Abstract
Background
Doxorubicin (DX) is used for the treatment of many types of cancer; however, a side effect of this agent is cardiotoxicity, which may lead to cardiomyopathy or cardiac failure. Oxidative stress is thought to play a major role in the development of cardiotoxic effects. Proanthocyanidins found in grapeseed (GS) extract may inhibit chemically induced lipid peroxidation and apoptosis caused by oxidative stress. We aimed to investigate the cardioprotective effects of GS extract against DX-induced cardiotoxicity.
Methods
A total of 28 male Sprague Dawley rats were grouped to receive: (a) standard nutrition (n = 7); (b) standard nutrition with an additional dose of 10 mg/kg DX (n = 7); (c) standard nutrition plus 100 mg/kg/day of GS (n = 7); (d) standard nutrition with 100 mg/kg/day of GS plus a single dose of 10 mg/kg DX. After 35 days the rats were decapitated and blood samples were taken for biochemical testing. Cardiac tissue samples were prepared for microscopy and histopathological evaluation.
Results
Rats in the DX group exhibited significant elevations in biomarkers such as troponin and NT-proBNP as well as in oxidative stress markers compared with all other groups. Histopathological examination corroborated these findings by demonstrating significant and severe structural injury in the cardiac tissue of DX rates. Moreover, rats in the DX + GS group had significantly lower cardiac injury than rats in the DX group according to both biochemical (troponin and NT-proBNP) and histopathological analyses. Serum malondialdehyde levels (a marker of oxidative stress) in the DX + GS rats were significantly lower than in the DX rats.
Conclusion
Our findings suggest that GS may reduce the severity of DX-induced cardiotoxicity and thus has the potential to prevent cardiac injury in this setting.
Zusammenfassung
Hintergrund
Doxorubicin (DX) wird zur Behandlung vieler Arten von Tumoren eingesetzt, eine seiner Nebenwirkungen ist allerdings die Kardiotoxizität, die zu Kardiomyopathie oder Herzinsuffizienz führen kann. Oxidativer Stress gilt als wesentlich für die Entstehung kardiotoxischer Wirkungen. Die in Traubenkernöl-Extrakt vorhandenen Proanthocyanidine können die chemisch induzierte Lipidperoxidation und die durch oxidativen Stress erzeugte Apoptose hemmen. Ziel der Autoren war es, die kardioprotektiven Wirkungen von Traubenkernöl-Extrakt gegen durch DX induzierte Kardiotoxizität zu untersuchen.
Methoden
Dazu wurden 28 männliche Sprague-Dawley-Ratten in Gruppen aufgeteilt, sie erhielten entweder (a) Standardnahrung (n = 7); (b) Standardnahrung mit einer zusätzlichen Dosis DX von 10 mg/kg (n = 7); (c) Standardnahrung plus Traubenkernöl-Extrakt in einer Dosierung von 100 mg/kg/Tag (n = 7) oder (d) Standardnahrung mit Traubenkernöl-Extrakt in einer Dosierung von 100 mg/kg/Tag plus eine Einzeldosis DX von 10 mg/kg. Nach 35 Tagen wurden die Ratten getötet und Blutproben für biochemische Untersuchungen entnommen. Herzgewebeproben wurden zur mikroskopischen und histopathologischen Untersuchung präpariert.
Ergebnisse
Die Ratten in der DX-Gruppe wiesen signifikante Erhöhungen von Biomarkern wie Troponin und NT-proBNP sowie von Markern für oxidativen Stress im Vergleich zu allen anderen Gruppen auf. In der histopathologischen Untersuchung bestätigten sich diese Befunde durch signifikante und schwere strukturelle Läsionen im Herzgewebe der DX-Ratten. Zudem waren die kardialen Schädigungen gemäß den biochemischen (Troponin und NT-proBNP) und histopathologischen Analysen bei den Ratten in der Gruppe mit DX plus Traubenkernöl-Extrakt signifikant geringer als bei den Ratten in der DX-Gruppe. Die Werte für Malondialdehyd im Serum (ein Marker für oxidativen Stress) waren in der Gruppe der Ratten mit DX plus Traubenkernöl-Extrakt signifikant geringer als bei den Ratten in der DX-Gruppe.
Schlussfolgerung
Die vorliegenden Ergebnisse sind ein Hinweis darauf, dass Traubenkernöl-Extrakt den Schweregrad der DX-induzierten Kardiotoxizität vermindern kann und somit das Potenzial hat, in diesem Rahmen eine kardiale Schädigung zu verhindern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Doxorubicin (DX) is an antineoplastic anthracycline that is used for the treatment of different types of cancer including leukemia, lymphoma, soft tissue and osseous sarcomas, Wilms tumor, neuroblastoma, and hepatoblastoma [1, 2]. However, a significant dose-limiting side effect of this agent is cardiotoxicity, which may lead to cardiomyopathy or cardiac failure [3]. Although the exact pathogenesis of DX-induced cardiotoxicity is unknown, oxidative stress is thought to play a major role in the development of cardiotoxic effects, as suggested by numerous studies.
