Abstract
Background and Purpose
Endovascular treatment (EVT) and stereotaxic gamma-knife radiosurgery (GKRS) can both effectively treat cavernous sinus dural arteriovenous fistulas (CSDAVF). This study compared the prognostic factors and treatment effectiveness of GKRS and EVT for different CSDAVF types.
Methods
The charts of 200 patients undergoing GKRS and 105 patients undergoing EVT were reviewed for data on symptoms (e.g. orbital, cavernous, ocular, and cerebral). The CSDAVFs were classified into proliferative, restrictive, and late restrictive types. The prognostic factors for complete obliteration (CO) were evaluated in both the GKRS and EVT groups and the latent period to CO was measured. For statistical analysis χ2-tests were used to compare final CO rates for EVT and GKRS across the three CSDAVF types.
Results
The EVT and cavernous symptoms were significant independent predictors of CO. The CO rate after EVT (97.9%) was significantly higher than that after GKRS (63.5%) for restrictive CSDAVFs (P < 0.001) but not for proliferative or late restrictive types. In the GKRS group, cavernous symptoms (hazard ratio, HR: 0.557) and target volume (HR: 0.853) predicted CO, but only target volume remained significant in multivariate analysis. In the EVT group, the latent period to CO was shortest for restrictive CSDAVFs (3.2 ± 1.6 months, P = 0.05).
Conclusion
Angioarchitecture did not affect treatment outcomes. Cavernous symptoms were strongly associated with lower complete obliteration rates in the GKRS but not the EVT group. The EVT method remains the treatment of choice, especially for restrictive CSDAVFs; however, compared to EVT, GKRS had lower complication rates and similar therapeutic effects for proliferative type fistulas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dural arteriovenous fistulas (DAVFs) make up 7–15% of all intracranial arteriovenous malformations [1]. Cavernous sinus dural arteriovenous fistulas (CSDAVFs) are the most common type of DAVF in Asian populations [2, 3]. Unlike DAVFs in other locations, CSDAVFs are relatively benign due to their location outside the dura and possession of multiple extracranial and intracranial venous outlets [4]. Like the Borden and Cognard classification, the Barrow classification of carotid cavernous fistulas has been adapted for the classification of CSDAVFs. To facilitate microsurgery, the Barrow system divides indirect CSDAVFs into types B, C, and D depending on whether the arterial blood supplier is the external carotid artery, internal carotid artery, or both. Among the endovascular treatment (EVT) options, the transvenous approach is considered to be the treatment of choice and is associated with a 73–90% cure rate and relatively low complication rate in studies of more than 100 patients [5, 6].
Using the relationship between symptoms and venous drainage routes in CSDAVFs as a basis for a classification system, Suh et al. divided CSDAVFs into proliferative, restrictive, and late restrictive types [7]. Proliferative CSDAVFs receive numerous arterial feeders and are drained by multiple venous outlets; therefore, they mostly cause cranial nerve deficits. Late restrictive CSDAVFs drain primarily into the superior ophthalmic veins, and patients tend to present with ocular symptoms such as increased orbital pressure. Restrictive CSDAVFs are transitional between proliferative and late restrictive CSDAVFs, and patients typically exhibit a combination of cranial nerve and ocular symptoms. If the inferior petrosal sinus (IPS) or superior petrosal sinus (SPS) is involved, tinnitus will develop; if the middle cerebral vein or perimesencephalic vein is involved, which is rare, the patient might have neurologic deficits such as altered memory function, ataxia, or intracranial hemorrhage [8].
