Abstract
Purpose
Carotid artery stenting (CAS) has been proven to decrease the risk of stroke in symptomatic patients with moderate/high-grade carotid stenosis; however, there is an increased periprocedural risk of stroke with CAS compared to carotid endarterectomy. The goal of this article is to report the utilization of endovascular optical coherence tomography (OCT) during CAS to aid in the identification of stent malapposition, plaque prolapse, and adjacent residual thrombus that could cause periprocedural stroke.
Methods
Approval was obtained for endovascular OCT imaging in patients undergoing CAS. Images were obtained before and after stenting. Images were acquired with proximal balloon occlusion and saline to clear luminal blood during acquisition.
Results
A total of seven patients provided informed consent for imaging. There were no complications during image acquisition or the stenting procedure. Optical coherence tomography imaging revealed free intraluminal red thrombi, fibrous cap dissections, and thin cap fibroatheromas with underlying ulcerative plaques. Plaque herniation through stents was also demonstrated along with thrombus. Poor stent apposition was clearly visible.
Conclusion
Optical coherence tomography image acquisition was found to be safe, effective, and to provide valuable information about plaque morphology and stent-vessel interactions. The role of perioperative anticoagulation after stenting should be re-evaluated given the new findings. A study comparing CAS with and without OCT with a clinical primary outcome such as ipsilateral stroke could help determine if OCT is an innovative tool to guide stenting and antithrombotic management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Roughly 15% of all ischemic strokes are caused by large-vessel cerebrovascular disease, with extracranial internal carotid atherosclerosis being the most common [1]. Carotid revascularization has been proven to decrease the risk of stroke in patients with carotid atherosclerotic disease, particularly those with high-grade stenosis [2, 3]. Carotid artery stenting (CAS) is well recognized as an alternative to carotid endarterectomy (CEA) for revascularization in certain patients [4].
Endovascular optical coherence tomography (OCT) has emerged as a valuable imaging tool for interventionalist, which utilizes near-infrared light with a wavelength of approximately 1300 nm and excellent blood vessel wall spatial resolution of 10–20 µm is achievable [5]. In comparison, 3‑Tesla Magnetic Resonance (MR) vessel wall imaging has a voxel size of 2.0 × 0.4 × 0.4 mm and intravascular ultrasound (IVUS) has a spatial resolution of 100 µm. The depth of vessel wall penetration is approximately 3 mm. The OCT technique has aided interventional cardiologists in characterizing coronary plaque composition, morphology, and neovascularization [6, 7]. More recently OCT has been utilized in patients with carotid atherosclerotic disease. The commercially available OCT devices are U.S. Food and Drugs Administration (FDA) approved for coronary atherosclerotic disease and studies reporting on carotid pathology have gained institutional research ethics board approval. Authors have reported on imaging carotid plaque morphology, composition and utilizing OCT during CAS as an aid to examine for plaque prolapse or stent malapposition after deployment [8,9,10]. The aim of this study was to report the authors institutional experience utilizing OCT as an adjunct during CAS for carotid stenosis. The article describes the OCT image acquisition protocol using saline only as a medium to clear the lumen, safety, and utility.
Methods
Study Design
Research ethics board approval was obtained from the institutional ethics committee for the use of OCT in patients with carotid stenosis undergoing CAS. All patients provided informed consent. All patients undergoing CAS for carotid stenosis were considered for inclusion granted the anatomy was not extremely tortuous and they could tolerate the additional 10 min required to obtain images. Data regarding patient specific characteristics were collected prospectively, along with procedural indication and degree of carotid stenosis. A complication was defined as any new/worsening neurological deficit during or after procedure.
Carotid Artery Stenting Protocol
The CAS procedure was performed with the patients awake with light sedation. Patients were treated with dual antiplatelet therapy before and after CAS for 3 months, at which point one antiplatelet agent was discontinued. During the procedure, patients were heparinized to target activating clotting time (ACT) of 300. Vascular access was obtained with an 8 F sheath placed in the right common femoral artery under ultrasound guidance. A FlowGate (Stryker, Kalamazoo, MI, USA) balloon guide catheter was navigated over a glidewire into the common carotid artery (CCA). An Emboshield (Abbott Vascular, Redwood City, CA) distal embolic protection device was deployed over a micro-guidewire into the distal cervical ICA. At this point, the first set of pre-stenting OCT images of the carotid plaque was captured. Next, the OCT microcatheter was removed and a Protege (Medtronic, Minneapolis, MN, USA) stent was deployed. After stent deployment, post-stenting OCT images were acquired. The Emboshield was captured and removed and post-stent balloon dilation was only done if stent-strut malapposition was identified using OCT.