A common mechanism proposed in these studies involves the formation of free oxygen radicals and lipid peroxidation [4]. This is associated with inadequate antioxidant capacity of cardiac myocytes with regard to protection against reactive oxygen species leading to mitochondrial injury and lipid peroxidation [5]. These findings suggest that treatments aimed at reducing oxidative stress may help prevent cardiac injury.
In this regard, it is noteworthy that several antioxidants have been shown to prevent the development of DX-induced cardiotoxicity [6]. Proanthocyanidins found in grapeseed (GS) extract are a specific subgroup of flavonoids, which are polyphenolic compounds. Experimental studies in mice have shown that proanthocyanidins may inhibit chemically induced lipid peroxidation as well as cranial and hepatic apoptosis caused by oxidative stress [7]. Proanthocyanidins owe their free radical scavenging and antioxidant effects to their vasodilator, anti-carcinogenic, anti-allergic, anti-inflammatory, cardioprotective, and immune-stimulating properties, in addition to their ability to inhibit phospholipase A2 and lipo-oxygenase [8].
In this study, our objective was to investigate the cardioprotective effects of GS extract against DX-induced cardiotoxicity.
Materials and methods
The study protocol was approved by the Institutional Ethics Committee of Firat University for Experimental Animals. A total of 28 male Sprague–Dawley rats, 3–4 months of age and weighing between 300 and 400 g, were grouped seven per cage and were provided with standard food and water ad libitum. The ambient temperature was maintained at 22 ± 0.5 ℃. The control group (n = 7) received standard nutrition. The same type of nutrition was also provided to the rats in the DX group (n = 7) for 35 days, and an additional 10 mg/kg of doxorubicin was administered as a single dose via intraperitoneal injection on day 28. Each rat in the GS group (n = 7) received standard nutrition plus 100 mg/kg/day of GS extract diluted with water and administered via an orogastric tube for 35 days. Again, each rat in the GS + DX group (n = 7) received 100 mg/kg/day of GS extract diluted with water and administered via an orogastric tube for 35 days in addition to 10 mg/kg of DX as a single dose administered via intraperitoneal injection on day 28 of the study. All rats were followed up for a total duration of 35 days, after which they were decapitated.
Specimen preparation and collection
After decapitation of the rats, cardiac tissue samples were removed, weighed, and placed in 10% formaldehyde solution. After fixation, transverse cardiac sections of 1 cm thickness were prepared and examined under microscopy after hematoxylin and eosin and Masson trichrome straining.
Assessment of biochemical parameters
Blood samples of 5 cc were collected in plain biochemistry tubes at the time of decapitation. After allowing a 10-min interval for clotting, the samples were centrifuged for 3 min at 5000 rpm. The sera obtained were placed into Eppendorf tubes and were stored at −20 ℃ until the time of analysis. Serum troponin levels were measured using an OLYMPUS 2700 (Olympus CO Ltd., Tokyo, Japan) auto-analyzer, while N‑terminal pro-B-type natriuretic peptide (NT-proBNP) assays were performed with an Immulite 2000 device (Siemens Healthcare Diagnostics, Foster City, CA, USA). For oxidative stress and antioxidant assays, the tissues were weighed and then homogenized using a homogenizer. The oxidation (total oxidative stress [TOS] and malondialdehyde [MDA]) and antioxidation parameters (total antioxidant capacity [TAC] and nitric oxide [NO]) were measured in serum and cardiac tissue samples. The level of MDA was determined using the methodology based on the reaction of MDA with thiobarbituric acid [9]. TOS [10] and TAC [11] was determined using the automated measurement method developed by Erel. Nitric oxide was assayed using a commercial kit (Nitrate/Nitrite Colorimetric Test kit, Cat. No. 780001; Cayman Chemicals, Ann Arbor, MI, USA).
Histopathological evaluations
Myocyte misalignment (MM), myocyte hypertrophy (MH), and small-vessel disease (SMD) were assessed in histological cross-sections stained with hematoxylin and eosin, while Masson trichrome staining was used for the assessment of fibrosis. Myocyte misalignment, MH, and SMD were scored on a 4-point scale as follows: 0, absent; +1, mild; +2, moderate; +3, severe.
Statistical analysis
The data were analyzed using SPSS Windows 22.0 software. All data are presented as mean ± standard error. For multiple group comparisons, one-way analysis of variance (ANOVA) was utilized with Tukey correction. A p value of less than 0.05 was considered statistically significant.
