Abstract
Background
Endovascular techniques for treatment of large vessel occlusions (LVO) in patients with acute ischemic stroke (AIS) have advanced in recent years. We report a multicenter experience using a combined aspiration and stent retriever technique for mechanical thrombectomy (MT).
Methods
We retrospectively analyzed 32 consecutive MT patients using a novel, combined approach of Stent retriever Assisted Vacuum-locked Extraction (SAVE) by 3 operators at 3 stroke centers. Primary endpoint was successful first-pass reperfusion with a modified Thrombolysis in Cerebral Infarction (mTICI) score of 3. Secondary endpoints were number of passes, time from groin puncture to reperfusion, embolization to new territories (ENT), postinterventional symptomatic intracranial hemorrhage (sICH) and clinical outcome at discharge.
Results
First-pass mTICI 3 reperfusion was achieved in 23 out of 32 patients (72%) with a mean groin puncture to reperfusion time of 36.0 min ± 15.8 and mTICI 3 was accomplished in 25 out of 32 cases (78%) with a maximum of 3 attempts. Successful reperfusion (mTICI ≥ 2b) was achieved in all patients (100%) with a mean time from groin puncture to reperfusion of 44.5 min ± 25.8 and an average of 1.2 ± 0.7 attempts. The rate of ENT was 0% and 1 patient with sICH after MT died on postoperative day 4. At discharge, the median National Institutes of Health Stroke Scale (NIHSS) score was 4 (range 0−17) and favorable neurological outcome by the modified Rankin score (mRS ≤ 2) was achieved in 19 out of 32 patients (59%).
Conclusion
SAVE is fast and appears to be very effective in terms of first-pass complete reperfusion in patients with LVO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Endovascular treatment with modern stent retrievers has been shown to be the most effective treatment option for acute ischemic stroke (AIS) caused by large vessel occlusion (LVO) in the anterior circulation [1–5]. Clinical outcome is improved when early revascularization of the primary occlusion is achieved [6]. In the past decade, development of neurointerventional equipment for endovascular therapy has advanced rapidly with overwhelming efficacy for current devices compared to previous technology, resulting in high reperfusion rates and shorter groin puncture to reperfusion times [1–5]. The question which current mechanical thrombectomy (MT) technique yields the best results in terms of safety and efficacy is subject to discussion. Principal MT techniques include (i) the use of stent retrievers by incorporation of the clot through the stent retriever struts after passive stent opening with subsequent withdrawal of the device under flow arrest by a balloon guide catheter (BGC), (ii) a direct aspiration first-pass thrombectomy (ADAPT) with a large bore aspiration catheter placed at the face of the thrombus and (iii) a combination of both, where the stent retriever is usually withdrawn into an intracranially placed aspiration catheter (termed the “Solumbra technique”) [7–10]. We present a multicenter experience using a novel endovascular technique. It comprises a combination of both procedures (i) and (ii) by Stent retriever Assisted Vacuum-locked Extraction (SAVE).
Methods
This multicenter retrospective study evaluated 32 patients treated consecutively by 3 operators in 3 different certified comprehensive stroke centers for AIS based on LVO. One operator with profound neurointerventional skills (>5 years experience in endovascular stroke treatment) per institution performed thrombectomy using the aforementioned novel technique. We analyzed the prospectively maintained neurointerventional databases to examine the technical and clinical outcome. The primary endpoint was defined as first-pass successful reperfusion defined as modified Thrombolysis in Cerebral Infarction (mTICI) 3. Secondary endpoints were total number of passes, time interval from groin puncture to reperfusion, rate of embolization to new territories (ENT), rate of postinterventional symptomatic intracranial hemorrhage (sICH) and clinical outcome at discharge assessed by the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin score (mRS). All values are presented as median and range or mean and standard deviation (SD) for continuous variables, and as frequency and percent for categorical variables. According to the guidelines of the respective local ethics committees, no approval was necessary for this anonymous retrospective study, which was conducted in accordance to the Declaration of Helsinki.
Patient Selection
Inclusion criteria were as follows: LVO and absence of ICH on baseline imaging, Alberta Stroke Program Early CT score (ASPECTS) ≥5, no age limit, baseline NIHSS score of ≥5 or aphasia. Patients with known symptom onset were eligible within a time frame of ≤8 h. In the case of unknown onset all patients with positive imaging profile evaluated with multimodal computed tomography (CT) or magnetic resonance imaging (MRI) were included. All patients received intravenous thrombolysis (IVT) according to the national guidelines of the Society of Neurology. Patients with tandem occlusions comprising a preceding extracranial occlusion or stenosis were not included in our study.