Recently, Luo et al. showed a significantly lower cure rate for proliferative (50%) than for restrictive and late restrictive CSDAVFs (50% versus 73% and 86%, respectively) [9]. Due to the relatively benign clinical course of most CSDAVFs, and the absence of reported hemorrhage or cranial paralysis during the latent period of stereotaxic gamma-knife radiosurgery (GKRS), GKRS is also considered to be an effective treatment for CSDAVFs [10]. The overall obliteration rate of CSDAVFs treated by GKRS is 70–80%, which is comparable to the rate for EVT [5, 9, 11]; however, the obliteration rate for specific types of CSDAVF as well as the impact of the angioarchitecture of the CSDAVFs is a relatively unexplored area [10, 12, 13]. The use of GKRS produces radiation necrosis of the vascular walls in the shunting to obliterate DAVFs, and theoretically the results of GKRS are less affected by venous outlet patterns than EVT [14]. Certain angioarchitectural features of CSDAVFs may have equivalent therapeutic responses to GKRS and EVT. So far, no direct comparison of the therapeutic effects of GKRS and EVT in treating CSDAVFs has been attempted. The aim of this study was thus to 1) compare the treatment effectiveness of GKRS and EVT, and 2) explore whether clinical symptoms or angiographic factors can help predict the outcomes of CSDAVFs treated with EVT or GKRS.
Materials and Methods
Patient Selection and Evaluation of Symptoms
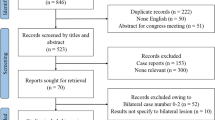
This study was approved by the local ethics committee, and the board waived the informed consent requirement. Retrospectively, CSDAVF patients from our GKRS logbook were consecutively included who had received GKRS between 2002 and 2016, and patients from the angio room logbook who had received EVT. Excluded were patients who did not have complete digital subtraction angiography (DSA) on the date of treatment recorded in the picture archive and communications system (n = 21), patients with direct type DAVFs (n = 9), patients who experienced spontaneous regression before treatment (n = 2), patients who had received prior treatment outside of the hospital (n = 5), and patients with known Klippel-Trenaunay-Weber syndrome (n = 1) or fibromuscular dysplasia (n = 1). Patients’ symptoms were identified by chart review and classified into orbital symptoms (chemosis, exophthalmos, periorbital pain and eyelid swelling), cavernous symptoms (ptosis, diplopia, anisocoria and ophthalmoplegia), ocular symptoms (ocular pain, glaucoma and retinal hemorrhage) and cerebral symptoms (altered memory function, ataxia and intracranial hemorrhage) [7].
Gamma-Knife Radiosurgery
The boundaries of the CSDAVFs targeted for irradiation were delineated using stereotactic magnetic resonance (MRA) and stereotactic digital subtraction angiography (DSA). Targets were generally delimited by the involved sinus wall, with arterial feeders and venous outflow excluded. Subsequent dose planning was based on findings from the integrated images. The dose planning strategy was to deliver an adequate radiation dose to the delineated target, while sparing the adjacent critical structures. Cranial nerve paralysis and brain stem injury are rare complications of GKRS [10]. Multiple isocenters were used to improve the dose conformity of the treatment volume (the median number of isocenters was 4) [15]. The average radiation volume was 6.97 ± 6.46 ml, the average target volume was 3.36 ± 4.96 ml, the average peripheral dose was 16.70 ± 3.08 Gy, and the average maximum dose was 23.22 ± 6.84 Gy. The abovementioned radiation parameters were used only in the subgroup analysis of the complete obliteration (CO) rate (see treatment outcome evaluation) for the GKRS group.
Endovascular Treatment
Detachable coils were available for use at the beginning of this study period. Intravenous access was preferred because it is relatively safe and associated with a low complication rate [6, 16]. A simple trans-inferior petrosal sinus (IPS) approach achieved successful embolization in 100 (95.2%) cases. If the occluded IPS could not be recanalized, which is common in late restrictive type CSDAVFs, attempts were made to navigate through the facial vein (n = 5, 0.9%), through direct puncture (n = 3, 2.8%), or by using an intra-arterial approach (n = 6, 5.6%) [17, 18]. In the next step one or two microcatheters were placed in the fistula compartment of the cavernous sinus and the fistula was filled with detachable coils until the shunt disappeared; if there was residual flow in the fistula Onyx (Covidien Vascular Therapies, Irvine, CA, USA) was used to obliterate the fistula (n = 15, 14.2%), or the procedure was terminated in cases in which using Onyx was considered too risky based on an assessment of the angiographic architecture (n = 19, 18.1%). Progressive thrombosis in nearly obliterated CSDAVFs is anticipated [19]. The average coil length used was 260.2 ± 179.3 cm. The average amount of Onyx used was 1.4 ± 0.74 ml in 15 patients (14.2%). The length of coil used and the amount of Onyx used in the subgroup analysis were included to determine the rate of complete obliteration (CO, see treatment outcome evaluation) for the EVT group.
