Abstract
Background
Current genetic association studies have reported conflicting results regarding the association between miRNA polymorphisms and myocardial infarction (MI) risk
Methods
Relevant studies were retrieved from the PubMed, EMBASE, ISI Web of Science, and Scopus databases. Eligible studies determining the association between miRNA polymorphisms and MI susceptibility were included and a meta-analysis was performed to quantify the associations between miRNA polymorphisms and MI risk.
Results
A total of eight studies with 2507 MI patients and 3796 healthy controls were included, dealing with nine miRNA genes containing 11 different loci, including miR-149 (rs71428439 and rs2292832), miR-126 (rs4636297 and rs1140713), miR-146a (rs2910164), miR-218 (rs11134527), miR-196a2 (rs11614913), miR-499 (rs3746444), miR-27a (rs895819), miR-26a‑1 (rs7372209), and miR-100 (rs1834306). miR-146a rs2910164 and miR-499 rs3746444 were determined to have a significant association with MI susceptibility, a finding that was supported by the meta-analysis (rs2910164: GG/CC, odds ratio [OR]: 1.40, 95% confidence interval [95% CI]: 1.05–1.74, p < 0.001; rs3746444: AA + AG/GG, OR = 2.04, 95% CI: 1.37–2.70, p < 0.001). Limited or conflicting data were found for the relationship between the other miRNA polymorphisms (rs71428439, rs4636297, rs1140713, rs11134527, rs11614913, rs895819, rs7372209, rs1834306, rs2292832) and MI risk.
Conclusion
There was a significant association between rs2910164 and rs3746444 and MI susceptibility. Further studies are required to investigate the role of miRNA polymorphisms in MI risk.
Zusammenfassung
Hintergrund
In aktuellen genetischen Assoziationsstudien wurde von widersprüchlichen Ergebnissen hinsichtlich der Assoziation zwischen Polymorphismen der Mikro-RNA (miRNA) und dem Risiko für einen Myokardinfarkt (MI) berichtet.
Methoden
Relevante Studien aus den Datenbanken PubMed, EMBASE, ISI Web of Science und Scopus wurden erfasst. In die Auswertung wurden geeignete Studien zur Bestimmung der Assoziation zwischen miRNA-Polymorphismen und der MI-Anfälligkeit einbezogen und eine Metaanalyse durchgeführt, um die Zusammenhänge zwischen miRNA-Polymorphismen und dem MI-Risiko zu quantifizieren.
Ergebnisse
Es wurden 8 Studien mit 2507 MI-Patienten und 3796 gesunden Kontrollen ausgewertet, dabei ging es um 9 miRNA-Gene mit 11 verschiedenen Loci, einschließlich miR-149 (rs71428439 und rs2292832), miR-126 (rs4636297 und rs1140713), miR-146a (rs2910164), miR-218 (rs11134527), miR-196a2 (rs11614913), miR-499 (rs3746444), miR-27a (rs895819), miR-26a‑1 (rs7372209) und miR-100 (rs1834306). Für miR-146a rs2910164 und miR-499 rs3746444 wurde festgestellt, dass ein signifikanter Zusammenhang mit der MI-Anfälligkeit bestand, ein Ergebnis, dass durch die Metaanalyse gestützt wurde (rs2910164: GG/CC, Odds Ratio [OR]: 1,40; 95%-Konfidenzintervall [95%-KI]: 1,05–1,74; p < 0,001; rs3746444: AA + AG/GG, OR = 2,04; 95%-KI: 1,37–2,70; p < 0,001). Eingeschränkte oder widersprüchliche Daten wurden für den Zusammenhang zwischen den anderen miRNA-Polymorphismen (rs71428439, rs4636297, rs1140713, rs11134527, rs11614913, rs895819, rs7372209, rs1834306, rs2292832) und dem MI-Risiko festgestellt.
Schlussfolgerung
Es bestand eine signifikante Assoziation zwischen rs2910164 und rs3746444 sowie der MI-Anfälligkeit. Weitere Studien sind erforderlich, um die Bedeutung der miRNA-Polymorphismen für das MI-Risiko zu untersuchen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Myocardial infarction (MI) is one of the most serve forms of coronary artery disease and a leading cause of death and disability worldwide [1]. Several risk factors have been confirmed to contribute to the susceptibility and progression of MI, including hypertension [2], diabetes [3], smoking, alcohol intake, hyperlipidemia, and overweight [4]. However, these modifiable factors cannot entirely explain the overall incidence of MI, and therefore it is necessary to investigate potential susceptibility factors that promote MI development, in particular hereditary factors [5]. Increasing evidence highlighted that complex interactions between susceptibility genes and environmental risk factors contribute to the occurrence of MI. In this context, an individual’s single-nucleotide polymorphisms (SNPs), as the most common genetic variations, are attracting increasing attention [6, 7].
