom no PES was found after TEE were categorized as truly undetermined embolism.
Classification of DWI Patterns
MRI/MRA
MRI scans were performed using 1.5‑T superconducting magnets (Gyroscan Intera Achieva 1.5 Pulsar, Philips). Each MRI was performed within 5 days after stroke. Diffusion-weighted scans (TR 1861 ms/TE 69 ms, EPI factor 37) and fluid attenuated inversion recovery (FLAIR) scans (TI 2000 ms,TR 6000 ms, TE 120 ms) were obtained at a slice thickness of 6 mm. Three-dimensional time-of flight images were acquired in the axial plane with a repetition time of 25 ms, echo time of 6.9 ms, flip angle of 18°, 210 mm field of view, partition of 64, 219 512r acquisition matrix and 1 signal average, for a total imaging time of 4 min 33 s. Patients underwent MRI/MRA within 24 h of symptom onset (78%) or between 24h and 72 h after stroke (22%). All MRI scans were assessed by two neurologists (Y.Y. and Y.N.) who were blinded to the clinical findings.
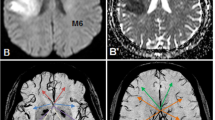
We hypothesized that acute DWI patterns on MRI may depend on characteristic histopathology of thrombi [14,15,16] and embolic trajectory determined by hydrodynamics [17] that may be associated with the vascular branching pattern. Based on the above, DWI lesion patterns were classified into 14 groups according to the main infarcts based on the dominant arterial territories proposed by Tatu et al. (Figure 1; [19]). The segments of MCA anatomically consisted of M1: horizontal segment, M2: insular segment, M3: opercular segment and M4: cortical segment [20]. The M2 territory was further dichotomized into superior and inferior branches. Thus 14 DWI groups consisted of the territories of (1) ICA, (2) M1, (3) superior M2, (4) inferior M2, (5) M3, (6) M4, (7) multiple lenticurostriate arteries with diameter > 20 mm, i.e., striatocapsular infarction (SCI) [20], (8) anterior cerebral artery (ACA), (9) posterior cerebral artery (PCA), (10) superior cerebellar artery (SCA), (11) PICA, (12) rostral basilar artery (RBA) that include rostral pons or midbrain with or without PCA or SCA, (13) multiple cortical branches (MCB) and (14) MSS: multiple scattered small infarcts.
Typical diffusion weighted image pattern of 14 different vascular territories. ICA internal carotid artery, M1, M2, M3 and M4 mean each segment of middle cerebral artery. Superior M2 M2 superior branch, inferior M2 M2 inferior branch, ACA anterior cerebral artery, PCA posterior cerebral artery, SCA superior cerebellar artery, PICA posterior inferior cerebellar artery, RBA rostral basilar artery, SCI atriatocapsular infarction. Every sections are axial
Inferior M2 branch included arteries of posterior parietal, angular, middle temporal, posterior temporal and temporo-occipital, while other branches ascending the parietal lobe were classified as superior M2 branch. The MCB mean at least one cortical infarct and additional cortical or subcortical infarcts in different territory. More than two small infarcts (all of them \(\leq\)15 mm) in the different vascular territories were categorized as MSS. Although the SCI can be categorized into M1 occlusion, we separately created this group because a different mechanism can be speculated from infarcts involving cortical area.
Analysis of Data
After describing the characteristics of all patients with embolic stroke, we analyzed patients with the ESUS group in the present study. The concept of the ESUS in the present study was made according to the design of the RESPECT-ESUS [11] because the RESPECT-ESUS included patients with Af that was found after 24h from admission. There is no consensus on the appropriate duration of monitoring after stroke in the ESUS group [12].
Statistical Analysis
Baseline characteristics and DWI patterns were compared between different PES groups setting pAf as reference. Descriptive demographic, clinical, and radiological data are shown as mean ± SD or numbers and frequencies. Discrete variables of ordinal scale were expressed as median and IQR. The χ2-test, Student’s t test or Mann-Whitney test was used as appropriate.