Angioarchitecture Analysis
The DSA performed on the same day as either GKRS or EVT was used for the angioarchitecture analysis. Barrow and Suh classifications were applied (Fig. 1) and patterns of arterial feeders and drainage veins were classified by 2 interventionists with 27 and 12 years of experience, respectively, working independently. Discrepancies were discussed by the two interventionists until agreement was reached. The label “fluffy” was applied to designate the presence of innumerable arterial feeders, especially from the internal maxillary artery and ophthalmic arteries. There were six venous outlets: the ipsilateral superior ophthalmic vein, ipsilateral superior petrosal sinus, ipsilateral inferior petrosal sinus (IPS), contralateral superior ophthalmic vein, contralateral superior petrosal sinus, and contralateral IPS. The venous outlet score (VOS) was defined as the number of venous outlets ranging from 0 (no venous outlets) to 6 (all 6 of the abovementioned venous outlets; Fig. 2). Cortical venous drainage (CVD) was defined as venous reflux into any of the following vessels: the superior middle cerebral vein, the perimesencephalic vein, and the cerebellar vein.
Suh’s classification of CSDAVFs into three different types: a, d The proliferative type CSDAVF. Note that the cavernous sinus has poorly delineated contours, innumerable arterial feeders, and multiple venous outlets. b, e The restrictive type CSDAVF. This type has fewer arterial feeders and venous outlets. c, f The late restrictive type CSDAVF shows drainage into a solitary venous outlet, usually the superior ophthalmic vein
Treatment Outcome Evaluation
Initial treatment outcome was only evaluated in the EVT group. Immediate CO was defined as complete disappearance of abnormal fistula flow in immediate control angiography following EVT. All patients with immediate CO were followed up with control DSA 1 year later. All patients in the GKRS group and those with incomplete obliteration in the EVT group received follow-up treatment on an outpatient basis as needed, with MRA every 6 months until the MRA showed CO, and then a final control digital subtraction angiography to confirm CO. Final CO was defined for both groups as complete disappearance of abnormal fistula flow on follow-up MRA or DSA. The negative predictive value of post-GKRS MRA is high [20]. If symptoms persisted or worsened, then timely imaging was arranged. If there was any residual fistula in the last follow-up DSA or MRA, the treatment outcome was not categorized as CO. The primary endpoint was defined as either CO or receiving continued treatment. Complications were defined as any newly developed post-treatment ischemic or intracranial hemorrhage, confirmed by brain CT or MRI. A T2-weighted hyperintensity in the perilesional white matter on MRI was considered indicative of acute radiation effects. Transient cranial paresis was defined as worsening of cranial nerve function after initial treatment. Recurrence was defined as reappearance of a CSDAVF after CO.
Statistical Analysis
Statistical analyses were conducted using SPSS for Windows (version 20; IBM-SPSS, Chicago, IL, USA). The results are presented as medians (ranges) and numbers (percentages) for categorical and continuous variables, respectively. The χ2-test was used to analyze the effects of gender, different symptoms, innumerable feeders, CVD, and immediate and final treatment outcomes. Kendall’s tau was used to analyze Barrow and Suh’s classifications. Mann-Whitney U-tests were used to evaluate VOS, and independent t-tests to compare the ages of patients in the GKRS and EVT groups. We also used Cox regression was also used as needed to determine the CO rate with respect to treatment options, symptoms, Suh’s classification, presence or absence of innumerable feeders, VOS, CVD, embolization agent and radiation profile, after adjustment for gender and age. Only those variables with P-values less than 0.10 in univariate analyses were subsequently included in multivariate analyses. The latent periods before CO among different angiographic types in the GKRS and EVT groups were determined using the Kaplan-Meier method and compared using the Breslow test. Final CO rates were compared with a χ2-test. Statistical significance was set at P < 0.05.
