Abstract
Background and Purpose
The Barrel device is an electrolytically detachable laser cut, closed-cell microstent that is used for neck reconstruction in wide-necked bifurcation and branching aneurysms to support coiling. The key feature is a barrel section that herniates over the aneurysmal ostium. The objective was to evaluate the safety, feasibility and the immediate and mid-term occlusion results of this new device.
Material and Methods
The databases of two tertiary care centers were screened for all Barrel-based stent-assisted intracranial coil embolization of wide-necked aneurysms between June 2015 and September 2016. Case files and imaging data were retrospectively analyzed for angiographic and clinical outcome parameters, including immediate and mid-term modified Raymond-Roy aneurysm occlusion classification (RROC) rates and procedural complications.
Results
A total of 21 patients comprising 21 intracranial aneurysms (20 unruptured, 1 ruptured) were treated with the Barrel device and additional coiling of the aneurysm sac. All aneurysms were wide-necked, saccular bifurcation aneurysms defined by a dome/neck ratio ≤2. Immediate complete occlusion (RROC1) was observed in 19/21 (90%). An intra-interventional in-stent thrombus formation in two cases (10%) was medically resolved without neurological sequelae. A single case of symptomatic in-stent stenosis (5%) was cleared by balloon angioplasty. Follow-up (FU) was available in 20/21 cases (95%) after a median of 282 days (range: 17–591 days). At follow-up 19/20 aneurysms (95%) were completely occluded (RROC1).
Conclusion
The Barrel device showed a satisfactory safety profile and a promising rate of immediate and mid-term complete aneurysm occlusion for stent-assisted coil embolization of wide-necked intracranial bifurcation aneurysms, warranting further investigation of the device.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endovascular coil embolization of ruptured and unruptured intracranial aneurysms has evolved into the treatment of choice in the majority of cases when compared to surgical clipping [1,2,3,4,5]. Bare coiling is not generally applicable to the treatment of so-called complex aneurysms bearing an unfavorable anatomy, e. g. wide-necked bifurcation and branching aneurysms, essentially due to the risk of coil protrusion from the aneurysm sac and unsatisfactory recurrence rates, reported to be as high as 10.8% in a recent prospective multicenter study [6]. The rationale for the use of stents in the treatment of intracranial aneurysms is that stents do not only provide a scaffold for supporting coils in a wide-necked or bifurcation aneurysm but also promote progressive intrasaccular thrombosis and endothelialization across the aneurysm neck over time [7, 8]. This technique yields higher rates of complete aneurysm occlusion while maintaining similar complication rates compared to coiling alone [9]; however, at times extensive techniques, e. g. the kissing Y stent- [10], crossing Y stent- [11], T‑stent-assisted coiling [12], or x‑stent assisted coiling [13] are required to achieve complete and long-term occlusion. Unfortunately, the high metal-to-artery ratio, bearing an immanent thromboembolic risk, is a main drawback of the aforementioned advanced techniques.
The Barrel device (Barrel Vascular Reconstruction Device VRD, Medtronic, Minneapolis, MN, USA) is a self-expandable, laser cut stent, featuring a bulged center section that herniates “belly-like” over the ostium of the aneurysm as an adjunct to facilitate coiling of the sac of complex wide-necked bifurcation and branching aneurysms (Figs. 1 and 2). The specific architecture of the Barrel allows the abstention from the common need of a second microstent resulting in a decrease of metal coverage and thrombogenicity compared to the use multiple conventional microstents [14].
Silicon model of a wide-necked bifurcation aneurysm. The Barrel Vascular Reconstruction Device (VRD) features a bulged, “belly-like” center section that herniates over the ostium of the aneurysm and can protect both parent vessels from coil protrusion. Exemplarily, the reddots tag the position of the platinum proximal center marker, the markers at the center section and the distal center marker (Fig. provided by Medtronic)
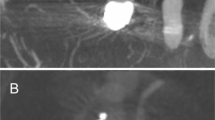
Unruptured aneurysm of the basilar tip, fetal origin of the left posterior cerebral artery (PCA) (not shown); a anterior view shows a saccular wide-necked aneurysm between right PCA and duplicated left superior cerebellar artery (SUCA). The white line represents the bifurcation span length for determination of the appropriate size of the device; b unsubtracted anterior view illustrates the positioning of the Barrel VRD; c anterior view shows complete occlusion of the aneurysm after final coil embolization through the interstices of the device
Materials and Methods
We screened the longitudinal neurointerventional databases of two tertiary care hospitals for all patients with wide-necked bifurcation and branching aneurysms, defined by a dome/neck ratio ≤2 that were treated with Barrel stent-assisted coil embolization on an intention-to-treat basis between June 2015 and September 2016. According to institutional guidelines, no ethics committee approval was required for this retrospective observational study. All imaging and patient data were reviewed by two experienced consultant neuroradiologists (C.K., C.L.). In addition, a third reviewer (V.M.), consultant neuroradiologist with >5 years of experience, who was not involved in the treatment, reviewed all imaging data independently and blinded.