Imaging and Clinical Assessment
All angiographic images were re-evaluated to determine the target downstream territory, new target downstream embolizations or ENT and the mTICI grade before and after MT according to the 2013 recommendations of the Cerebral Angiographic Revascularization Grading collaborators (CARG). Native cranial computed tomography (NCCT) was performed postinterventionally after 24 h or earlier in case of secondary worsening. SICH was defined according to the European Cooperative Acute Stroke Study (ECASS) criteria. A consultant neurologist assessed all NIHSS and mRS scores independently.
Technical Description of SAVE
MT is either conducted with the patient under conscious sedation or general anesthesia as per the institutional practice. A short or long 8‑French (F) sheath (8 F Destination, Terumo, Leuven, Belgium) is placed in the groin. In anterior circulation AIS the internal carotid artery (ICA) is catheterized with an 8 F guide catheter (8 F Cordis Vista, Johnson & Johnson, New Brunswick, NJ) or with the aforementioned long sheath followed by an initial angiogram to depict the status of the intracranial vasculature. In cases of uncooperative anxious patients, the angiography table is swayed towards the ventilation machine and the anesthesiologist for easier initiation of general anesthesia. Meanwhile, an aspiration catheter, a microcatheter and a microwire are prepared and connected with permanent saline flush lines. In cases of LVO in the anterior circulation we predominantly use an AXS Catalyst 6 aspiration catheter (0.060″ inner diameter; Stryker Neurovascular, Fremont, CA) or a Sofia Plus aspiration catheter (0.070″ inner diameter; Microvention, Tustin, CA). The AXS Catalyst 6 aspiration catheter is also used in cases of basilar artery occlusion with the support of a 7-French guide catheter or 80 cm sheath, which is placed in the subclavian artery/origin of the vertebral artery. A 0.021″ microcatheter is advanced past the occlusion site in a M2/A2/PCA vessel with the help of a 0.014″ microwire (see online video). Position of the microcatheter tip distally to the thrombus is confirmed with a careful contrast injection through the microcatheter after removing the microwire. At this point the aspiration catheter is not advanced past the origin of the ophthalmic artery, as this process can be time-consuming in elongated vessels. A stent retriever usually Trevo ProVue, (Stryker Neurovascular), Solitaire FR (Medtronic, Minneapolis, MN) or EmboTrap (Neuravi, Galway, Ireland) is then advanced through the microcatheter and placed primarily distally to and with the proximal third across the occlusion site (Fig. 1b) by using the active push deployment technique described by Wiesmann et al. [11]. At this point it is important to tighten the compression seal of the rotating hemostatic valve in order to maintain tension and support incorporation of the thrombus by the stent. After waiting for 2–3 min the valve is loosened and the microcatheter is slowly removed (bare wire technique) in order to maximize the inner volume of the aspiration catheter (Fig. 1c; [12]). At the same time, by pulling the microcatheter, the aspiration catheter usually advances past the origin of the ophthalmic artery (grapple hook technique, Fig. 1c, black arrow). We then connect the aspiration catheter to an aspiration pump (Penumbra, Alameda, CA) for permanent aspiration, and further advance the tip of the aspiration catheter towards the occlusion site, while gently retracting the stent retriever. We continue carefully with minimal pulling of the stent retriever while pushing the aspiration catheter until we have reached a wedge position (Fig. 1d, white arrow). After reaching the wedge position and seeing no flow within the aspiration tube, permanent aspiration with the pump is switched to the guide catheter or long sheath while negative pressure is retained within the aspiration catheter with the use of a 60 ml vacuum pressure syringe (VacLok, Merit Medical, South Jordan, UT). The stent retriever and the aspiration catheter are retrieved outright back inside the guide catheter/sheath as a unit, in contrast to current techniques, such as Solumbra (Figs. 2 and 3). The unit retrieval process is done slowly under fluoroscopy and can last up to 30 s in order to prevent distal emboli or ENT. During this process, the stent retriever and thrombus are frequently further retracted inside the aspiration catheter because of the negative pressure and the stretching of the previously compressed catheter tip (Fig. 1e, black arrow). Pump aspiration on the guide catheter/sheath stays on for another 30 s after removal of the stent retriever/aspiration catheter unit in order to minimize distal emboli or ENT (Fig. 4). In exceptional cases, where no back-flow is observed within the guide catheter we also retrieve the guide catheter under permanent aspiration to remove the entrapped thrombus. The removable hemostatic valve of the guide catheter/sheath is then flushed forcefully with a 20 ml saline flush syringe to remove any remaining clots from the compression seal. Control angiograms are acquired (Fig. 1f and g) and if necessary the whole procedure is repeated. Technical details are presented in Table 1.