Results
Patient Demographics
There were no significant between-group differences in any of the clinical symptoms. There were significantly more Barrow type D CSDAVFs, and fewer late restrictive type CSDAVFs in the EVT group than in the GKRS group. The imbalance of patients with late restrictive morphology between the two groups was largely a result of the belief during the first 11 years included in the database (2002–2012) that transvenous embolization was unlikely to be successful due to occlusion of the IPS; during this period patients with late restrictive morphology were predominantly referred for GKRS. The VOS scores were significantly lower in the GKRS group than in the EVT group (Table 1).
Associations between Angiographic Factors and Complete Obliteration
After adjusting for age and sex, Cox regression showed that treatment option was an independent predictor of CO. The use of EVT was more highly associated with CO (hazard ratio, HR: 3.828, 95% confidence interval, CI: 2.723–5.383; P < 0.001) than GKRS. Having cavernous symptoms was also an independent predictor of CO (HR: 0.584, 95% CI: 0.410–0.832; P = 0.003). Neither Suh’s classification, VOS, CVD, nor multiple feeders were associated with the likelihood of CO (Table 2). In the EVT group, the initial CO rate was highest for restrictive CSDAVFs (74.1%), followed by late restrictive CSDAVFs (57.1%), and proliferative CSDAVFs (27.3%). The effect of EVT was greater than that of GKRS for all three types of CSDAVF; however, the final CO rate was significantly higher only for restrictive type CSDAVFs for the EVT group (97.9%) than for the GKRS group (63.5%; P < 0.001).
Treatment Response in the GKRS Group
The overall CO rate was 63.5%, and the median duration to CO was 15 months (interquartile range, IQR 11–31 months, n = 98) in the GKRS group. The median duration to CO was longer for proliferative CSDAVFs (23 months, IQR 11–62 months, n = 26) than for restrictive CSDAVFs (14 months, IQR 11–26 months, n = 47) and late restrictive CSDAVFs (14 months, IQR 12–33 months, n = 25), but the differences were not significant (P = 0.532; Fig. 3). Independent negative predictors of CO included having cavernous symptoms (HR: 0.557, 95%, CI: 0.363–0.854; P = 0.007) and target volume (HR: 0.853, 95% CI: 0.739–985; P = 0.031). No association was found between radiation volume, peripheral dose, maximum dose, symptoms, or angiographic parameters and CO (Table 3). Of the patients two (1%) developed a post-procedural 6th nerve palsy, but no occurrences of acute radiation effects or intracranial hemorrhage, and no recurrence.
Treatment Responses in the EVT Group
The overall initial CO rate was 57.1% in the EVT group. The Suh et al. classification was the only predictor of immediate CO for CSDAVFs: the initial CO rate was significantly higher for restrictive CSDAVFs (74.1%) than for late restrictive (57.1%) and proliferative CSDAVFs (27.3%, P < 0.001). The overall final CO rate was 92.6% in the EVT group. There were significant differences in the CO rate between the Suh et al. classification types (Fig. 4). The duration of CO was significantly shorter for restrictive type CSDAVFs (3.3 ± 1.6 months) than late restrictive (8.4 ± 1.5 months) and proliferative type CSDAVFs (9.1 ± 2.7 months, P = 0.05). No associations were found between VOS, CVD, innumerable feeder arteries, coil length, or onyx amount used and CO (Table 4). There were 3 (2.8%) minor thromboembolic events without major neurologic deficits or mortality, 35 (33.3%) cases had periprocedural transient cranial 6th (n = 28) and/or 3rd nerve (7) paresis, all resolving spontaneously within 2 months, no cases of intracranial hemorrhage and 1 case of a restrictive type that recurred 2 months after EVT treatment but subsequently spontaneously occluded.