OCT Image Acquisition Protocol
The DragonflyTM OCT catheter (St. Jude Medical, Minneapolis, MN) was used for image acquisition (Fig. 1a). This device generates cross-sectional luminal images with a spatial resolution of 10–20 µm. The depth of tissue penetration is approximately 3 mm. Navigation of the catheter into the carotid artery is generally straightforward; however, the stiffness of the catheter prevents reliable navigation beyond the cavernous carotid into the tortuous cerebral vessels. The catheter is connected to heparinized saline flush to prevent thrombus formation.
Illustration of the distal end of the optical coherence tomography (OCT) catheter and positioning the catheter in the carotid artery. a There is one radiopaque marker located near the tip of the catheter and one marker at the lens spaced 23 mm apart. The lens marker is located 50 mm distal to the proximal marker. The lens motorized pullback length is 54 mm. b In order to ensure imaging of the entire plaque, place the lens marker distal to the stenosis before starting the pump injection. The balloon should be inflated in the distal common carotid artery
The following steps were followed for image acquisition: (1) flush the OCT catheter with saline using the provided 5ml syringe, (2) load an automated injection pump with 150ml of heparinized saline. This is used to clear the blood within the lumen during image acquisition, (3) mount the catheter on a rapid exchange micro-guidewire and position such that the lens radiopaque marker is distal to the stenosis (Fig. 1b), (4) inflate the dual-lumen balloon in the CCA, (5) infuse 6ml per second for 5 s via the automated pump. The OCT catheter automatically detects luminal blood clearing and performs the motorized automated pullback of 54 mm, (6) deflate the balloon and remove the OCT catheter.
Results
A total of 7 patients (6 men) provided informed consent for OCT imaging (Table 1). The mean age was 61 years (range 45-71 years). Adequate images were acquired for each patient using only saline as a medium to clear intraluminal blood with proximal balloon occlusion. Contrast was not needed. There were no complications during image acquisition or the CAS procedure in general.
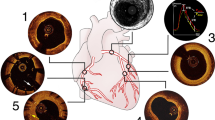
The OCT imaging revealed several plaque features not visible with other imaging modalities, e.g. Doppler ultrasound, computed tomography (CT) angiography, contrast enhanced magnetic resonance (MR) angiography, and cerebral angiography done before CAS. In all patients, normal vessel wall architecture beyond the plaque was visible, with all three layers and internal/external lamina apparent (Fig. 2a). Of the patients one had evidence of free intraluminal red thrombus with signal attenuation beyond the thrombus (Fig. 2b) and irregular thin fibrous cap dissection (Fig. 2c). Other patients had evidence of thin cap fibroatheroma and ulcerative plaque below (Figs. 3c and 4c).
Optical coherence tomography (OCT) images of a 45-year-old man. a Atherosclerotic changes beginning in cervical internal carotid artery (ICA) with areas of thick fibrous plaque (yellow arrowhead) present along with normal vessel wall architecture (red arrowhead) with visible internal/externa lamina. b Intraluminal red thrombus (yellow arrowhead) with signal attenuation beyond the thrombus (green arrowhead). c–d Irregular fibrous plaque with dissection causing luminal narrowing (yellow arrowheads). Asterisk denotes the shadow from the guidewire
Angiographic and optical coherence tomography (OCT) images from a 61-year-old man. a Cerebral angiogram showing moderate stenosis (black arrowhead). b Post-stenting angiogram showing luminal expansion. c OCT image showing a thin fibrous cap (yellow arrowhead) over an ulcerative plaque core. d Post-stenting OCT imaging showing satisfactory stent-wall apposition (green arrowhead) and plaque protrusion/thrombus through the stent struts in two locations (yellow arrowheads). Asterisk denotes the shadow from the guidewire
Angiographic and optical coherence tomography (OCT) images from a 71-year-old man. a Cerebral angiogram showing proximal common carotid artery (CCA) irregular stenosis (black arrow). b Post-stenting angiogram showing luminal expansion. c OCT image showing a very irregular plaque (yellow arrowheads). d Post-stenting OCT imaging showing satisfactory stent-wall apposition (yellow arrowhead) and collapse of the irregular plaque, with no evidence of protrusion. Asterisk denotes the shadow from the guidewire
The use of OCT was able to characterize stent/vessel wall and stent/plaque interactions clearly. For one patient, plaque herniation through the stent was demonstrated along with thrombus (Fig. 3d). Another patient had poor stent apposition as visible on OCT and underwent subsequent balloon angioplasty. All other patients had satisfactory stent apposition (Fig. 4d).