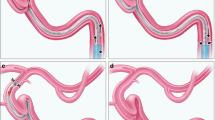
a Initial angiogram showing an mTICI 0 occlusion of the right M1 segment. b After advancing a 0.021″ microcatheter past the occlusion site to a distal M2 segment, the microwire is removed and a 4 × 30 mm stent retriever (Trevo ProVue) is placed primarily distally (black arrow) and with the proximal third across the thrombus (white arrow) by using the active push deployment technique. Up to this point, we lose no time with navigating the AXS Catalyst 6 aspiration catheter past the origin of the ophthalmic artery. After a waiting period of 2–3 min the microcatheter is slowly pulled back out of the catheter to maximize aspiration volume inside the distal catheter (bare wire thrombectomy). Simultaneously, by pulling the microcatheter the aspiration catheter usually advances past the origin of the ophthalmic artery (c black arrow). We continue with synchronized pulling of the stent retriever and pushing of the aspiration catheter until we reach a wedge position (d white arrow) under continuous aspiration with a pump. Note that the stent retriever has not actually changed position during this maneuver. After no flow is observed in the aspiration catheter, we switch the pump aspiration to the guide catheter and start pulling the unit stent retriever/aspiration catheter back towards the guide catheter while vacuum is preserved in the aspiration catheter with the help of a negative pressure syringe. Continuous aspiration on the guide catheter is needed during retraction of the stent retriever/aspiration-catheter unit in order to warrant flow reversal within the internal carotid artery and to prevent migration of thrombus fragments in distal vessels. Flow reversal with large-bore guide catheters can only be warranted for a couple of seconds with a negative pressure syringe. The unit retrieval process is done slowly under fluoroscopy and can last up to 30 s. It is not unusual to see the stent retriever and thrombus being further retracted inside the aspiration catheter during this process, due to initial compression of the distal part of the aspiration catheter during the wedge maneuver (e black arrow). f,g Control angiograms show successful reperfusion after one attempt with the SAVE technique. h Picture of the stent retriever after successful thrombectomy with the main part of the clot being incorporated by the proximal/middle part of the stent and a clot fragment being caught by the far distal part of the stent
a, b Depict the technique most interventionalists associate with the term Solumbra. A stent-retriever is placed centrally across the clot, while an aspiration catheter is advanced to the clot face. The stent retriever is then retracted back inside the aspiration catheter, while negative pressure within the aspiration catheter is preserved with pump aspiration or a negative pressure syrinx (curved arrows on aspiration catheter tip). Usually 6 F long sheaths or BGCs are used with Solumbra and no proximal aspiration (depicted by x at the guide tip) is applied during the critical moments of stent retriever retraction. This results in antegrade blood flow within the A1 and M1 segments during retraction of the stent retriever, as the aspiration catheter is clogged with the clot. Clot fragments cannot be controlled with this technique. c, d Delineate a recently proposed technique called aspiration retriever technique for stroke (ARTS). Similarly to Solumbra the stent is placed centrally across the clot. The aspiration catheter is then advanced to the face of the thrombus under permanent aspiration and retracted as a unit with the stent retriever. In the publication by Massari et al. BGCs were usually used as guide catheters without the use of additional proximal aspiration with the BGC [13]. This leads to flow arrest (X on the ICA) within the ICA, but retrograde blood flow in the A1 segment or posterior communicating artery (Pcom) and antegrade flow in the M1 segment. Clot fragments cannot be controlled with this technique in the critical moments of clot immobilization and retraction, as the aspiration catheter is clogged with the clot. A similar technique called continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE) has been recently published and while the idea of aspiration with the aspiration catheter during deployment of the stent-retriever is intriguing, the name of the technique is misleading as again no proximal aspiration is being proposed. As the authors of CAPTIVE describe they monitor the drip rate of the aspiration tubing until it has stopped. This leads to a clogged aspiration catheter and no control over clot fragments in the critical seconds of clot immobilization. e–h Depict the steps of the SAVE technique. The stent retriever is deployed primarily distally to the clot and only the proximal third interacts with the clot. Immediately after deployment of the stent retriever we usually observe anterograde flow within the target territory, while the stent controls clot fragments. The aspiration catheter is then advanced to the