MicroRNA (miRNA) is a highly conserved class of small noncoding RNA with a length of 18 ~ 25 nucleotides that regulates gene expression at the post-transcriptional level [8, 9]. Typically, miRNA functions as a suppressor by binding the 3′ untranslated regions (3′ UTRs) of mRNA targets and results in mRNA cleavage and translational repression [10]. It has been suggested that miRNAs directly or indirectly participate in a variety of biological processes and are closely correlated with diverse human diseases, including cancer [11], Alzheimer’s disease [12], and cardiovascular diseases [13]. Recently, evidence has accumulated that miRNAs play a crucial role in the regulation of fundamental biological processes of MI, including proliferation [14], differentiation [15], and apoptosis [16]. Primary miRNAs (pri-miRNAs) are first transcripted by RNA polymerase II and then processed into miRNA precursors (pre-miRNAs). Ultimately, the pre-miRNAs are exported out of the nucleus and processed into mature miRNAs [17]. Previous studies demonstrated that SNPs located on pri-miRNAs, pre-miRNAs, or miRNAs might influence the binding capacity of miRNAs to target genes [18, 19], which eventually leads to abnormal expression of target genes.
Due to the alteration of gene expression caused by functional SNPs in miRNAs, the associations between SNPs in miRNAs and disease susceptibility have been widely investigated. Previous studies have demonstrated that SNPs in miRNAs were correlated with susceptibility to various diseases, including carcinoma [20] and psoriasis [21], among others. Several studies revealed that miRNA polymorphisms were also associated with MI susceptibility [22]. Considering the small sample size of these dependent studies and the inconsistency of previous results, we found it necessary to conduct a meta-analysis to explore the associations between miRNA SNPs and MI risk.
The present study collected data from the current body of evidence to identify miRNA SNPs that could alter the risk of MI. Eligible studies reporting on the associations between miRNA polymorphisms and MI susceptibility were retrieved form public databases. The data in the included studies were extracted and a meta-analysis was performed to estimate the associations between miRNA polymorphisms and MI susceptibility. Sensitivity analysis was carried out to assess the robustness of the meta-analysis results, and publication bias was also evaluated.
Methods
Search strategy
A comprehensive literature search was carried out systematically on four online databases, including PubMed, EMBASE, ISI Web of Science, and Scopus, from inception to March 18, 2020. The following terms were used: (“Myocardial infarction” or “myocardial infarct” or “heart infarction” or “cardiovascular stroke”) and (miRNA or microRNA or pre-mir or miR) and (“Single nucleotide polymorphism” or SNP or variant or variation or polymorphism or mutation or locus). In addition, the reference lists of the retrieved articles were manually examined for eligible studies. This process was accomplished independently by two investigators. All analyses were performed based on prior published articles. Therefore, patient consent or ethical approval were not necessary.
Inclusion and exclusion criteria
Eligibility criteria included the following: (1) case–control studies focused on the associations between miRNA polymorphisms and MI risk; (2) studies conducted with human participants; (3) the data provided in the articles concerning genotype frequencies should be sufficient to estimate odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) in both case and control groups; and (4) studies with MI patients were allocated into a subgroup under cases with detailed information.
Case reports, letters, reviews, editorials, and article comments were excluded.
Data extraction and quality assessment
Two researchers independently extracted the essential information, including first author, year of publication, study region, ethnicity, sample size, number of cases and controls, and genotype frequency in cases and controls for each miRNA polymorphism. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to evaluate the quality of the included studies, and a score of ≥ 6 was considered as high quality. Studies with low quality (score < 6) were excluded from the final analysis. Any uncertainties about the data set or about the quality assessment were evaluated by a senior author.
Statistical analysis
Hardy–Weinberg equilibrium (HWE) for each study was determined with the chi-square test. The correlations between miRNA polymorphisms and MI risk were measured using crude OR accompanied by 95% CI. The “best-evidence” synthesis method in previous studies was used to assess the association between miRNA polymorphisms and MI risk [23]. The evidence was defined as “generally consistent” if ≥ 75% of the included studies/cohorts reported consistent results. Strong evidence was defined as two or more studies with low risk of bias and “generally consistent”; moderate evidence was defined as one study with low risk of bias, or ≥ 2 studies with a high risk of bias and “generally consistent”; limited evidence was defined as one study with low risk of bias or > 2 studies with a high risk of bias, and “generally consistent”; insufficient evidence was defined as a finding in one study with a high risk of bias. If < 75% studies reported consistent findings, then that evidence was defined as conflicting evidence.