Discussion
Angioarchitecture of CSDAVF
The vigorous blood flow in proliferative type CSDAVFs causes swelling of the cavernous sinus and therefore leads mainly to cavernous symptoms, followed by orbital symptoms [7]. The fact that outcomes are worse in proliferative type CSDAVFs than in the other two types, regardless of treatment, suggests that cavernous symptoms are predictors of incomplete obliteration. Certain restrictive and all proliferative type CSDAVFs were also found to shared the feature of innumerable arterial feeders. Conversion from restrictive to late restrictive type with less venous outlets is part of the natural history or post-treatment response of the CSDAVF [21,22,23]. The study confirmed that the natural course of CSDAVF evolution is from proliferative to restrictive type and subsequently to late restrictive type but the duration of the evolution varies from one individual to another. It appears that timely diagnosis can affect the therapeutic outcome given that the CO rate is highest for restrictive type CSDAVFs.
Venous Outflow
The characteristic multiple channels of the cavernous sinus (CS) represent a two-edged sword for embolization: unlike DAVFs in other locations, CSDAVFs cured by occluding the CS rarely cause neurologic sequelae because the sinus has multiple potential venous drainage outlets [24]. On the other hand, it is also technically demanding to obliterate all venous outflow. Although the risk of diverting flow from a posterior collateral to the pontomesencephalic vein via a bridging vein is low, a few cases have been reported of worsening symptoms or brain stem edema due to alteration of the flow direction following embolization [25]. Recurrence or redirection of flow should be taken into account if the symptoms change during the latent period of treatment. Satomi et al. observed that the possibility of developing CVD was low after partial treatment because the posterior drainage routes of CSDAVFs tend to close before the anterior drainage routes [26, 27]. It is necessary to ensure that there is no CVD at the end of embolization. In contrast to much of the existing literature, Liu et al. reported that venous outflow score (VOS) was not predictive of treatment outcome since the treatment of late restrictive type CSDAVFs (with a solitary venous outlet) had a lower success rate [28]. Compartmentalization of the cavernous sinus was associated with less venous outflow and fewer cases of CO in the EVT group. Compartmentalization is of less concern with GKRS because the cranial III, IV and VI nerves have a high tolerance for radiation [29].
EVT Treatment
The immediate CO rate for restrictive CSDAVFs is higher than that for proliferative and late restrictive CSDAVFs because the chronicity of the late restrictive type is longer than that of the restrictive type. Therefore, the risk of chronically occluded IPS recanalization is lower [23,24,25,26]. Closing the extensive shunting zone of the proliferative type is equivalent to obliteration of almost the whole cavernous sinus. Additionally, interventionists need to close all venous outlets before embolization to achieve CO. These dual challenging tasks explain the lower initial CO rate for proliferative CSDAVFs. Onyx has the potential to improve the immediate CO rate but it carries the potential risks of trigeminocardiac reflex-induced bradycardia or damage to the cranial III, IV and V nerves [30, 31]. Using detachable coils alone can also achieve a satisfactory final CO rate but might result in transient cranial paresis due to a mass effect [21,22,23,24,25,26,27,28,29,30,31,32]. In the only case of CSDAVF recurrence in the EVT group, no residual shunt was detected in the immediate control angiography. Previously reported cases of recurrence after morphologic resolution of the CSDAVF were associated with overlooked residual shunting hidden by the embolization agent in immediate control angiography or arising de novo from an adjacent region [33, 34]. Progressive sinus thrombosis, angiogenesis after manipulation, or regrowth of a pre-existing second angiographic occult DAVF have been hypothesized [35].