face of the clot and aspiration with the pump is started. We continue carefully with minimal pulling of the stent retriever while pushing the aspiration catheter until we have reached a wedge position. In this position the aspiration catheter is obviously clogged with clot, thus there is no need for pump aspiration and vacuum can be preserved with a negative pressure syringe. The permanent aspiration with the pump is then connected to the guide catheter, which leads clot fragment controlling in the critical moments of clot immobilization (f) and retrieval of the unit inside the guide catheter. Our preferred set-up for the SAVE technique at the moment is an 8 F long sheath, a large-bore aspiration catheter and a long stent-retriever. See the Methods for a more detailed description of the SAVE technique
Angiographic images of the patient used for the drawings in Fig. 2. a shows an M1 occlusion while b depicts antegrade flow after placement of the stent retriever. Angiographic series after deployment of the stent retriever are not necessary and should only be performed through the guide/sheath and with low pressure/strongly diluted contrast media in order to minimize clot fragmentation risk and contrast media uptake of the already infarcted downstream territory. Injections through the aspiration catheter should be strictly avoided. Successful reperfusion (mTICI 3) of the left middle cerebral artery (MCA) is delineated on c and d after 1 SAVE maneuver
Angiographic images of a patient with a carotid-T occlusion. a Shows the initial angiogram with minimal flow past the occlusion towards the MCA and ACA territories as the clot expands towards both the M1 and the A1 segments. b Depicts a magnified, later image of the first angiogram. c, d Show the successful recanalization of the carotid-T/M1/A1 segments and an mTICI 2b reperfusion of the downstream territories after 1 SAVE maneuver. Our initial experience with the SAVE technique for bifurcation clots has been very positive, as there was no need for dual stent (so-called kissing stents technique) deployment since starting to use SAVE as our first-line technique
Results
A total of 32 patients (20 males) with LVO received MT with the SAVE technique. Baseline characteristics are presented in Table 2. Out of the 32 patients 31 had a LVO of the anterior circulation (97%): ICA in 3/32 (9%), MCA M1 in 26/32 (81%), MCA M2 in 1/32 (3%) and anterior cerebral artery (ACA) in 1/32 (3%). One patient suffered from basilar artery (BA) occlusion (3%). Median age of the patients was 74 years (range 34–93 years) and 21/32 patients received concomitant IVT (66%). Baseline median NIHSS score was 15 (range 5–25) on admission and mean time from groin puncture to reperfusion was 44.5 min ± 25.8 (median 41, range 18–148). In one patient procedure time was nearly 2.5 h due to elongated supra-aortic vessels and massive extent of thrombus, so that after two ineffective maneuvers the 4 × 20 mm Solitaire FR stent retriever was exchanged for a 4 × 40 mm with subsequent reperfusion to mTICI 2b. The pretreatment mTICI score was 0 in 31/32 (97%) and mTICI 1 in 1/32 cases (3%). A final reperfusion result of mTICI ≥2b was achieved in all cases with an average of 1.2 ± 0.7 attempts and mTICI 3 in 25/32 cases (78%) with a maximum of 3 attempts. Primary endpoint of first-pass mTICI 3 reperfusion was reached in 23/32 patients (72%) with a mean groin to reperfusion time of 36.0 min ± 15.8 (median 30.5, range 18–68 min). A total of 19 out of 26 (73%) occlusions in MCA M1 and 2 of 3 carotid-T occlusions were successful recanalized with 1 attempt. Rate of ENT was 0% and no new downstream territory occlusions were observed in the final angiographic control runs. A procedure-related death occurred in one patient with sICH at the 4th postoperative day and two patients died from pneumonia. Median NIHSS score at discharge was 4 (0–17) and favorable neurological outcome (mRS ≤2) was achieved in 19/32 patients (59%) at discharge.
Discussion
Multiple randomized controlled trials (RCT) comprising the newest generation of stent retrievers have demonstrated success in neurothrombectomy with favorable clinical outcome [1–5]. Based on these studies stent retrievers are nowadays the standard of care in MT and can be safely and effectively combined with flow-arrest or aspiration techniques. The use of aspiration catheters alone is a reliable alternative (level II evidence) [7–9, 14–17]. In the aforementioned RCTs stent retrievers were predominantly used (81.5–100%), although a small number of patients received MT with aspiration alone or older generation devices, such as the MERCI device [1]. The specific technique of MT, such as the use of a flow-arresting guide catheter or aspiration catheter or the maximum of allowed retrieval passes [3–6] varied between trials. From a technical perspective, it remains unclear which concept yields the highest rate of first-pass complete reperfusion. The inherent design of the stent-retriever itself did not influence reperfusion rates in the MR CLEAN trial [18].