Furthermore, a meta-analysis approach was employed for a better understanding of the association between miRNA polymorphisms and MI risk. The best-fitting genetic model was confirmed by a model-free approach to avoid an inflated false-positive error [24, 25]. If A variant was the polymorphism of interest that could potentially alter the MI risk, then OR1, OR2, and OR3 were calculated for genotypes AA vs. aa, Aa vs. aa, and AA vs. Aa for each polymorphism to capture the magnitude of genetic effect and to identify the most appropriate genetic model. The most plausible genetic model used for meta-analysis was confirmed according to the relationships between the three pairwise comparisons as follows:
-
1.
Recessive model: if OR1 = OR3 ≠ 1 and OR2 = 1
-
2.
Dominant model: if OR1 = OR2 ≠ 1 and OR3 = 1
-
3.
Complete over-dominant model: if OR1 = 1, OR2 = 1 / OR3 ≠ 1
-
4.
Co-dominant model: if OR1 > OR2 > 1 and OR1 > OR3 > 1, or OR1 < OR2 < 1 and OR1 < OR3 < 1
The meta-analysis was performed using STATA/SE 15 software (Stata Corporation, College Station, TX, USA) based on the genetic model that was confirmed by the model-free approach. Heterogeneity among the included studies was measured using the Q statistical test and I2 test [26]. The fixed-effect model was used if I2 > 50% and p > 0.05. Otherwise, the random-effect model was used [27]. Sensitivity analysis was carried out by omitting one study in turn to test the robustness of the association between miRNA polymorphisms and MI risk. Egger regression and Begg rank correlation tests were run to assess the publication bias. The research methodology is shown in Fig. 1.
Results
Included studies and main characteristics
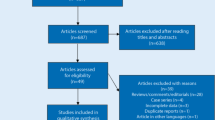
The literature selection process is detailed in Fig. 2. The initial search of four online databases yielded 765 potentially relevant records (331 from Web of Science, 211 from EMBASE, 173 from Scopus, and 50 from PubMed). After the first screening, 298 duplicated records were excluded. Of the remaining 467 records, a further 459 studies were eliminated upon screening titles and abstracts. The other nine articles [28,29,30,31,32,33,34,35,36] were considered eligible for full-text review, and one article was removed due to the lack of available data. Ultimately, eight studies [28,29,30,31,32,33,34,35] were incorporated into the qualitative synthesis and five studies were further included in the meta-analysis.
The major characteristics of the included studies are summarized in Table 1. Eight case-control studies comprising 2507 MI patients and 3796 healthy controls were included. All articles were published between 2013 and 2020. The sample size of the individual studies ranged from 80 to 1109. Nine miRNAs containing 11 different loci were included in our study, including miR-149 (rs71428439 and rs2292832), miR-126 (rs4636297 and rs1140713), miR-146a (rs2910164), miR-218 (rs11134527), miR-196a2 (rs11614913), miR-499 (rs3746444), miR-27a (rs895819), miR-26a‑1 (rs7372209), and miR-100 (rs1834306). Additionally, all included studies were considered to be of high quality because the NOS scores were greater than 6 for each study (Table 2). According to the “best-evidence” synthesis method, these SNPs were divided into groups of strong evidence, limited evidence, and conflicting evidence.
SNPs with strong evidence
Strong evidence was found for the association between rs3746444 polymorphism in miR-499 and MI risk. Genotype AA appeared to significantly increase the MI risk under the homozygous model (AA/GG) in two individual cohorts from two studies [33, 34]. The results of each cohort were OR: 1.873, 95% CI: 1.238–2.834, p = 0.003; OR: 2.629, 95% CI: 1.274–5.424, p = 0.008; and OR: 4.6, 95% CI: 2.22–9.84, p < 0.001, respectively. Significant associations were observed under the recessive model (AA/AG + GG, OR: 1.874, 95% CI: 1.083–3.224; p = 0.032) and the dominant model (AA + AG/GG, OR: 2.048, 95% CI: 1.074–3.902, p = 0.035; [28]). Statistical analysis suggested that individuals carrying allele A of rs3746444 had a significant increase in MI risk [28, 33]. However, these associations were not validated in another cohort [29].
In addition, the rs11134527 polymorphism in MiR-218 was examined by two studies [32, 34], and the results from each cohort were: OR: 0.897, 95% CI:0.73–1.102, p = 0.301 and OR: 1.27, 95% CI: 0.87–1.85, p = 0.213 for the homozygous model (AA/GG), respectively. Nonsignificant associations were observed under the homozygous model (AA/GG) in each cohort (cohort 1: 1.108, 0.846–1.45; cohort 2: 1.08, 0.65–1.8). Moreover, the associations between rs11134527 and MI risk under the recessive model (AA/AG + GG, OR: 1.08, 95% CI:0.69–1.7; p = 0.744) and dominant model (AA + AG/GG, OR: 1.22, 95% CI: 0.85–1.76, p = 0.273) were also not significant.