GKRS Treatment
The incidence (33%) of cortical venous drainage (CVD) from CSDAVFs in the GKRS group was similar to that from DAVFs in other locations [10, 36]. The CVD was more often associated with venous outflow restriction and more aggressive behavior of the DAVF in other locations [3, 36] and was associated with a lower likelihood of CO following GKRS in an earlier pooled analysis [10] but not in the present study. The explanation is that venous outflow from multiple sites in the cavernous sinus decreased the severity of the CVD. No patients experienced worsening of neurologic deficits or intracranial hemorrhage in the GKRS group during the latent period, confirming the safety of GKRS as an alternative treatment for CSDAVF, even in the presence of CVD. The use of GKRS induces a wide spectrum of radiobiological responses in small-size vessel walls such as perivascular or subendothelial edema, fissuring of the wall, intraluminal hemorrhage, thrombus formation, necrosis of endothelial cells, increased interstitial colloids, and increased fibroblastic activity, subsequently leading to therapeutic effects on arteriovenous malformations as well as fistulas [14, 37]. These arterial feeders are located on the sinus wall and should be the target of irradiation. In the current study, no significant differences were found in final CO rates for the three types of CSDAVF or for the presence of innumerable feeders. This suggests that the therapeutic effect of GKRS is due primarily to the radiation profile rather than the angioarchitecture. In patients with a similar disease, cerebral arteriovenous malformation, Taeshineetanakul et al. found an association between the obliteration rate and higher flow through feeding arteries but not with venous morphology [38].
Limitations
There were several limitations to this study. First of all, this was a retrospective study covering a long period of time. A prospective randomized trial is warranted to confirm the results. Secondly, liquid embolic agents such as Onyx became treatment options part way through this period; however, the treatment strategy consistently involved the use of detachable coils as the initial strategy and therefore the study population was relatively homogeneous. Although Onyx used as the first-line embolization agent for proliferative CSDAVFs might potentially increase the immediate CO rate, it also increases the risk of complications. Further evaluation of long-term outcomes is needed. Thirdly, a higher percentage of patients in the GKRS group than in the EVT group experienced symptom relief and did not return for follow-up, possibly leading to an underestimation of the therapeutic effects of GKRS. Fourthly, the complication rates for both groups were too low to analyze the contribution of CSDAVF types. To the best of our knowledge, this is the first study to compare the effects of EVT and GKRS based on symptomology and angioarchitecture. Finally, the relatively small sample size of late restrictive type CSDAVFs (n = 14) in the EVT group may have resulted in a type II error and it is difficult to draw any conclusions on the comparative efficacy of the two treatments for this particular morphology. This could be assessed further in a prospective cohort study.
Conclusion
Cavernous symptoms were found to be a useful clinical predictor of a low complete obliteration rate. The EVT remains the treatment of choice to resolve CSDAVF, especially for restrictive type CSDAVFs; however, in this study, GKRS had a lower complication rate and had a relatively homogeneous therapeutic effect on all types of CSDAVFs. The use of GKRS had therapeutic effects on proliferative type CSDAVFs that were similar to those of EVT.
Abbreviations
- CSDAVF:
-
Cavernous sinus dural arteriovenous fistula
- CO:
-
Complete obliteration
- EVT:
-
Endovascular treatment
- GKRS:
-
Gamma knife radiosurgery
- VOS:
-
Venous outflow score
References
Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163–9.
Kiyosue H, Hori Y, Okahara M, Tanoue S, Sagara Y, Matsumoto S, Nagatomi H, Mori H. Treatment of Intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24:1637–53.
Gross BA, Albuquerque FC, Moon K, McDougall CG. Evolution of treatment and a detailed analysis of occlusion, recurrence, and clinical outcomes in an endovascular library of 260 dural arteriovenous fistulas. J Neurosurg. 2017;126:1884–93.
Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural Arteriovenous fistulae. Stroke. 2017;48:1424–31.
Howard BM, Grossberg JA, Prater A, Cawley CM, Dion JE, Tong FC. Incompletely obliterated cranial arteriovenous fistulae are safely and effectively treated with adjuvant ε‑aminocaproic acid. J Neurointerv Surg. 2018;10:698–703.
Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC, Lefler JE, Higashida RT. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134:85–92.
Suh DC, Lee JH, Kim SJ, Chung SJ, Choi CG, Kim HJ, Kim CJ, Kook M, Ahn HS, Kwon SU, Kim JS. New concept in cavernous sinus dural arteriovenous fistula: correlation with presenting symptom and venous drainage patterns. Stroke. 2005;36:1134–9.