The proposed approach for clot retrieval combines a distally placed stent retriever and a proximally placed aspiration catheter which act as a unit, providing a distal (stent retriever) and proximal capture of the clot (aspiration catheter) while being withdrawn simultaneously under continuous proximal aspiration into the cervical guide catheter. The addition of other techniques/steps (active push deployment for better clot caption, bare wire technique for maximizing flow inside the aspiration catheter, grapple hook technique for time saving) resulted in an excellent overall rate of successful reperfusion (mTICI ≥ 2b) of 100% and an exceptionally high rate of first-pass complete reperfusion (mTICI 3) of 72%. These preliminary data are promising compared to previous MT techniques reported in the literature: stent retriever thrombectomy trials have shown core laboratory controlled reperfusion rates (mTICI ≥ 2b) of up to 88% [1–5] with first-pass mTICI 3 success up to 25% while self-reported large series have reported reperfusion rates of up to 94% [15, 19–23]. The use of an extracranial BGC in combination with a stent retriever reveals overall mTICI ≥ 2b success in 89% with a first-pass thrombectomy rate of 63.7% [24]. The use of an intracranial large-bore aspiration catheter (ADAPT) which is placed at the face of the thrombus yields successful reperfusion only in part of the treated patients [14, 15]. The self-reported rate of first-pass mTICI 3 is as high as 64%, but only for a subgroup of patients where ADAPT alone was feasible, resulting in 35% first-pass mTICI 3 overall [15]. This technique was initially described by Turk et al. who revealed an overall successful reperfusion rate (mTICI ≥ 2b) of 75% [9]. The prospective randomized THERAPY trial identified patients with poor prognosis due to a clot burden of >8 mm and revealed a successful reperfusion rate of 70% when using an aspiration catheter alone [25]. Withdrawal of the stent retriever into an intracranial aspiration catheter (Solumbra technique) achieves successful reperfusion (mTICI ≥ 2b) up to 88% with a one-pass thrombectomy rate of 37% [8]. The first-pass reperfusion rate using the active push deployment or push and fluff technique with the Trevo stent retriever described by Haussen et al. was 54% [26]. In summary, there are to date no reports in the literature demonstrating such high first-pass mTICI 3 results, which are known to be a premise of favorable clinical outcome (Table 3; [19]). The time of reperfusion from groin puncture (44.5 min) lies within the range of previously reported series in which stent retrievers were used (20–120 min) [16, 24, 27–29] and is superior compared to the technique with a BGC (120 min) or non-BGC (161 min) published in the North American Solitaire Acute Stroke registry [16]. In the study of Velasco et al. reperfusion time with BGC was 20.5 min vs. 41 min in the non-BGC group, but the time interval is not comparable to the widely used groin puncture to reperfusion time, due to the definition of the first carotid angiogram as the starting time point [24]. The ADAPT technique achieves the fastest reperfusion times with a median time between 20 and 30 min from groin puncture [9, 10, 15]; however, the rate of successful reperfusion is inferior compared to SAVE. As we included consecutive cases, including the first SAVE cases treated in each institution, we expect procedural times to further shorten in the future.
Withdrawal of the stent retriever within the aspiration catheter has been described previously, but the essential point of our technique is that, contrary to previous approaches, where the stent was fully retrieved inside the aspiration catheter, we wedge the thrombus between aspiration catheter tip and stent retriever based on the idea of increasing clot entrapment while simultaneously maintaining local aspiration (Fig. 2; [8]). Similar to our technique, Massari et al. described an Aspiration Retriever Technique for Stroke (ARTS) which was initially used as a bail out method in cases of failed ADAPT attempts and is based in a more thrombus-centered implantation of the stent retriever [13]. This technique exhibited a similar overall successful reperfusion rate of 97.6%; however, their first-pass favorable reperfusion rate, defined as mTICI 2b/3, in 43% of their patients was clearly inferior to our results of 72% first-pass mTICI 3 efficacy. Furthermore, their reported mean time from groin puncture to reperfusion was longer (65 min vs. 45 min). Both ARTS and a recently published similar technique called continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE) do not include proximal aspiration with the guide catheter/sheath in their described steps. This leads to reduced control of clot fragments during the critical moments of clot immobilization as the aspiration catheter becomes clogged with clot material and the propagated continuous aspiration is just a continuous negative pressure inside the aspiration catheter [34]. The ARTS procedure tries to control clot fragments with the frequent use of a BGC but the absence of proximal aspiration means collateral flow from the A1 segment cannot be reduced (Fig. 2b). Altogether, the combination of BGC and large-bore aspiration catheter is very problematic as the Sofia Plus (Microvention) catheter is not compatible with BGCs (residual radius of 0 mm with a 9 F BGC) and the newly introduced ACE 68 (Penumbra) has a very small radius difference with all BGCs (Table 4). This means that even with proximal aspiration on the BGC flow reversal in the ICA is minimal, leading to antegrade flow in the M1 segment depending on the Acom and Pcom configuration. Additionally, the authors of CAPTIVE describe Solumbra as an incorrect technique because “as the embolus is withdrawn into the distal catheter, small fragments may break off and embolize to the distal territory” but omit to say that the same risk applies to the moments of the unit embolus/stent retriever/aspiration catheter being withdrawn inside the guide catheter/sheath. The aspiration catheter cannot control thrombus fragments in this position as it is clogged with the clot. SAVE addresses both issues (fragments during initial clot immobilization and during unit retrieval inside the guide) with the inclusion of distal stent retrieval placement and of proximal/continuous aspiration with a large-bore guide catheter or sheath.