SNPs with limited evidence
Limited evidence was found for the association between the rs71428439 polymorphism in miR-149 and MI risk. Statistical analysis suggested that genotype AA significantly increased MI risk under the homozygous model (AA/GG, OR: 2.323, 95% CI: 1.432–3.77, p = 0.001), and the risk effect of genotype CC was detected under the recessive model (AA/AG + GG, OR: 1.635, 95% CI: 1.165–2.293, p = 0.004) and the dominant model (AA + AG/GG, OR: 1.922, 95% CI: 1.235–2.993; [35]). However, Chen et al. reported that rs71428439 was not linked to MI risk [34]. Two loci (rs4636297 and rs1140713) in the miR-126 gene were sequenced from 350 patients with MI and 350 healthy controls [30]. Statistical analysis suggested that individuals carrying allele A of rs4636297 had a significant decrease in MI risk by 21% (OR: 0.79, 95% CI: 0.65–0.93; p = 0.004). Carriers of genotype GG had a significantly lower risk (72%) of MI (OR: 0.28; 95% CI: 0.09–0.83; p = 0.017). However, Cai et al. [28] reported that rs4636297 in miR-126 was not associated with MI risk among five genetic models.
Additionally, only one study with a low risk of bias reported associations between rs895819, rs7372209, rs1834306, rs2292832 and MI risk. Cai et al. reported that genotype GG of rs895819 acted as a protective factor against MI risk (GG/AA, OR: 0.4, 95% CI: 0.22–0.75, p = 0.004; [32]). No significant association was observed between genotype distribution and allelic frequencies for rs7372209 (C/T: 0.98, 0.74–1.29, p = 0.879; TT/CT: 1.26, 0.58–2.76, p = 0.556; CC/CT + TT: 1.01, 0.72–1.42, p = 0.947; TT/CT + CC: 1.23, 0.58–2.61, p = 0.585), rs1834306 (C/T: 0.97, 0.77–1.23, p = 0.816; TT/CT: 1.28, 0.8–2.05, p = 0.303; CC/CT + TT: 1.22, 0.78–1.9, p = 0.391; TT/CT + CC:1.06, 0.75–1.51, p = 0.733), and rs2292832 (C/T:1.062, 0.7237–1.560, p = 0.769; CC/CT + TT: 1.031, 0.611–1.738, p = 1.000; TT/CT + CC: 1.181, 0.564–2.474, p = 0.700).
SNPs with conflicting evidence
Conflicting evidence was found for the association of the rs2910164 polymorphism with the risk of MI. Chen et al. found that genotype CC acted as a protective factor against MI risk (GG/CC, OR: 1.368, 95% CI: 1.044–1.791, p = 0.023; [34]), but the association was not validated in other cohorts [28, 29]. In addition, conflicting evidence was also found for the association between the rs11614913 polymorphism in miR-196a2 and MI risk. The rs11614913 polymorphism in miR-196a2 was reported by five individual cohorts from four studies. Osmak et al. [31] found a significant association between rs11614913 and MI risk in their two cohorts. Genotype TT appeared to significantly increase the risk of MI under the homozygous model. The results for each cohort were CC/TT: OR: 0.57, 95% CI 0.34–96; p = 0.023 and TT/CC: OR: 1.43, 95% CI 1.00–2.07; p = 0.036, respectively. A risk effect of genotype TT was detected under the recessive model: CC + TC/TT, OR: 0.67, 95% CI 0.47–0.95; p = 0.025 [29]. Nevertheless, these associations were not validated by Chen et al. [34]. Contrasting the findings by Osmak et al. [31], Agiannitopoulos et al. [28] reported that individuals carrying allele C of rs11614913 had a significant increase in MI risk by 68.9% (OR: 1.689, 95% CI: 1.147–2.487; p = 0.008).
Meta-analysis results
In addition to the “best-evidence” synthesis method, a meta-analysis was carried out to quantify the association between miRNA polymorphisms and MI risk. Before combining data from each individual study, it was assumed that a genetic model of inheritance was not made. A model-free approach was used to dictate the best-fitting genetic model for the meta-analysis, to avoid an inflated type I error rate. Three polymorphisms, rs3746444 in miR-499, rs11614913 in miR-192a2, and rs2910164 in miR-146a, were included in the meta-analysis, as these were evaluated in not less than three studies and thereby enabled a meta-analysis to be performed.
To determine the association between rs11614913 in miR-196a2 and MI risk, a model-free approach was utilized. First, OR1, OR2, and OR3 were calculated for genotypes CC vs. TT, CC vs. TC, and TC vs. TT for rs11614913 (Table 3). The results showed that there were no significant associations between rs11614913 in miR-196a2 and MI risk (OR1: 0.867, 95% CI: 0.733–1.027, p = 0.098; OR2: 0.911, 95% CI: 0.783–1.059, p = 0.225; OR3: 0.952, 95% CI: 0.822–1.103, p = 0.513), and indicated that rs11614913 alters the risk of MI, which were consistent with “best-evidence” synthesis results.