Logistic regression analysis was performed to evaluate the association of acute DWI on MRI with different PES. Additional variables included in this model were age (age 1: 70–80 years vs. < 70 years old and age 2: > 80 years vs. < 70 years old), male sex, vascular risk factors (yes vs. no) and left atrial diameter (\(\geq\) 4 cm vs. < 4 cm). The OR and 95% CI of each variable and DWI (said pattern vs. the others) for different PES were first calculated as univariate analysis. Then, OR and 95% CI of each DWI for 3 different PESs were calculated after the adjustment of additional variables that were shown to be positive in univariate analysis (Table 3). All statistical analyses were performed using JMP 12 software (SAS Institute Inc., Cary, NC, USA).
Results
Total Patients
Out of 2915 patients with consecutive acute ischemic stroke, 992 patients were diagnosed with embolic stroke (34.0%). PSE was finally identified in 878 patients out of 992 patients with embolic stroke (88.5%). There were 560 patients in the general group and 366 patients in the ESUS group. The general group consisted of 464 patients with cAf, 39 with myocardial infarction, 27 with Trousseau syndrome, 9 with thrombocytosis, 9 with valvular disease and 12 with various causes were identified. The ESUS group consisted of 168 patients with pAf, 77 with paradoxical embolism, 71 with aortogenic embolism and 50 with truly undetermined embolism in whom no PES was identified even after TEE (Table 1).
Analysis in Patients with the ESUS Group
Patients with pAf showed older age and higher score of NIHSS, while those with paradoxical embolism showed younger age and lower prevalence of vascular risk factors. Patients with aortogenic embolism showed male predominance and high prevalence of vascular risk factors. The left atrial diameter was longest in pAf followed by aortogenic embolism and shortest in paradoxical embolism (Table 1b; [20]).
Comparison was made setting pAf as a reference in individual DWI of the total 14 DWI groups. In the DWI of M1, superior M2, inferior M2 and MCB, the prevalence of paradoxical embolism and aortogenic embolism were significantly lower than pAf. In SCI and PICA, paradoxical embolism was significantly higher than pAf. In MSS, paradoxical embolism and aortogenic embolism were significantly higher than pAf (Table 2b). The percentage of different PES in each DWI is depicted in Fig. 2.
Logistic regression analysis of individual DWI pattern for 3 different PES was performed as follows:
-
1.
For pAf
Variables that showed positively significantly high ORs for pAf by univariate analysis were age 1 (4.7, p = 0.0004), age 2 (12.2, p < 0.0001), hypertension (1.7, p = 0.023) and left atrial diameter ≥ 4 cm (2.5, p = 0.0001). After adjustment of such variables, the DWI of ICA (12.1, p = 0.037), M1 (4.2, p = 0.001), inferior M2 (7.5, p = 0.0041) and MCB (12.6, p < 0.0001) were independently associated with pAf. ACA showed close to significant difference.
-
2.
For paradoxical embolism
There were no variables that showed positively significantly high ORs for paradoxical embolism. The DWI of SCI and PICA showed significantly high ORs (12.5, p < 0.0001, 3.6, p = 0.018, respectively).
-
3.
For aortogenic embolism
Variables that showed positively significant high ORs for aortogenic embolism were age 1 (3.6, p = 0.016), male sex (3.3, p < 0.0001), hypertension (1.7, p = 0.023), diabetes mellitus (2.5, p = 0.0015), hyperlipidemia (1.7, p = 0.039) and smoking (2.3, p = 0.0028). After adjustment of such variables, MSS was independently associated with aortogenic embolism (8.1, p < 0.0001).
Discussion
The present study identified specific PSE for 88.5% of all patients with embolic stroke.
The associations of each DWI with different PES have their distinctive characteristics in addition to clinical variables and may help in predicting underlying PES in patients with the ESUS group. Prominent characteristics in pAf were high ORs of ICA, M1, inferior M2 and MCB along with high age and large left atrial diameter. Although large infarct volume and MCB in pAf were previously reported as found in the present study, [7, 8] the high prevalence of inferior M2 was a unique finding in this study.