In the present cohort, clinical outcome was favorable (mRS ≤ 2) in 59% of patients at discharge which is in the upper range compared with results published to date [10, 14, 15, 27, 32]. One patient (3%) presenting with unknown symptom onset died due to the occurrence of sICH after MT. This was possibly related to vulnerable brain tissue (baseline ASPECT score of 5) and pre-existing antiplatelet medication. The data from RCTs support this assumption, as these are known risk factors of postinterventional sICH [1–5]. There were two deaths from pneumonia, both in octogenarians with pre-existing chronic cardiac failure and renal impairment, which are also known to increase the risk of postinterventional morbidity and mortality [35–37]. A possible explanation for the high percentage of good clinical outcome in our study could be the absence of ENT. Up to 6% of ENT were described in the MR CLEAN and EXTEND IA trials or when using the ADAPT technique [10, 15]. In our study, no ENT was observed, albeit admittedly our sample size is too small to prove superiority and our data lacks core laboratory control. A probable explanation to the absence of ENT might be the aforementioned technical features: SAVE provides distal as well as proximal protection from clot migration as the stent retriever and aspiration catheter are removed as a unit under additional continuous aspiration with the guide catheter, reinforcing complete removal of the thrombus. Additionally, the distal implantation of the stent retriever in SAVE (Fig. 1b), compared to the more thrombus-centered implantation in similar techniques like the Solumbra or the recently described ARTS, probably prevents fragments from migrating distally and occluding downstream vessels (Fig. 1h). Data by Chueh et al. support the first assumption as their risk of distal embolization was dependent on the catheterization technique: the Solumbra technique seemed to be the most efficient method for reducing hard fragment-prone clots in contrast to direct aspiration, which increased the risk for soft elastic clot fragmentation [38, 39].
The high rate of first-pass reperfusion confirmed our hypothesis, but there might also be drawbacks including the loss of aspiration catheter access within the distal cerebral vasculature after removing the coaxial system, resulting in further catheter navigation if a second pass is necessary. This issue affects other techniques as well, e. g. ADAPT or a standard BGC and stent retriever approach [10, 14, 15, 19, 24]. On the other hand, the overall time from groin puncture to reperfusion was short in our cohort due to very high rates of complete first-pass reperfusion. Tandem occlusions comprising a preceding extracranial occlusion/stenosis were not included in our study. Theoretically, the same limitation applies to SAVE as to the standard BGC plus stent retriever approach: as the stent retriever has direct contact with the vessel walls in both techniques during retrieval it should not be withdrawn through an acutely placed extracranial stent as this may cause entanglement of the stent struts. This problem can be solved by executing angioplasty of the proximal stenosis initially with a balloon, then performing thrombectomy intracranially with SAVE and finally placing an extracranial stent if necessary or by advancing the guide catheter distally to the carotid stent.
A major limitation of this study is the retrospective design with the attendant selection bias. The small sample size precludes profound statistical analysis; therefore, larger scale prospective studies are needed. Evaluation of clinical outcome has to be interpreted with caution because of the missing 90-day follow-up; however, the study’s primary focus was on the angiographic reperfusion result, an undisputed prerequisite of favorable clinical outcome. We further included occlusions both in the anterior and posterior circulation. Angiographic data were self-reported and may be less favorable after core laboratory adjudication.
In our opinion, the SAVE technique represents a superior technical approach in MT for patients with LVO in terms of first-pass efficacy. Preliminarily, it also seems to translate into a high rate of favorable clinical outcome. All three participating centers have therefore switched to SAVE as their primary endovascular approach in MT. A prospective, multicenter, core laboratory controlled study is warranted.
Conclusion
The SAVE technique is fast and appears to be very effective in terms of first-pass complete reperfusion in patients with LVO.
References
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t‑PA vs. t‑PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306.
Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–73.
Deshaies EM. Tri-axial system using the solitaire-FR and penumbra aspiration microcatheter for acute mechanical thrombectomy. J Clin Neurosci. 2013;20:1303–5.
Humphries W, Hoit D, Doss VT, Elijovich L, Frei D, Loy D, Dooley G, Turk AS, Chaudry I, Turner R, Mocco J, Morone P, Fiorella D, Siddiqui A, Mokin M, Arthur AS. Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg. 2015;7:90–4.