For the association between rs3746444 in miR-499 and MI risk, OR1 (2.321, 95% CI: 1.767–3.048, p < 0.001) and OR2 (2.13, 95% CI: 1.595–2.84, p < 0.001) were statistically significant while OR3 (1.089, 95% CI: 0.932–1.273, p = 0.28) was not, suggesting that the dominant model (AA + AG/GG) could be the best-matching genetic model for meta-analysis. In the dominant model, the data showed that the AA + AG genotype was significantly associated with an increased MI risk, compared with the GG genotype (OR = 2.04, 95% CI: 1.37–2.70, p < 0.001). The fixed-effect model was employed for data combination due to the statistically significant heterogeneity between the studies (I2 = 0.0%, p = 0.597; Fig. 3). The Egger test (t = 0.24, p = 0.852) and the Begg test (z = 1.04, p = 0.296) suggested the absence of statistically significant publication bias.
For the association between rs2910164 in miRNA-146a and MI risk, the co-dominant model was deemed as the most plausible genetic model for meta-analysis because OR1 was 1.374 (95% CI: 1.125–1.678, p = 0.002) and was statistically significant, whereas OR2 at 0.932 (95% CI: 0.786–1.106, p = 0.421) and OR3 at 1.073 (95% CI:0.904–1.273, p = 0.421) were not. The pooled data suggested that there was no association between the GG genotype of rs3746444 and the risk of MI compared with the CC genotype (OR: 1.40, 95% CI: 1.05–1.74, p < 0.001) in a fixed-effect model (I2 = 0.0%, p = 0.478; Fig. 4). The Egger test (t = 1.52, p = 0.370) and Begg test (z = 1.57, p = 0.117) results suggested the absence of statistically significant publication bias.
Discussion
We summarized the available literature from eight studies and presented an analysis of the associations between genetic polymorphisms reported in several miRNA genes and the risk of MI. Although several miRNA polymorphisms were accessed for a possible association with the risk of MI, there was a lack of sufficient data regarding most of these polymorphisms and only three polymorphisms (rs11614913, rs3746444, and rs2910164) were evaluated in the meta-analysis. Among these included SNPs, strong evidence suggested that rs3746444 in miR-499 significantly correlated with MI risk, which was further supported by meta-analysis with a recessive model. In addition, the association between rs2910164 in miR-16a and MI risk was also confirmed by the meta-analysis. However, rs11614913 in miR-196a2 was not associated with an altered MI risk, as suggested by the “best-evidence” synthesis and meta-analysis. Limited evidence was found to support an association between other polymorphisms and MI susceptibility.
Intrinsic factors, in association with external factors, have been shown to drive the occurrence and development of MI, and genetic factors play crucial roles during this process [37, 38]. miRNAs have wide-ranging effects on mRNA transcripts, and polymorphisms in miRNA genes have attracted a great deal of global attention over the past decade due to their roles in regulating miRNA functions and disease susceptibility. Although the occurrence of polymorphisms in miRNA sequences is relatively rare, it is of great importance. miRNA SNPs were associated with diverse complex diseases including cancer [39], diabetes [40], coronary heart disease [41], stroke [42], and several others [43]. In addition, miRNAs SNPs were also demonstrated to be involved in MI [34].
miR-146a plays an important role in the regulation of innate immunity and inflammatory responses [44, 45]. rs2910164 in miRNA-146a results in a C > G substitution in the stem structure of the miR-146a precursor. The C allele of rs2910164 was correlated with elevated expression of mature miR-146a compared with the G allele. However, in type 2 diabetes and thyroid carcinoma, the G allele has been shown to upregulate miR-146a expression [46]. These discrepancies indicated that disease-specific, cell-type-specific, or tissue type-specific factors modified the effects of rs2910164 on miR-146a functions. Here, the GG phenotype of rs2910164 in miR-146a was correlated with a higher risk of MI compared to the CC phenotype, evidenced by both the “best-evidence” synthesis and meta-analysis. Compared with healthy individuals, increased miR-146a expression was found in patients with MI [47]. Therefore, rs2910164 with G allele decreases the expression of miR-146a in MI patients, which eventually contributes to MI risk by weakening the anti-inflammatory function of miR-146a.
miR-499 is located in intron 20 of the MYH7B gene and is expressed primarily in cardiac cells and skeletal muscles. miR-499 can protect cardiomyocytes from ischemia/reperfusion-induced apoptosis by inhibiting calcineurin-mediated dephosphorylation of dynamin-related protein‑1, while the silencing of miR-499 induced myocardial apoptosis and increased the infarct size [48]. miR-499 can also regulate the expression of inflammatory cytokines, such as interleukin (IL)-6, IL‑8, and IL-2R [49]. rs3746444 in miR-499, which resides in the seed region of miR-499, can damage the secondary structure of miR-499 and thus affect the miRNA maturation process and binding affinities to target genes. It is plausible that rs3746444 may contribute to the susceptibility of various diseases by regulating different sets of downstream genes. Although the “best evidence” synthesis yielded conflicting results, further meta-analysis demonstrated a significant association between rs3746444 and MI susceptibility, under a dominant model. The results suggested that the GG and AG genotypes of rs3746444 conferred an increased risk for MI. The potential mechanism for such an association could be due to the impaired ability of miR-499 with the G allele in rs3746444 to inhibit apoptosis and inflammation.