The Barrel Vascular Reconstruction Device
The Barrel device is a laser-cut closed-cell microstent composed of nitinol featuring a bulged center section, “the barrel”, that herniates over the ostium of the aneurysm sac in order to facilitate coiling. The device has 12 radio-opaque platinum markers: one longer marker at the proximal tip, one proximal and distal center marker, six markers at the center section, that improve visibility especially in the mid-portion of the device to enhance visualization during device deployment across the aneurysm neck and three distal tip markers. The Barrel VRD is accredited to be deployed in parent arteries with diameters between 2 and 4 mm. According to the size of the bulged center section diameter (5–7.5 mm) and length (5–8 mm) the device has a usable length from 20–22 mm. The Barrel VRD is compatible with any 0.021″ microcatheter, is up to three times re-sheathable over its full length and ultimately electrolytically detachable. To determine the appropriate size of the device, in every case bifurcation span length was measured according to the manufacturer’s guidelines: a diagonal line was drawn from the distal shoulder of the aneurysm neck to the opposite wall of the parent vessel (Fig. 2a).
Endovascular Technique
Every intervention was performed via a transfemoral approach with the patient under general anesthesia. A 6F guiding catheter was introduced through a short femoral sheath into the internal carotid or vertebral artery. In addition to standard bi-plane angiographic imaging, a three-dimensional rotational angiography was obtained for the procedural planning in every case. The appropriate Barrel VRD was delivered through an 0.021″ microcatheter (Prowler Select Plus, Cordis, Miami Lakes, FL, USA; Orion21 or Rebar18, Medtronic, Irvine, CA, USA) followed by probing the aneurysm sac with a 0.017″ microcatheter (Excelsior SL10, Stryker, Fremont, CA, USA; Echelon10, Medtronic) through the stent interstices in order to ultimately embolize it with coils.
Antiplatelet Regimen
In all cases of elective treatment, patients started 5 days prior to the intervention with acetylsalicylic acid (ASA) 100 mg and clopidogrel 75 mg daily. Platelet function testing was performed immediately before the interventional procedure by using ASA and P2Y12 assays (Verify Now, Accumetrics, San Diego, CA, USA). A therapeutic platelet inhibition level between 30%–60% for P2Y12 and 350–550 aspirin reaction units (ARU) for ASA was basic prerequisite before stenting. A life-long treatment with ASA 100 mg/day and clopidogrel 75/day for 3 months was prescribed after the procedure. A bolus of heparin (5000 IU) was applied after groin puncture, followed by aliquots of 1000 IU/h until the end of the procedure. In the single case of emergency treatment of an acutely ruptured, wide-necked basilar tip aneurysm, tirofiban, dosage according to the manufacturer’s recommendations, was administered immediately prior to the deployment of the Barrel VRD. Next day, tirofiban was discontinued after 24 h and the antiplatelet regimen was continued with life-long ASA and clopidogrel for 3 months as described before.
Results
Procedural Data
A total of 21 intracranial aneurysms in 21 patients, 12 female and 9 male (median age 56 years, range 34–81 years) were treated. Of the 21 cases 1 (5%) presented with a ruptured basilar tip aneurysm and subarachnoid hemorrhage (SAH) Fisher grade 4, H&H grade 4 and was treated as an emergency. All aneurysms were saccular wide-necked bifurcation aneurysms. All aneurysms, except a precoiled recurring aneurysm of the anterior communicating artery, were virgin, previously untreated cases (95%). Barrel VRD deployment was attempted 21 times and it was technically feasible in all attempts (100%). Table 1 gives an overview of the anatomical and technical data of the procedures. The “jailing” technique was not performed; secondary to Barrel deployment, in every case the aneurysm sac was probed with microwire and 0.017″ microcatheter through the interstices of the stent and ultimately coiled.