Results
All rats survived until completion of the study.
Serum biochemistry assessments
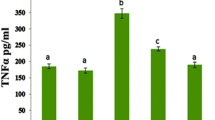
A significant increase in troponin and NT-proBNP levels was observed in DX rats compared with other groups. While troponin was significantly higher in DX + GS rats compared with the control and GS groups, it was significantly lower than that of the DX group. Levels of NT-proBNP were significantly lower in the DX + GS group than in the DX group. Serum creatinine levels were comparable among the study groups. Table 1 shows the serum biochemistry parameters in the study groups.
Serum oxidant and antioxidant parameters
Levels of MDA and TOS were significantly higher in the DX group. There was a significant elevation of TAC in the DX + GS rats compared with the control rats. Although TOS was lower and TAS was higher in DX rats compared with DX + GS rats, the differences were not statistically significant (p = 0.30 and p = 0.24, respectively). Nitric oxide in the DX + GS rats was significantly higher than in control and DX rats. While MDA was significantly higher in DX + GS rats than in control rats, it was significantly lower compared with the DX rats. Table 2 shows the oxidant and antioxidant parameters in the study groups.
Tissue oxidant and antioxidant parameters
Levels of MDA and TOS were significantly higher in the DX group compared with the control and other groups. There was a significant elevation of TAC in DX + GS rats compared with the control rats. Total antioxidant capacity was higher in DX + GS rats when compared with the DX group; however, the difference did not reach statistical significance (p = 0.063). Levels of MDA and TOS in DX + GS rats were significantly lower compared with DX rats. Nitric oxide in the DX + GS rats was significantly higher than in the control and DX rats. Tissue oxidant and antioxidant parameters are shown in Table 3.
Histopathological assessments
All values in DX rats (MM, MH, SVD, and fibrosis) were significantly higher than in controls. Myocyte misalignment, MH, SVD, and fibrosis scores were significantly lower in DX + GS rats than in DX rats, while they were not significantly different from controls. Table 4 and Fig. 1 show the histopathological assessments.
Histopathological images (H&E, × 400). a Normal myocyte alignment in control rats, b severe myocyte misalignment and hypertrophy in doxorubicin (DX) group, c normal myocyte alignment in grapeseed extract (GS) group, d significantly milder myocyte alignment and hypertrophy in DX + GS group compared with DX group
Discussion
Doxorubicin is an anthracycline cytotoxic agent that is commonly used for the treatment of several types of cancer including leukemia and lymphoma. However, despite its significant antitumor activity, cardiotoxicity associated with its use represents a major dose-limiting factor, and may lead to cardiomyopathy or cardiac failure [12, 13]. Many previous studies have established the fact that the fundamental mechanism responsible for DX-induced cardiotoxicity involves oxidative stress due to formation of free oxygen radicals [12, 14].
As shown in the study by Sardao et al., oxidative stress causes injury in the mitochondrial membrane, resulting in mitochondrial dysfunction [15]. In another study performed by Zhang et al., DX injection was followed by elevation in cardiac biomarkers, oxidative injury in cardiac tissue due to MDA production and lipid peroxidation, and reduction in antioxidant parameters [16]. In line with these observations, rats in the DX group in our study exhibited significant elevations in biomarkers such as troponin and NT-proBNP as well as in oxidative stress markers such as MDA and TOS. In this context, several previous studies have been carried out based on the assumption that substances with antioxidant properties may prevent such effects. In some of these studies, antioxidants of plant origin were able to protect tissue against oxidative stress when used in conjunction with DX [16, 17].
Grapeseed extract, one such antioxidant substance of plant origin, contains a group of biologically active flavonoids such as oligomeric proanthocyanidins, gallic acid, monomeric flavan-3-ols-cathecin, and polymeric proanthocyanidins. In a study by Cetin et al., it was found that the antioxidant properties of GS could be related to these biophenols in its composition [18]. Procyanidins in GS have been suggestive to have significant protective effects against myocardial ischemia-reperfusion injury owing to their ability to scavenge the reactive oxygen species produced during ischemia and reperfusion [19]. Grapeseed extract has potent free radical scavenging capabilities and may provide significant cardiac protection against myocardial infarction [20]. In this study, our aim was to investigate the role of GS in preventing DX-induced cardiotoxicity on the basis of its beneficial antioxidant properties.
In our study, DX was associated with cardiac toxicity as evidenced by significant elevations in troponin and NT-proBNP levels as compared with all other study groups. Similarly, histopathological examination corroborated these findings by demonstrating significant and severe structural injury in cardiac tissue. On the other hand, rats in the DX + GS group had significantly lower biochemical (troponin and NT-proBNP) and histopathological cardiac injury as compared with rats in the DX group.