Unfortunately, our meta-analysis failed to demonstrate a significant association between rs11614913 and MI susceptibility, and “best evidence” synthesis also generated conflicting results. The rs11614913 T → C variant is located in the 3p strand of mature miR-196a2, which could influence pre-miR maturation and interaction between 3p mature miRNAs and target mRNAs. Several studies suggested that the rs11614913 T > C polymorphism was a protective factor against MI. However, Agiannitopoulos et al. reported the opposite result, i.e., that the T allele in rs11614913 increased the susceptibility of MI in a Greek cohort. miR-196a2 could regulate annexin A1 (ANXA1) to control inflammation, and the elevated expression of ANXA1 might be a protective factor for MI by mediating the inflammatory response [50]. In addition, miR-196a2 rs11614913 CC could promote the expression of mature miR-196a in cardiac tissue, and the higher expression of miR-196a could lead to a decreased mRNA target of homeobox B8 [22]. Thus, the miR-196a2 rs11614913 T > C polymorphism may influence the risk of CAD by increasing miR-196a2 expression and decreasing the mRNA level of homeobox B8. Considering the controversial results for rs11614913, the potential role of miR-196a2 rs11614913 may be diluted or masked by the interaction of gene–environment factors. In future, more studies with detailed environmental factors are needed to support our findings.
In addition to the mechanisms discussed earlier, other miRNA polymorphisms were also involved in MI susceptibility. For instance, Ding et al. demonstrated that miR-149 prevented mitochondrial fission in cardiomyocytes by regulating the puma gene, and the miR-149 rs71428439 polymorphism was closely associated with MI susceptibility and executed functional influence on apoptosis by affecting the production of mature miR-149 [35]. Hu et al. found that miR-126 was a potential biomarker of MI and had the potential to be reused in the development of MI therapies. Two functional SNPs (rs4636297 and rs1140713) were revealed to contribute to the risk of MI [30]. In addition, miR-27a played a unique role in endothelial cell (EC) dysfunction, which may contribute to the development of MI [51]. Research by Cai et al. suggested that the AG and AA genotype of rs895819 in miR-27a decreased the risk of MI [32].
Limitations
Nevertheless, some limitations should be pointed out in this review. First, all of the studies included were designed as case–control and population-based studies in the hospital, which could result in a selection bias and a low evidence level in the present study. Second, all relevant studies were retrieved from English databases and a potential language bias may exist. Third, our combined effects were based on initial data and were not adjusted by environmental factors due to the lack of relevant data across eligible studies, which is extremely significant because gene–environment interactions can modify the risk of diseases. Fourth, the relationship between miRNA SNPs and MI risk may be different in different ethnicities, and the selection of different ethnicities may lead to some bias when a conclusion is drawn. Therefore, for those controversial SNPs or even SNPs that are not controversial, it is necessary to expand the study population for each ethnicity. Finally, many polymorphisms lack extensive research, and therefore enable a systematic review, but were not appropriate for a meta-analysis.
Conclusion
Taken together, this study systematically identified 11 miRNA polymorphisms and assessed their possible contribution to the risk of myocardial infarction (MI). A conclusion could not be drawn about the contribution of most miRNA polymorphisms to the risk of MI because of insufficient data. Importantly, rs2910164 and rs3746444 were found to contribute to MI susceptibility. The results of the meta-analysis suggested that the miR-146a rs2910164‑C allele had a protective effect against the development of MI, and the miR-499 rs3746444‑G was associated with an increased risk of MI. Confirmation of the relationship between miRNA polymorphisms and MI susceptibility can provide advice for clinical diagnosis and treatment, and promote the development of new targeted drugs. However, further studies with larger populations of different ethnicities are warranted to obtain a better understanding of the possible roles of miRNA polymorphisms in MI risk.