Discussion
This article reports the institutional experience using endovascular OCT during carotid artery stenting for carotid atherosclerotic disease. The appeal of utilizing OCT imaging as an adjunct during CAS stems from the unmatched spatial resolution provided. The OCT image acquisition was found to be safe, effective, providing valuable information about plaque morphology and stent-vessel interaction that can alter management.
The landmark NASCET and subsequent trials showed that surgical intervention is highly beneficial for symptomatic patients with moderate and high-grade stenosis, and these findings remain influential today as most societies recommend carotid revascularization for symptomatic patients with moderate/severe stenosis [3, 4]. There is mounting evidence that the degree of radiographic stenosis can fail to identify those patients with vulnerable carotid plaques [11]. Nighoghossian et al. defined vulnerable plaques as those with high likelihood of thrombotic complications and rapid progression and identified several vulnerable morphologic features including thin fibrous cap, thrombus, ulceration, and erosion [12]. Shindo et al. showed using OCT that fibrous cap thickness <130 µm was associated with increased risk of rupture in 36 patients with high-grade stenosis [9]. Jones et al. found that American Heart Association (AHA) type VI plaques were more common in symptomatic patients using OCT and that there was no association between degree of stenosis and complicated plaque features [8].
It is clear from the literature and this study that OCT can provide information about the carotid plaque that other imaging modalities cannot achieve. It could be argued that plaque morphologic features should be considered in stroke risk stratification for certain patients; however, no imaging modality has proven reliable in identifying vulnerable plaques in relation to future stroke development [13]. Consequently, it is difficult determine if there is a role for invasive diagnostic imaging for carotid revascularization. For example, a symptomatic patient with stenosis ≥70% will undergo treatment regardless of plaque morphology. Conversely, OCT could certainly help clarify ambiguous/discordant non-invasive imaging findings.
The utility of OCT becomes more apparent for patients already selected to undergo CAS. Studies have described the use of OCT imaging for detecting plaque prolapse and stent strut malapposition during CAS, and clinicians have altered management based on these findings [10, 14,15,16,17,18,19,20]. Dohad et al. conducted the largest North American prospective study comparing patients undergoing CAS utilizing OCT and CAS without OCT [10]. They reported several instances of OCT altering the treatment plan, including post-treatment dilation. In the present study, one patient underwent subsequent balloon angioplasty after OCT revealed poor stent-strut apposition.
Instinctively, studies describing utilizing OCT as a tool to decrease the amount of plaque prolapse or correct strut malapposition sounds promising; however, the endpoints in these studies were not clinical outcomes but proxies such as plaque protrusion or malapposition. Furthermore, given the small number of patients and patient selection bias, there are characteristics that will be uncorrectable on multivariate analysis. Nonetheless, OCT appears to be a powerful adjunct for the interventionalist performing CAS to decrease poor technical results such as malapposition and significant tissue prolapse through the stent. It could also be used to evaluate new carotid stents, particularly the interaction between the stent mesh and underlying atherosclerotic disease.
A meta-analysis comprised of several large randomized trials concluded that there was an increased periprocedural risk of stroke with CAS compared to CEA. It is reasonable to consider that plaque prolapse/thrombus through the stent or stent-strut malapposition could contribute to this increased risk. One could intuitively suggest that any adjunct that could identify plaque prolapse/thrombus or malapposition during CAS could be of clinical utility. Furthermore, given the presence of visible thrombus and plaque prolapse in this study, the authors suggest that there may be a role for periprocedural anticoagulation. The current standard in most centers is dual antiplatelets before/after CAS [21]. Of the early trials comparing CAS and CEA, the CAVATAS study required that patients undergoing CAS be fully heparinized during and after CAS for minimum of 24 h, and they found no difference in perioperative stroke risk [22]. Given the new findings revealed by OCT in the present study, clinicians should re-evaluate the role of perioperative anticoagulation after CAS on a case by case basis.
There are limitations to intravascular OCT imaging. First, the current device is not designed to image larger vessels, and if the diameter is >10 mm the entire vessel lumen will not be captured. Second, the depth of penetration is 3 mm, and thus the entire burden of disease cannot be characterized.
In conclusion, OCT image acquisition was found to be safe, effective, and to provide valuable information about plaque morphology and stent-vessel interactions. The role of perioperative anticoagulation after CAS should be re-evaluated given the new findings observed with OCT. A study comparing CAS with and without OCT with a clinical primary outcome such as ipsilateral stroke could help determine if OCT is an innovative tool to guide CAS and the medical management after.
Abbreviations
- AHA:
-
American Heart Association
- CAS:
-
Carotid artery stenting
- CEA:
-
Carotid endarterectomy
- OCT:
-
Optical coherence tomography
References
Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–6.
Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379–87.
North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, Eliasziw M. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53.
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236.
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Räber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G; International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72.
Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol. 2006;97:1172–5.
Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F, Samady H, Akasaka T, Carlson EB, Leesar MA, Matsumura M, Ozan MO, Mintz GS, Ben-Yehuda O, Stone GW; ILUMIEN III: OPTIMIZE PCI Investigators. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–28.
Jones MR, Attizzani GF, Given CA 2nd, Brooks WH, Ganocy SJ, Ramsey CN, Fujino Y, Bezerra HG, Costa MA. Intravascular frequency-domain optical coherence tomography assessment of carotid artery disease in symptomatic and asymptomatic patients. Jacc Cardiovasc Interv. 2014;7:674–84.
Shindo S, Fujii K, Shirakawa M, Uchida K, Enomoto Y, Iwama T, Kawasaki M, Ando Y, Yoshimura S. Morphologic features of carotid plaque rupture assessed by optical coherence tomography. AJNR Am J Neuroradiol. 2015;36:2140–6.
Dohad S, Zhu A, Krishnan S, Wang F, Wang S, Cox J, Henry TD. Optical coherence tomography guided carotid artery stent procedure: technique and potential applications. Catheter Cardiovasc Interv. 2018;91:521–30.
Brinjikji W, Huston J 3rd, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. 2016;124:27–42.
Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005;36:2764–72.
Huibers A, de Borst GJ, Wan S, Kennedy F, Giannopoulos A, Moll FL, Richards T. Non-invasive carotid artery imaging to identify the vulnerable plaque: current status and future goals. Eur J Vasc Endovascular Surg. 2015;50:563–72.
Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8.
Yoshimura S, Kawasaki M, Hattori A, Nishigaki K, Minatoguchi S, Iwama T. Demonstration of intraluminal thrombus in the carotid artery by optical coherence tomography: technical case report. Neurosurgery. 2010;67(3 Suppl Operative):onsE305; discussion onsE305.
Jones MR, Attizzani GF, Given CA 2nd, Brooks WH, Costa MA, Bezerra HG. Intravascular frequency-domain optical coherence tomography assessment of atherosclerosis and stent-vessel interactions in human carotid arteries. AJNR Am J Neuroradiol. 2012;33:1494–501.
de Donato G, Setacci F, Sirignano P, Galzerano G, Cappelli A, Setacci C. Optical coherence tomography after carotid stenting: rate of stent malapposition, plaque prolapse and fibrous cap rupture according to stent design. Eur J Vasc Endovascular Surg. 2013;45:579–87.
Umemoto T, de Donato G, Pacchioni A, Reimers B, Ferrante G, Isobe M, Setacci C. Optical coherence tomography assessment of newgeneration mesh-covered stents after carotid stenting. EuroIntervention. 2017;13:1347–54.
Harada K, Oshikata S, Kajihara M. Optical coherence tomography evaluation of tissue prolapse after carotid artery stenting using closed cell design stents for unstable plaque. J Neurointerv Surg. 2018;10:229–34.
Yamada K, Yoshimura S, Miura M, Kanamaru T, Shindo S, Uchida K, Shirakawa M, Shibuya M, Imanaka T, Ishihara M, Masuyama T, Ishikura R, Kawasaki M. Potential of new-generation double-layer Micromesh Stent for carotid artery Stenting in patients with unstable plaque: a preliminary result using OFDI analysis. World Neurosurg. 2017;105:321–6.
McKevitt FM, Randall MS, Cleveland TJ, Gaines PA, Tan KT, Venables GS. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur J Vasc Endovascular Surg. 2005;29:522–7.
Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–37.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study concept and design: Pasarikovski, da Costa, Black, Yang. Acquisition, analysis, or interpretation of data: Pasarikovski, Ramjist, da Costa, Black, Cardinell, Yang. Drafting the article: Pasarikovski, da Costa, Black, Yang. Administrative, technical, or material support: Pasarikovski, Ramjist, da Costa, Black, Cardinell, Yang. Study supervision: Yang.
Corresponding author
Ethics declarations
Conflict of interest
C.R. Pasarikovski, J. Ramjist, L. da Costa, S.E. Black, J. Cardinell and V.X. Yang declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pasarikovski, C.R., Ramjist, J., da Costa, L. et al. Optical Coherence Tomography as an Adjunct During Carotid Artery Stenting for Carotid Atherosclerotic Disease. Clin Neuroradiol 30, 503–509 (2020). https://doi.org/10.1007/s00062-019-00799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00799-9