Periprocedural Complications
In case no. 3 a thrombus formation inside the Barrel VRD and in case no. 8 a peripheral M2 thromboembolism was detected during the procedure. A prompt complete dissolution of the thrombi, without clinical sequelae, was achieved after intra-arterial administration of abciximab.
Immediate Imaging Results
At the end of the procedure a complete aneurysm occlusion (RROC1) was observed in 19/21 cases (90%), a residual neck (RROC2) in 2/21 (10%) and no residual aneurysm (RROC3). The wall adaptation of the Barrel stent was satisfactory in all cases without the need for any adjunctive in-stent balloon angioplasty. All aneurysms were embolized with either Axium (ev3, Irvine, CA, USA) or Target coils (Stryker, Fremont, CA, USA).
Angiographic-Follow-up
A DSA-based angiographic FU was available in 20/21 cases (95%). The final FU was performed after a median of 282 days (range 17–591 days). In 19/20 cases (95%) FU angiography depicted a complete aneurysm occlusion (RROC1) and in 1 case (5%) a neck remnant without necessity of retreatment. In quintessence, a relevant difference in FU grading between blinded and non-blinded reviewers could not be detected (Tables 1 and 2).
Patient no. 16 presented with two episodes of temporary right body paresthesia 4 weeks after the procedure. Magnetic resonance (MR) angiography demonstrated a significant in-stent stenosis (70%) due to intimal hyperplasia. While on dual antiplatelet treatment, in-stent balloon angioplasty was performed, leading to substantial improvement of the stenosis and cessation of the relapsing temporary neurologic symptoms without new clinical sequelae. The FU DSA after 10 months showed full patency of the Barrel stent and complete aneurysm occlusion (RROC1). Beyond that, additional manifestations of in-stent stenosis/intimal hyperplasia were not recorded and none of the patients developed any new transient or permanent neurological deficit immediately after the procedure or during follow-up.
Discussion
Stent-assisted coiling was initially developed for wide-necked aneurysms on the hypothesis that a stent can provide the scaffold to hold the coils within the aneurysm sac and prevent coil herniation into the parent vessel [15,16,17]. Furthermore, due to a minor flow diversion effect and facilitation of neo-endothelialization of the stent, stent-assisted coiling might promote delayed intra-aneurysmal thrombosis leading to higher occlusion rates and less recurrence compared to coiling alone [9]; however, in cases of wide-necked bifurcation aneurysms that incorporate ≥2 parent vessels, coiling in combination with a single stent is generally insufficient to protect the parent vessels from coil protrusion and advanced techniques using two microstents might be necessary, resulting in a high metal load and an increased potential risk of thromboembolic complications [9,10,11, 13]. Bartolini et al. reported a rate of 19% procedure-related neurologic deficits and 10% permanent neurologic deficits in a case series of 100 treated complex and wide neck intracranial aneurysms with y‑stent and x‑stent assisted coiling [13]. The Barrel VRD owing to its bulged “belly-like” center section that herniates over the ostium of the aneurysm enables the common need of a second stent to be omitted resulting in a decrease of metal coverage. This should theoretically lower the potential of thrombogenicity compared to the use multiple conventional microstents [14]; however, in our small series, we detected a not insignificant rate of 2/21 (10%) periprocedural thromboembolic events that compares unfavorably to a recent study that focuses on sole aneurysm coiling [6]. After medical dissolution, both events did not lead to transitory or permanent neurologic deficits. All elective cases were mRS 0 at final FU. The immediate complete aneurysm occlusion rates (90%) and at final FU (95%) compare favorably to y‑stent and x‑ stent assisted coiling studies [13, 18] and to a recently published Barrel single center study [14].