Abbreviations
- 3′UTRs:
-
3′ Untranslated regions
- CIs:
-
Confidence intervals
- ECs:
-
Endothelial cells
- HWE:
-
Hardy–Weinberg equilibrium
- MI:
-
Myocardial infarction
- miRNAs:
-
MicroRNAs
- pre-miRNAs:
-
miRNA precursors
- pri-miRNAs:
-
Primary miRNAs
- NOS:
-
Newcastle–Ottawa Quality Assessment Scale
- OR:
-
Odds ratio
- SNPs:
-
Single-nucleotide polymorphisms
References
Lu L, Liu M, Sun R, Zheng Y, Zhang P (2015) Myocardial infarction: symptoms and treatments. Cell Biochem Biophys 72:865–867
Nicoleau S, Wojciak-Stothard B (2020) Beyond thrombosis: the role of platelets in pulmonary hypertension. SciMed J 2:243–271
Lae KZW, Su SS, Nwet NW, Ni NT, Hla N (2019) Isolation of lasiodiplodin and evaluation of some biological activities of the stem barks of phyllanthus albizzioides (Kurz) hook.f. SciMed J 4:199–216
Rørholm Pedersen L, Frestad D, Mide Michelsen M et al (2016) Risk factors for myocardial infarction in women and men: a review of the current literature. Curr Pharm Des 22:3835–3852
Kosvyra A, Maramis C, Chouvarda I (2019) Developing an integrated genomic profile for cancer patients with the use of NGS data. Emerg Sci J 3:157–167
Li X, Lin Y, Zhang R (2019) Associations between endothelial nitric oxide synthase gene polymorphisms and the risk of coronary artery disease: a systematic review and meta-analysis of 132 case-control studies. Eur J Prev Cardiol 26:160–170
Pandey CP, Misra A, Negi MPS et al (2019) Aspirin & clopidogrel non-responsiveness & its association with genetic polymorphisms in patients with myocardial infarction. Indian J Med Res 150:50
Vasudevan S, Tong Y, Steitz JA (2007) Switching from repression to activation: microRNas can up-regulate translation. Science 318:1931–1934
Amini Khorasgani M, Mohammady Nejad P, Moghani Bashi MM, Hedayati M (2019) Evaluation of mir-377-3p expression in patients with multiple sclerosis. SciMed J 2:48–54
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK (2016) miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov 6:235–246
Swarbrick S, Wragg N, Ghosh S, Stolzing A (2019) Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol Neurobiol 56:6156–6167
Samanta S, Balasubramanian S, Rajasingh S et al (2016) MicroRNA: a new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc Med 26:407–419
Arif M, Pandey R, Alam P et al (2017) MicroRNA-210-mediated proliferation, survival, and angiogenesis promote cardiac repair post myocardial infarction in rodents. J Mol Med 95:1369–1385
Yao R, Ma Y, Du Y et al (2011) The altered expression of inflammation-related microRNAs with microRNA-155 expression correlates with Th17 differentiation in patients with acute coronary syndrome. Cell Mol Immunol 8:486–495
Huang W, Tian S, Hang P et al (2016) Combination of microRNA-21 and microRNA-146a attenuates cardiac dysfunction and apoptosis during acute myocardial infarction in mice. Mol Ther Nucleic Acids 5:e296
Zheng L, Tu Q, Meng S et al (2017) Runx2/DICER/miRNA pathway in regulating osteogenesis. J Cell Physiol 232:182–191
Wilk G, Braun R (2018) Single nucleotide polymorphisms that modulate microRNA regulation of gene expression in tumors (arXiv:1803.03189)
Wang X, Ren H, Zhao T et al (2016) Single nucleotide polymorphism in the microRNA-199a binding site of HIF1A gene is associated with pancreatic ductal adenocarcinoma risk and worse clinical outcomes. Oncotarget 7:13717
Gao Y, Yan H, Ding J et al (2009) An insertion/deletion polymorphism at miRNA-122-binding site in the interleukin-1alpha 3′ untranslated region confers risk for hepatocellular carcinoma. Carcinogenesis 30:2064–2069
Wu LS, Li F, Sun L et al (2011) A miRNA-492 binding-site polymorphism inBSG(basigin) confers risk to psoriasis in Central South Chinese population. Hum Genet 130:749–757
Xu J, Hu Z (2010) Functional variant in microRNA-196a2 contributes to the susceptibility of congenital heart disease in a Chinese population. Hum Mutat 30:1231–1236
Kaynak M, Nijman F, van Meurs J, Reijman M, Meuffels DE (2017) Genetic variants and anterior cruciate ligament rupture: a systematic review. Sports Med 8:1637–1650
Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24:1291–1306
Li X, Chen K, Lv Z (2019) APRISMA-compliant systematic review and meta-analysis determining the association of miRNA polymorphisms and risk of congenital heart disease. Medicine 98:e17653
Higgins TJP (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Nathan M, William H (1959) Statistical aspects of the analysis of data from retrospective studies of disease. JNCI J Natl Cancer Inst 22:719–748
Agiannitopoulos K, Samara P, Papadopoulou M et al (2021) miRNA polymorphisms and risk of premature coronary artery disease. Hellenic J Cardiol 62(4):278–284. https://doi.org/10.1016/j.hjc.2020.01.005