The relationship between pAf and large infarct volume may partly be explained from characteristic histopathology of thrombi [14,15,16,17]. The thrombus composition retrieved after thrombectomy revealed AF-related cardioembolic thrombi to be higher proportions of fibrin/platelets, less erythrocytes, and more leucocytes than non-cardioembolic thrombi [14,15,16,17]. Erythrocyte-rich areas are entangled in a thin fibrin meshwork, while platelet-rich areas are characterized by dense fibrin structures [17]. As fibrin/platelet-rich thrombi are highly organized and exhibit dense and compact structure, it may cause the occlusion of larger caliber arteries. High prevalence of MCB may be associated with thrombus fragmentation. The degree of inflammatory cell invasion within the thrombus including neutrophil elastase-positive cells [22] or the higher percentage of lymphocytes [23] were suggested to promote thrombus degradation. Given higher percentages of white blood cells in the thrombus of AF-related cardioembolisms, [14,15,16,17] MCB may be naturally associated with pAf.
The present study found inferior M2 was specifically associated with pAF. There were no patients with inferior M2 that were associated with paradoxical embolism or aortogenic embolism. It means inferior M2 infarcts are exclusively caused by pAf-related cardioembolism. The course of emboli is suggested to be determined by the hydrodynamic low or embolus properties [18, 24, 25]. The angular artery that represents the inferior M2 was reported to be the most frequent recipient site of emboli [18]. It could be possible that the agglomerate of fibrin/platelet-rich thrombi associated with pAf may be high density and preferentially flow into the inferior division of M2 branches that may be hydrodynamically less resistant [25]. Embolus trajectory through the branching vessels should be further investigated.
It should also be emphasized that SCI showed a high OR of 12.5 for paradoxical embolism. SCI is mainly caused by MCA embolic occlusion that is succeeded by early recanalization [21]. Venous thromboembolisms that are rich in fibrin and red blood cells may be more easily resolved spontaneously or by thrombolysis than in the case of Af-related cardioembolism [26]. Boutet et al. found paradoxical embolisms more often have subcortical single lesions larger than 15 mm compared with cardiogenic embolisms [27]. Ryoo et al. studied 321 patients with cryptogenic stroke and found subcortical lesions more frequently in paradoxical embolism (58 patients, 37.9%) than in pAf (15 patients, 11.7%) [8]. Such results support our findings. These findings should be further confirmed in a larger cohort study.
In addition to SCI, PICA was significantly associated with paradoxical embolisms. Chung et al. studied 2702 consecutive patients with acute ischemic stroke and found cardioembolism was most frequent in SCA territory infarcts accounting for 60%, while only 15.3% was in PICA infarcts [28]. The prevalence of pAf in SCA (54.5%) and PICA (6.6%) in our study was similar to their findings. It could be possible that highly organized fibrin/platelet-rich thrombi tend to go straight to the basilar top, while fibrin and red blood cell-rich thrombi with light density may hydrodynamically stray into the PICA that branch off forming various angles to the vertebral artery [29]. Patients with paradoxical embolism showed younger age and less vascular risk factors resulting in high RoPE (risk of paradoxical embolism) scores [30].
Most characteristic DWI in aortogenic embolism were MSS. It has consistently been reported that DWI patterns in aortogenic embolisms are characterized by multiple small scattered lesions in multiple vascular territories [31, 32]. Patients with aortogenic embolisms showed older age and preponderance of male sex and various vascular risk factors in line with previous studies [31, 32].
Limitations and Strengths of the Present Study
Limitations should be addressed. The correlation of DWI and PES can result by chance; however, the number is substantial and the correlation of DWI with cAF, which was not a focus of the paper, was similar to that of DWI with pAf. If more than two underlying PES were possible, we prioritized a PES based on an arbitrarily ranking. A strength of this study is applying TEE as exclusively as possible for patients in whom PES was not found after general examination.