Turk AS, Spiotta A, Frei D, Mocco J, Baxter B, Fiorella D, Siddiqui A, Mokin M, Dewan M, Woo H, Turner R, Hawk H, Miranpuri A, Chaudry I. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg. 2014;6:231–7.
Kabbasch C, Möhlenbruch M, Stampfl S, Mpotsaris A, Behme D, Liebig T. First-line lesional aspiration in acute stroke thrombectomy using a novel intermediate catheter: Initial experiences with the SOFIA. Interv Neuroradiol. 2016;22:333–9.
Wiesmann M, Brockmann MA, Heringer S, Müller M, Reich A, Nikoubashman O. Active push deployment technique improves stent/vessel-wall interaction in endovascular treatment of acute stroke with stent retrievers. J Neurointerv Surg. 2016 Mar 14. [Epub ahead of print]
Nikoubashman O, Alt JP, Nikoubashman A, Büsen M, Heringer S, Brockmann C, Brockmann MA, Müller M, Reich A, Wiesmann M. Optimizing endovascular stroke treatment: removing the microcatheter before clot retrieval with stent-retrievers increases aspiration flow. J Neurointerv Surg. 2016 Apr 15. [Epub ahead of print]
Massari F, Henninger N, Lozano JD, Patel A, Kuhn AL, Howk M, Perras M, Brooks C, Gounis MJ, Kan P, Wakhloo AK, Puri AS. ARTS (Aspiration-Retriever Technique for Stroke): Initial clinical experience. Interv Neuroradiol. 2016;22:325–32.
Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, Spiotta A, Mokin M, Dewan M, Quarfordt S, Battenhouse H, Turner R, Chaudry I. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014;6:260–4.
Kowoll A, Weber A, Mpotsaris A, Behme D, Weber W. Direct aspiration first pass technique for the treatment of acute ischemic stroke: initial experience at a European stroke center. J Neurointerv Surg. 2016;8:230–4.
Nguyen TN, Malisch T, Castonguay AC, Gupta R, Sun CH, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Linfante I, Dabus G, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Taqi M, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Abou-Chebl A, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Masoud H, Nogueira RG, Norbash AM, Zaidat OO. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke. 2014;45:141–5.
Lee JS, Hong JM, Lee SJ, Joo IS, Lim YC, Kim SY. The combined use of mechanical thrombectomy devices is feasible for treating acute carotid terminus occlusion. Acta Neurochir (Wien). 2013;155:635–41.
Dippel DW, Majoie CB, Roos YB, van der Lugt A, van Oostenbrugge RJ, van Zwam WH, Lingsma HF, Koudstaal PJ, Treurniet KM, van den Berg LA, Beumer D, Fransen PS, Berkhemer OA; MR CLEAN Investigators. Influence of device choice on the effect of intra-arterial treatment for acute Ischemic stroke in MR CLEAN (Multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands). Stroke. 2016;47:2574–81.
Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin C, Holloway WE, Mueller-Kronast N, English JD, Linfante I, Dabus G, Malisch TW, Marden FA, Bozorgchami H, Xavier A, Rai AT, Froehler MT, Badruddin A, Nguyen TN, Taqi MA, Abraham MG, Janardhan V, Shaltoni H, Novakovic R, Yoo AJ, Abou-Chebl A, Chen PR, Britz GW, Kaushal R, Nanda A, Issa MA, Nogueira RG. North American Solitaire Stent Retriever Acute Stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg. 2014;6:584–8.
Delgado Almandoz JE, Kayan Y, Young ML, Fease JL, Scholz JM, Milner AM, Hehr TH, Roohani P, Mulder M, Tarrel RM. Comparison of clinical outcomes in patients with acute ischemic strokes treated with mechanical thrombectomy using either Solumbra or ADAPT techniques. J Neurointerv Surg. 2015;8:1123–8.
Turk AS, Turner R, Spiotta A, Vargas J, Holmstedt C, Ozark S, Chalela J, Turan T, Adams R, Jauch EC, Battenhouse H, Whitsitt B, Wain M, Chaudry MI. Comparison of endovascular treatment approaches for acute ischemic stroke: cost effectiveness, technical success, and clinical outcomes. J Neurointerv Surg. 2015;7:666–70.