Qiu H, Chen Z, Lv L, Tang W, Hu R (2020) Associations between microRNA polymorphisms and development of coronary artery disease: a case-control study. Dna Cell Biol 39:25–36
Hu H, Yuan H, Li C, Yu H, Chen Y (2019) Association of gene polymorphisms in the human microRNA-126 gene with plasma-circulating microRNA-126 levels and acute myocardial infarction. Genet Test Mol Biomarkers 23:460–467
Osmak GJ, Matveeva NA, Titov BV, Favorova OO (2018) The myocardial infarction associated variant in the MIR196A2 gene and presumable signaling pathways to involve miR-196a2 in the pathological phenotype. Mol Biol 52:1006–1013
Cai MY, Cheng J, Zhou MY et al (2018) The association between pre-miR-27a rs895819 polymorphism and myocardial infarction risk in a Chinese Han population. Lipids Health Dis 17:7
Fawzy MS, Toraih EA, Hamed EO, Hussein MH, Ismail HM (2018) Association of MIR-499a expression and seed region variant (rs3746444) with cardiovascular disease in Egyptian patients. Acta Cardiol 73:131–140
Chen C, Hong H, Chen L et al (2014) Association of microRNA polymorphisms with the risk of myocardial infarction in a Chinese population. Tohoku J Exp Med 233:89–94
Ding SL, Wang JX, Jiao JQ et al (2013) A Pre-microRNA-149 (miR-149) genetic variation affects miR-149 maturation and its ability to regulate the puma protein in apoptosis. J Biol Chem 288:26865–26877
Baulina N, Kiselev I, Osmak G et al (2017) miRNA expression and polymorphism of miRNA genes in the myocardial infarction. J Biotechnol 256:S89
Russell MW, Chung WK, Kaltman JR, Miller TA (2018) Advances in the understanding of the genetic determinants of congenital heart disease and their impact on clinical outcomes. J Am Heart Assoc 7:e6906
Spearman DA (2017) Epigenetics for the pediatric cardiologist. Congenit Heart Dis 6:828–833
Wu J, Wang Y, Shang L, Qi L, Song M (2018) Five common functional polymorphisms in microRNas and susceptibility to breast cancer: an updated meta-analysis. Genet Test Mol Biomarkers 22:350–358
Zhao X, Ye Q, Xu K et al (2013) Single-nucleotide polymorphisms inside microRNA target sites influence the susceptibility to type 2 diabetes. J Hum Genet 58:135–141
Zhang Y, Li H, Yang R, Yang B, Gao Z (2017) Association between circulating microRNA-208a and severity of coronary heart disease. Scand J Clin Lab Invest 5:1–6
Darabi H, Salmaninejad A, Jaripour ME et al (2018) Association of the genetic polymorphisms in immunoinflammatory microRNAs with risk of ischemic stroke and subtypes in an Iranian population. J Cell Physiol 4:3874–3886
Sethupathy P, Collins FS (2008) MicroRNA target site polymorphisms and human disease. Trends Genet 24:489–497
Roos J, Enlund E, Funcke J et al (2016) miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci Rep 6:38339
Chassin C, Kocur M, Pott J et al (2010) miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 8:358–368
Jazdzewski K, Chapelle ADL (2009) Genomic sequence matters A SNP in microRNA-146a can turn anti-apoptotic. Cell Cycle 8:1642–1643
Zidar N, Boštjančič E, Glavač D, Stajer D (2011) MicroRNAs, innate immunity and ventricular rupture in human myocardial infarction. Dis Markers 31:259–265
Wang JX, Jiao J, Li Q et al (2011) miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein‑1. Nat Med 17:71–78
Hashemi M (2013) Association of pre-miRNA-146a rs2910164 and pre?miRNA-499 rs3746444 polymorphisms and susceptibility to rheumatoid arthritis. Mol Med Rep 1:287–291
Qin CX, Finlayson SB, AI-Sharea A et al (2017) Endogenous annexin-A1 regulates haematopoietic stem cell mobilisation and inflammatory response post myocardial infarction in mice in vivo. Sci Rep 7:16615
Chistiakov DA et al (2016) The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol 97:47–55. https://doi.org/10.1016/j.yjmcc.2016.05.007
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YY performed the database search, data extraction, quality assessment, analysis, and writing and drafting of the manuscript. ZXD and GDZ performed the database search, data extraction, and writing. GDZ and XHZ were involved in the database search and writing. XJS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y. Yang, X. Shi, Z. Du, G. Zhou and X. Zhang declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
The authors Yang Yang and Xiajun Shi contributed equally to this work.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Yang, Y., Shi, X., Du, Z. et al. Associations between genetic variations in microRNA and myocardial infarction susceptibility: a meta-analysis and systematic review. Herz 47, 524–535 (2022). https://doi.org/10.1007/s00059-021-05086-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-021-05086-3