Furthermore, in a single case (no. 16), we detected a symptomatic, relevant in-stent stenosis (70%) due to intimal hyperplasia that could be cleared by performing balloon angioplasty without permanent clinical sequelae. In-stent stenosis is a relatively well-known phenomenon of atherosclerotic coronary artery stenosis treatment with an incidence of 10–50% [19]; however, in intracranial aneurysm treatment the stent is placed in a non-atherosclerotic parent artery and findings might not be transmittable [20]. In a study of 99 aneurysms that were treated with stent-assisted coiling, Kim et al. could show that moderate luminal narrowing due to intimal hyperplasia is not uncommon and appears to be a dynamic process that peaks between 2–8 months after stent placement and generally resolves spontaneously without need of further treatment [20]. Remarkably, in our case, the stenosis was detected after a short time interval of only 4 weeks after Barrel placement and under sufficient dual antiplatelet treatment. As a new device, there is obviously limited knowledge of the long-term effects of the Barrel stent on the cerebral vasculature and it remains unclear whether our finding represents an exceptional case or not, until a larger volume of data becomes available.
The use of the Barrel VRD in the single case of an acutely ruptured, wide-necked aneurysm that could not be treated by coil embolization only (case no. 1), was feasible; the procedure was conducted without technical or clinical sequelae under antiaggregation with tirofiban in the acute setting.
Other design strategies have recently emerged to assist the coil embolization of wide-necked bifurcation aneurysms with a single device: the pCONus (Phenox, Bochum, Germany) is a electrolytically detachable stent-like device consisting of a stent shaft that is positioned in the parent vessel and its distal end part featuring four petals that open outwardly inside the aneurysm sac, followed by final coil embolization [21]. In an initial series of patients, treatment with the pCONus turned out to be feasible with satisfactory clinical and anatomical outcomes [22, 23]. The Pulse Rider (Pulsar Vascular, San Jose, CA, USA) is an extra-aneurysmal “wing-like” device deployed outside the aneurysm in the parent vessel bifurcation covering the aneurysm orifice. Through the interstices of the Pulse Rider the aneurysm can be probed with a microcatheter and finally coil embolized [21]. Preliminary studies suggest the Pulse Rider to be a feasible treatment option [24, 25] but its value has yet to be elaborated.
Our pilot study bears several limitations that have to be taken into account when interpreting the results. These are mainly caused by a small sample size and the heterogeneity of such small cohorts as presented here; all except one case were virgin, previously untreated aneurysms. Case no. 11 represents a pre-coiled recurrent aneurysm that was retreated by Barrel stent-assisted coiling and might therefore differ in its clinical course. Angiographic outcomes were not assessed by a core laboratory. This might bias the interpretation of imaging results [26]. To diminish this potential bias at least in part, all imaging data were re-reviewed independently by a blinded third experienced consultant neuroradiologist, who was not involved in the treatment. Finally, completion of long-term FU is warranted to evaluate the occlusion and recanalization rate in comparison to established techniques as double stent-assisted coiling.
Conclusion
In our preliminary series stent-assisted coiling of wide-necked aneurysms with the Barrel VRD was feasible, showed a satisfactory safety profile and a promising rate of immediate and mid-term complete aneurysm occlusion that compares favorably with standard double-stent-assisted coiling, warranting further long-term investigation of the device.
References
Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke. 2010;41(7):1471–6.
Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001–2008. AJNR Am J Neuroradiol. 2011;32(6):1071–5.
Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, Partovi S, Nakaji P, Wallace RC. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. 2015;123(3):609–17.
Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–74.
Cognard C, Pierot L, Anxionnat R, Ricolfi F; Clarity Study Group. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: clinical results of the Clarity GDC study. Neurosurgery. 2011;69(4):837-41; discussion 842.
Brinjikji W, Amar AP, Delgado Almandoz JE, Diaz O, Jabbour P, Hanel R, Hui F, Kelly M, Layton KD, Miller JW, Levy E, Moran C, Suh DC, Woo H, Sellar R, Ho B, Evans A, Kallmes DF. GEL THE NEC: a prospective registry evaluating the safety, ease of use, and efficacy of the HydroSoft coil as a finishing device. J Neurointerv Surg. 2018;10(1):83-7.
Piotin M, Blanc R. Balloons and stents in the endovascular treatment of cerebral aneurysms: vascular anatomy remodeled. Front Neurol. 2014;5:41.
Shapiro M, Becske T, Sahlein D, Babb J, Nelson PK. Stent-supported aneurysm coiling: a literature survey of treatment and follow-up. AJNR Am J Neuroradiol. 2012;33(1):159–63.
Hong Y, Wang YJ, Deng Z, Wu Q, Zhang JM. Stent-assisted coiling versus coiling in treatment of intracranial aneurysm: a systematic review and meta-analysis. PLoS One. 2014;9(1):e82311.