Vargas J, Spiotta A, Fargen K, Turner R, Chaudry I, Turk A. Long term experience using the ADAPT technique for the treatment of acute ischemic stroke. J Neurointerv Surg. 2016 Apr 18. [Epub ahead of print]
Möhlenbruch MA, Kabbasch C, Kowoll A, Broussalis E, Sonnberger M, Müller M, Wiesmann M, Trenkler J, Killer-Oberpfalzer M, Weber W, Mpotsaris A, Bendszus M, Stampfl S. Multicenter experience with the new SOFIA Plus catheter as a primary local aspiration catheter for acute stroke thrombectomy. J Neurointerv Surg. 2016 Dec 20. [Epub ahead of print]
Velasco A, Buerke B, Stracke CP, Berkemeyer S, Mosimann PJ, Schwindt W, Alcázar P, Cnyrim C, Niederstadt T, Chapot R, Heindel W. Comparison of a balloon guide catheter and a non-balloon guide catheter for mechanical thrombectomy. Radiology. 2016;280:169–76.
Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, Lopes D, Frei D, Shownkeen H, Budzik R, Ajani ZA, Grossman A, Altschul D, McDougall C, Blake L, Fitzsimmons BF, Yavagal D, Terry J, Farkas J, Lee SK, Baxter B, Wiesmann M, Knauth M, Heck D, Hussain S, Chiu D, Alexander MJ, Malisch T, Kirmani J, Miskolczi L, Khatri P; THERAPY Trial Investigators*. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–8.
Haussen DC, Rebello LC, Nogueira RG. Optimizating clot retrieval in acute stroke: the push and fluff technique for closed-cell stentrievers. Stroke. 2015;46:2838–42.
Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS; TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40.
Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, Liebeskind DS, Nogueira RG, Arnold M, Sztajzel R, Liebig T, Goyal M, Besselmann M, Moreno A, Schroth G. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44:2802–7.
Almekhlafi MA, Menon BK, Freiheit EA, Demchuk AM, Goyal M. A meta-analysis of observational intra-arterial stroke therapy studies using the Merci device, Penumbra system, and retrievable stents. AJNR Am J Neuroradiol. 2013;34:140–5.
Bracard S, Ducrocq X, Mas JL et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016;15:1138–47.
Broderick JP, Palesch YY, Demchuk AM et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893–903.
Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO; SWIFT Trialists. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9.
Singer OC, Berkefeld J, Nolte CH et al. Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. ANN NEUROL 2015;77:415–424.
McTaggart RA, Tung EL, Yaghi S, Cutting SM, Hemendinger M, Gale HI, Baird GL, Haas RA, Jayaraman MV. Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J Neurointerv Surg. 2016 Dec 16. [Epub ahead of print]
Brinjikji W, Rabinstein AA, Kallmes DF, Cloft HJ. Patient outcomes with endovascular embolectomy therapy for acute ischemic stroke: a study of the national inpatient sample: 2006 to 2008. Stroke. 2011;42:1648–52.
Zhu W, Xiao L, Lin M, Liu X, Yan B. Large-vessel occlusion is associated with poor outcome in stroke patients aged 80 years or older who underwent intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2016;25:2712–6.
Sauer EM, Sauer R, Kallmunzer B, Blinzler C, Breuer L, Huttner HB, Schwab S, Köhrmann M. Impaired renal function in stroke patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2014;23:1225–8.
Chueh JY, Puri AS, Wakhloo AK, Gounis MJ. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg. 2016;8:197–202.
Chueh JY, Kühn AL, Puri AS, Wilson SD, Wakhloo AK, Gounis MJ. Reduction in distal emboli with proximal flow control during mechanical thrombectomy: a quantitative in vitro study. Stroke. 2013;44:1396–401.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
V. Maus reports minor travel grants from Stryker Neurovascular. D. Behme reports minor travel grants from Stryker Neurovascular. C. Kabbasch reports minor travel grants from Stryker Neurovascular. J. Borggrefe has nothing to disclose. I. Tsogkas has nothing to disclose. O. Nikoubashman has nothing to disclose. M. Wiesmann reports grants and personal fees from Stryker Neurovascular, non-financial support from Covidien, non-financial support from Penumbra, non-financial support from Microvention/Terumo. M. Knauth has nothing to disclose. A. Mpotsaris reports personal fees for consultancy from Neuravi and Penumbra. M.N. Psychogios has nothing to disclose.
Ethical standards
According to the guidelines of the respective local ethics committees, no approval was necessary for this anonymous study, which was conducted in accordance to the Declaration of Helsinki (in its current revised form). Due to the retrospective nature of the study informed consent from participants was deemed unnecessary. Consent was obtained from any patients identifiable from images or other information in the manuscript.
Additional information
A. Mpotsaris and M.N. Psychogios contributed equally.
Caption Electronic Supplementary Material
Illustrative video case of SAVE technique
Rights and permissions
About this article
Cite this article
Maus, V., Behme, D., Kabbasch, C. et al. Maximizing First-Pass Complete Reperfusion with SAVE. Clin Neuroradiol 28, 327–338 (2018). https://doi.org/10.1007/s00062-017-0566-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-017-0566-z