Lee RJ, Chen CF, Hsu SW, Lui CC, Kuo YL. Cerebellar hemorrhage and subsequent venous infarction followed by incomplete transvenous embolization of dural carotid cavernous fistulas: a rare complication. J Neurosurg. 2008;108:1245–8.
Luo CB, Chang FC, Wang AG, Lin CJ, Guo WY, Ting TW. Transvenous coil embolization of cavernous sinus dural arteriovenous fistula on a revised classification. World Neurosurg. 2016;95:357–67.
Chen CJ, Lee CC, Ding D, Starke RM, Chivukula S, Yen CP, Moosa S, Xu Z, Pan DH, Sheehan JP. Stereotactic radiosurgery for intracranial dural arteriovenous fistulas: a systematic review. J Neurosurg. 2015;122:353–62.
Gemmete JJ, et al. Endovascular techniques in the treatment of carotid-cavernous fistulas. Philadelphia: Lippincott Williams & Wilkins; 2000.
Tonetti DA, Gross BA, Jankowitz BT, Atcheson KM, Kano H, Monaco EA, Niranjan A, Lunsford LD. Stereotactic radiosurgery for dural arteriovenous fistulas without cortical venous reflux. World Neurosurg. 2017;107:371–5.
Yang HC, Kano H, Kondziolka D, Niranjan A, Flickinger JC, Horowitz MB, Lubsfird LD. Stereotactic radiosurgery with or without embolization for intracranial dural arteriovenous fistulas. Neurosurgery. 2010;67(2010):1276–83. discussion 1284–5.
Steiner L, Lindquist C, Adler JR, Torner JC, Alves W, Steiner M. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1–8.
Wu HM, Pan DH, Chung WY, Guo WY, Liu KD, Shiau CY. Gamma Knife surgery for the management of intracranial dural arteriovenous fistulas. J Neurosurg. 2006;105(Suppl):43–51.
Luo CB, Chang FC, Teng MM, Guo WY, Ting TW. Transvenous embolization of cavernous sinus dural arteriovenous fistula via angiographic occlusive inferior petrous sinus. J Chin Med Assoc. 2015;78:526–32.
Biondi A, Milea D, Cognard C, Ricciardi GK, Bonneville F, van Effenterre R. Cavernous sinus dural fistulae treated by transvenous approach through the facial vein: report of seven cases and review of the literature. AJNR Am J Neuroradiol. 2003;24:1240–6.
Yu SC, Cheng HK, Wong GK, Chan CM, Cheung JY, Poon WS. Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization throught the superior ophthalmic vein. Neurosurgery. 2007;60:1032–7. discussion 1037–8.
Jung KH, Kwon BJ, Chu K, Noh Y, Lee ST, Cho YD, Han MH, Roh JK. Clinical and angiographic factors related to the prognosis of cavernous sinus dural arteriovenous fistula. Neuroradiology. 2010;53:983–92.
Young CS, Schwartz ML, O’Brien P, Ramaseshan R. Stereotactic radiotherapy for AVMs: the University of Toronto experience. Acta Neurochir Suppl (Wien). 1995;63:57–9.
Ducruet AF, Albuquerque FC, Crowley RW, McDougall CG. The evolution of endovascular treatment of carotid cavernous fistulas: a single-center experience. World Neurosurg. 2013;80:538–48.
Sasaki H, Nukui H, Kaneko M, Mitsuka S, Hosaka T, Kakizawa T, Kimura R, Nagaseki Y, Naganuma H. Long-term observations in cases with spontaneous carotid-cavernous fistulas. Acta Neurochir (Wien). 1988;90:117–20.
Kurata A, Miyasaka Y, Kunii M, Nagai S, Ohmomo T, Morishima H, Fujii K, Kan S. The value of long-term clinical follow-up for cases of spontaneous carotid cavernous fistula. Acta Neurochir (Wien). 1998;140:65–72.
Kobkitsuksakul C, Jiarakongmun P, Chanthanaphak E, Pongpech S. Radiographic evaluation and clinical implications of venous connections between dural arteriovenous fistula of the cavernous sinus and cerebellum and the Pontomedullary venous system. World Neurosurg. 2015;84:1112–6.
Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, Chung TS. Results of transvenous embolization of cavernous dural arteriovenous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol. 2006;27:2078–82.
Satomi J, Satoh K, Matsubara S, Nakajima N, Nagahiro S. Angiographic changes in venous drainage of cavernous sinus dural arteriovenous fistulae after palliative transarterial embolization or observational management: a proposed stage classification. Neurosurgery. 2005;56:494–502.
Satomi J, van Dijk JM, Terbrugge KG, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002;97:767–70.
Liu HM, Wang YH, Chen YF, Cheng JS, Yip PK, Tu YK. Long-term clinical outcome of spontaneous carotid cavernous sinus fistulae supplied by dural branches of the internal carotid artery. Neuroradiology. 2001;43:1007–14.
Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E 3rd, Kooy HM, Flickinger JC. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215–21.
Wang J, Wu HC, Wang WW, Zhao HS, Dao RN, Liu WM, Zhou DZ, Wang HY, Du C. Trigeminal cardiac reflex caused by onyx embolization of intracranial dural arteriovenous fistula. Turk Neurosurg. 2016;26:325–30.
Zhang X, Guo W, Shen R, Sun J, Yin J, Chen X, Gao L, Chen Z, Zhang Q. Combined use of Onyx and coils for transarterial balloon-assisted embolization of traumatic carotid-cavernous fistulas: a report of 16 cases with 17 fistulas. J Neurointerv Surg. 2016;8:1264–7.
Cognard C, Januel AC, Silva NA Jr., Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008;29:235–41.
Ambekar S, Gaynor BG, Peterson EC, Elhammady MS. Long-term angiographic results of endovascularly “cured” intracranial dural arteriovenous fistulas. J Neurosurg. 2016;124:1123–7.
Kiyosue H, Tanoue S, Okahara M, Yamashita M, Nagatomi H, Mori H. Recurrence of dural arteriovenous fistula in another location after selective transvenous coil embolization: report of two cases. AJNR Am J Neuroradiol. 2002;23:689–92.
Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781–6.
Hu YS, Lin CJ, Wu H‑M, Guo WY, Luo CB, Wu CC, Chung WY, Liu KD, Yang HC, Lee CC. Lateral sinus dural arteriovenous fistulas: sinovenous outflow restriction outweighs cortical venous reflux as a parameter associated with hemorrhage. Radiology. 2017;285:528–35.
Lunsford LD, editor. Modern stereotactic neurosurgery. Boston: Martinus Nijhoff; 1988.
Taeshineetanakul P, Krings T, Geibprasert S, Menezes R, Agid R, Terbrugge KG, Schwartz ML. Angioarchitecture determines obliteration rate after radiosurgery in brain arteriovenous malformations. Neurosurgery. 2012;71:1071–8. discussion 1079.
Acknowledgements
The authors thank Hsin-Yi Huang (Biostatistics Task Force, Taipei Veterans General Hospital) for statistical assistance, and Wallace Academic Editing for manuscript editing.
Funding
This study was funded by Taipei Veterans General Hospital (grant number: V107C-170, 023) and the Ministry of Science and Technology (grant number: MOST-106-2314-B-010-015-MY2, MOST-106-2314B-075-011).
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of entire study, C.B.L, C.J.L, H.C.Y; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, C.B.L, H.M.W; clinical studies, W.Y.C, C.C.L, K.D.L; statistical analysis, C.J.L; manuscript editing, C.B.L, C.J.L, H.C.Y.
Corresponding author
Ethics declarations
Conflict of interest
H.-C. Yang, C.-J. Lin, C.-B. Luo, C.-C. Lee, H.-M. Wu, W.-Y. Guo, W.-Y. Chung and K.-D. Liu declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yang, HC., Lin, CJ., Luo, CB. et al. Treatment Outcomes of Cavernous Sinus Dural Arteriovenous Fistulas: Comparison of Radiosurgery and Endovascular Embolisation. Clin Neuroradiol 30, 321–330 (2020). https://doi.org/10.1007/s00062-019-00787-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00787-z