Brassel F, Melber K, Schlunz-Hendann M, Meila D. Kissing-Y stenting for endovascular treatment of complex wide necked bifurcation aneurysms using Acandis Acclino stents: results and literature review. J Neurointerv Surg. 2016;8(4):386–95.
Ko JK, Han IH, Cho WH, Choi BK, Cha SH, Choi CH, Lee SW, Lee TH. Crossing Y‑stent technique with dual open-cell stents for coiling of wide-necked bifurcation aneurysms. Clin Neurol Neurosurg. 2015;132:54–60.
Aydin K, Sencer S, Barburoglu M, Berdikhojayev M, Aras Y, Sencer A, İzgi N. Midterm results of T‑stent–assisted coiling of wide-necked and complex intracranial bifurcation aneurysms using low-profile stent. J Neurosurg. 2017;127(6):1288–96.
Bartolini B, Blanc R, Pistocchi S, Redjem H, Piotin M. “Y” and “X” stent-assisted coiling of complex and wide-neck intracranial bifurcation aneurysms. AJNR Am J Neuroradiol. 2014;35(11):2153–8.
Mühl-Benninghaus R, Simgen A, Reith W, Yilmaz U. The Barrel stent: new treatment option for stent-assisted coiling of wide-necked bifurcation aneurysms-results of a single-center study. J Neurointerv Surg. 2016;9(12):1219–22.
Benitez RP, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004;54(6):1359–67. discussion 1368.
Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007;61(3):460–9.
Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, Halbach VV. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87(6):944–9.
Jeon P, Kim BM, Kim DJ, Kim DI, Park KY. Y‑configuration double-stent-assisted coiling using two closed-cell stents for wide-neck basilar tip aneurysms. Acta Neurochir (Wien). 2014;156(9):1677–86.
Lowe HC, Oesterle SN, Khachigian LM. Coronary in-stent restenosis: current status and future strategies. J Am Coll Cardiol. 2002;39(2):183–93.
Kim YS, Lee SW, Yeom JA, Yoon CH, Baik SK. Angiographic findings of in-stent intimal hyperplasia after stent-assisted coil embolization: are they permanent findings? J Neurosurg. 2016;124(2):328–33.
Henkes H, Weber W. The Past, Present and Future of Endovascular Aneurysm Treatment. Clin Neuroradiol. 2015;25 Suppl 2:317-24.
Labeyrie PE, Gory B, Aguilar-Perez M, Pomero E, Biondi A, Riva R, Turjman F, Henkes H. The pCONus Device for Treatment of Complex Wide-Neck Anterior Communicating Artery Aneurysms. World Neurosurg. 2017;101:498–505.
Gory B, Aguilar-Pérez M, Pomero E, Turjman F, Weber W, Fischer S, Henkes H, Biondi A. One-year Angiographic Results After pCONus Stent-Assisted Coiling of 40 Wide-Neck Middle Cerebral Artery Aneurysms. Neurosurgery. 2017;80(6):925–33.
Gory B, Spiotta AM, Di Paola F, Mangiafico S, Renieri L, Consoli A, Biondi A, Riva R, Labeyrie PE, Turjman F. PulseRider for Treatment of Wide-Neck Bifurcation Intracranial Aneurysms: 6-Month Results. World Neurosurg. 2017;99:605–9.
Mukherjee S, Chandran A, Gopinathan A, Putharan M, Goddard T, Eldridge PR, Patankar T, Nahser HC. PulseRider-assisted treatment of wide-necked intracranial bifurcation aneurysms: safety and feasibility study. J Neurosurg. 2017;127(1):61–8.
Rezek I, Lingineni RK, Sneade M, Molyneux AJ, Fox AJ, Kallmes DF. Differences in the angiographic evaluation of coiled cerebral aneurysms between a core laboratory reader and operators: results of the Cerecyte Coil Trial. AJNR Am J Neuroradiol. 2014;35(1):124–7.
Funding
Data sharing statement: All data will be made available on request in an anonymized manner.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Kabbasch, A. Mpotsaris, V. Maus, J.C. Altenbernd and C. Loehr declare that they have no competing interests.
Additional information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Kabbasch, C., Mpotsaris, A., Maus, V. et al. The Barrel Vascular Reconstruction Device. Clin Neuroradiol 29, 295–301 (2019). https://doi.org/10.1007/s00062-017-0660-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-017-0660-2