In conclusion, the DWI of ICA, M1, inferior M2 and MCB along with older age, large left atrial diameter were independently associated with subsequent pAf. The DWIs of SCI and PICA were significantly associated with paradoxical embolism and that of MSS with aortogenic embolism. The DWI appears to be determined by characteristic histopathology of thrombi and the embolic trajectory is determined by hydrodynamics. Topographic DWI patterns in acute patients may assist with identifying underlying PES in patients with ESUS.
Abbreviations
- ACA:
-
Anterior cerebral artery
- cAf:
-
Continuous atrial fibrillation
- CMB:
-
Cortical multiple branch infarcts
- DWI:
-
Topographic diffusion-weighted imaging patterns
- ESUS:
-
Embolic stroke of undetermined source
- ICA:
-
Internal carotid artery
- MCA:
-
Middle cerebral artery
- MSS:
-
Multiple scattered small infarcts
- NIHSS:
-
National Institutes of Health Stroke Scale
- pAf:
-
Paroxysmal atrial fibrillation
- PCA:
-
Posterior cerebral artery
- PES:
-
Potential embolic sources
- PFO:
-
Patent foramen ovale
- PICA:
-
Posterior inferior cerebellar artery
- RBA:
-
Rostral basilar artery
- SCA:
-
Superior cerebellar artery
- SCI:
-
Striatocapsular infarction
- TEE:
-
Transesophageal echocardiography
- TTE:
-
Transthoracic echocardiography
References
Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–38.
Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Manios E, Spengos K, et al. Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis. Stroke. 2015;46:176–81.
Hart RG, Catanese L, Perera KS, Makaritsis K, Manios E, Spengos K, et al. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke. 2017;48:867–72.
Saliba W, Gronich N, Barnett-Griness O, Rennert G. Usefulness of CHADS2 and CHA2DS2-VASc Scores in the Prediction of New-Onset Atrial Fibrillation: A Population-Based Study. Am J Med. 2016;129:843–9.
Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of Atrial Fibrillation in a Racially Diverse Cohort: The Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2016;5:e3077.
Hulme OL, Khurshid S, Weng LC, Anderson CD, Wang EY, Ashburner JM, et al. Development and Validation of a Prediction Model for Atrial Fibrillation Using Electronic Health Records. JACC Clin Electrophysiol. 2019;5:1331–41.
Sharobeam A, Churilov L, Parsons M, Donnan GA, Davis SM, Yan B. Patterns of Infarction on MRI in Patients with acute ischemic stroke and cardio-embolism: A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:606521.
Ryoo S, Chung J, Lee MI, Kim SJ, Lee JS, Kim GM, et al. An approach to working up cases of embolic stroke of undetermined source. J Am Heart Assoc. 2016;5:e2975.
Chen CH, Lee M, Weng HH, Lee JD, Yang JT, Tsai YH, et al. Identification of magnetic resonance imaging features for the prediction of unrecognized atrial fibrillation in acute ischemic stroke. Front Neurol. 2022;13:952462.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Diener HC, Easton JD, Granger CB, Cronin L, Duffy C, Cotton D, RE-SPECT ESUS Investigators. Design of Randomized. Evaluation in secondary Stroke Prevention comparing the EfficaCy and safety of the oral Thrombin inhibitor dabigatran etexilate vs. acetylsalicylic acid in patients with Embolic Stroke of Undetermined Source (RE-SPECT ESUS). Int J Stroke. 2015;10:1309–12.
Mahagne MH, Lachaud S, Suissa L. Letter by Mahagne et al regarding article, “Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis”. Stroke. 2015;46:e69.
Tateishi Y, Ueno Y, Tsujino A, Kuriki A, Kamiya Y, Shimizu T, et al. Cardiac and Echocardiographic Markers in Cryptogenic Stroke with Incidental Patent Foramen Ovale. J Stroke Cerebrovasc Dis. 2021;30:105892.
Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic Stroke: What Does the Histological Composition Tell Us About the Origin of the Thrombus? Stroke. 2017;48:2206–10.
Jolugbo P, Ariëns RAS. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke. 2021;52:1131–42.
Boeckh-Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, et al. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke. 2016;47:1864–71.
Staessens S, Denorme F, Francois O, Desender L, Dewaele T, Vanacker P, et al. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica. 2020;105:498–507.
Gács G, Mérei FT, Bodosi M. Balloon catheter as a model of cerebral emboli in humans. Stroke. 1982;13:39–42.
Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: brainstem and cerebellum. Neurology. 1996;47:1125–35.
Gibo H, Carver CC, Rhoton AL, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151–69.
Donnan GA, Bladin PF, Berkovic SF, Longley WA, Saling MM. The stroke syndrome of striatocapsular infarction. Brain. 1991;114:51–70.
Kaesmacher J, Boeckh-Behrens T, Simon S, Maegerlein C, Kleine JF, Zimmer C, et al. Risk of Thrombus Fragmentation during Endovascular Stroke Treatment. Am J Neuroradiol. 2017;38:991–8.
Goebel J, Gaida BJ, Wanke I, Kleinschnitz C, Koehrmann M, Forsting M, et al. Is Histologic Thrombus Composition in Acute Stroke Linked to Stroke Etiology or to Interventional Parameters? Am J Neuroradiol. 2020;41:650–7.
Mukherjee D, Shadden SC. Inertial particle dynamics in large artery flows—Implications for modeling arterial embolisms. J Biomech. 2017;52:155–64.
Fabbri D, Long Q, Das S, Pinelli M. Computational modelling of emboli travel trajectories in cerebral arteries: influence of microembolic particle size and density. Biomech Model Mechanobiol. 2014;13:289–302.
Aleman MM, Walton BL, Byrnes JR, Wolberg AS. Fibrinogen and red blood cells in venous thrombosis. Thromb Res. 2014;133(Suppl 1):S38–40.
Boutet C, Rouffiange-Leclair L, Garnier P, Quenet S, Delsart D, Varvat J, et al. Brain magnetic resonance imaging findings in cryptogenic stroke patients under 60 years with patent foramen ovale. Eur J Radiol. 2014;83:824–8.
Chung JW, Park SH, Kim N, Kim WJ, Park JH, Ko Y, et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. J Am Heart Assoc. 2014;3:e1119.
Lee SH, Cha JH, Jung IE, Yu SW, Moon JS, Cho KH, et al. Relationship between the Angle of the Posterior Inferior Cerebellar Artery and Cardioembolic Stroke. J Stroke Cerebrovasc Dis. 2019;28:693–8.
Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–25.
Kim SW, Kim YD, Chang HJ, Hong GR, Shim CY, Chung SJ, et al. Different infarction patterns in patients with aortic atheroma compared to those with cardioembolism or large artery atherosclerosis. J Neurol. 2018;265:151–8.
Ezzeddine MA, Primavera JM, Rosand J, Hedley-Whyte ET, Rordorf G. Clinical characteristics of pathologically proved cholesterol emboli to the brain. Neurology. 2000;54:1681–3.
Author information
Authors and Affiliations
Contributions
YY contributed to the conception and design of the study. YN, ET, TY, JF and TO contributed to the acquisition and analysis of data. YY and YN contributed to drafting the text or preparing the figures.
Corresponding author
Ethics declarations
Conflict of interest
Y. Yamamoto, Y. Nagakane, E. Tanaka, T. Yamada, J. Fujinami and T. Ohara declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamamoto, Y., Nagakane, Y., Tanaka, E. et al. How Topographic Diffusion-Weighted Imaging Patterns can Predict the Potential Embolic Source. Clin Neuroradiol 34, 363–371 (2024). https://doi.org/10.1007/s00062-023-01366-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-023-01366-z