Examination of oxidative stress markers such as TOS and MDA showed significantly higher TOS in the DX group compared with the controls, and significantly higher MDA in the DX group compared with all other study groups. On the other hand, TAS and NO (markers of antioxidant capacity), were significantly higher in the DX + GS rats compared with the control and DX rats. Furthermore, serum MDA in DX + GS rats was significantly lower than in DX rats.
Examination of tissue samples showed significant elevation of TOS and MDA in DX rats than in other groups. Nitric oxide levels in the DX + GS rats were significantly higher compared with the control and DX rats. In the same group, TAC was significantly higher than in controls, while its elevation reached near significance when compared with DX rats. Again, TOS and MDA in the DX + GS rats were significantly lower compared with DX rats. Our results with respect to oxidant and antioxidant markers are consistent with the observations of Zhang et al. [16] and Ahmed et al. [21]. Again, an examination of the oxidant and antioxidant markers in serum and tissue samples in all study groups showed a strong tendency toward oxidative stress in DX rats, while antioxidant markers were significantly elevated in the DX + GS rats. Oxidative injury induced by DX was significantly reduced by the addition of GS to DX.
Limitations
A limitation of our study was that intraperitoneal DX injection was performed in the DX and DX + GS groups, but there were significant differences between the two groups. Although DX-induced cardiac injury is likely to have occurred in these two groups, septic cardiac injury due to peritonitis may also have developed. The significant decrease in cardiac injury in the DX + GS group compared with the DX group suggests that GS could prevent DX-induced cardiac injury and also prevent peritonitis-induced cardiac injury. In order to better illustrate the difference between these two conditions, we plan to perform intraperitoneal injection of NaCl in the control group and a herbal antioxidant group in subsequent experimental studies. We believe that our new study, which will be completed shortly, will provide better information. In our present study, subjects were followed up for 35 days and the effects of GS were significant. Longer follow-up was not possible because of the risk of losing the animals.
Conclusion
In conclusion, our findings suggest that grapeseed extract may reduce the severity of doxorubicin-induced cardiotoxicity. Thus, grapeseed extract has the potential to be used as a cardioprotective agent to prevent cardiac injury in this setting.
References
Muggia FM, Green MD (1991) New anthracycline antitumor antibiotics. Crit Rev Oncol Hematol 11:43–64
Hideg K, Kálai T (2007) Novel antioxidants in anthracycline cardiotoxicity. Cardiovasc Toxicol 7:160–164
Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS (2004) Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 109:3122–3131
Biner B (2000) Çocukluk Çağı Akut Lösemilerinde Erken ve Geç Dönem Antrasiklin Kardiyotoksisitesinin Tanısında Kardiyak Troponin T ve Ekokardiyografi. Uzmanlık Tezi. İstanbul Üniversitesi İstanbul Tıp Fakültesi, Sağlık Bilimleri Estitüsü, İstanbul
Menna P, Salvatorelli E, Minotti G (2008) Cardiotoxicity of antitumor drugs. Chem Res Toxicol 21:978–989
Xin H, Liu XH, Zhu YZ (2009) Herba leonurine attenuates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells. Eur J Pharmacol 612:75–79
Bagchi D, Garg A, Krohn RL (1998) Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol 30:771–776
Rice-Evans CA, Miller NJ, Paganda G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid per-oxidation products: malonaldehyde and 4hydroxynonenal. Methods Enzymol 186:407–421
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37:112–119
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229
Singal PK, Deally CM, Weinberg LE (1987) Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol 19:817–828
Doroshow JH (1983) Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res 43:4543–4551
Sardao VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB (2009) Morphological alterations induced by doxorubicin on H9c2 myoblasts: nuclear, mitochondrial, and cytoskeletal targets. Cell Biol Toxicol 25:227–243
Zhang XY, Li WG, Wu YJ, Gao MT (2005) Amelioration of doxorubicininduced myocardial oxidative stress and immunosuppression by grape seed proanthocyanidins in tumour-bearing mice. J Pharm Pharmacol 57:1043–1052
Du Y, Lou H (2008) Catechin and proanthocyanidin B4 from grape seeds prevent doxorubicininduced toxicity in cardiomyocytes. Eur J Pharmacol 591:96–101
Cetin A, Kaynar L, Kocyigit I, Hacioglu SK, Saraymen R, Ozturk A, Sari I, Sagdic O (2008) Role of grape seed extract on methotrexate induced oxidative stress in rat liver. Am J Chin Med 36:861–872
Chang WT, Shao ZH, Yin JJ, Mehendale S, Wang CZ, Qin Y et al (2007) Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur J Pharmacol 566:58–66
Uchida S, Hirai K, Hatanaka J, Hanato J, Umegaki K, Yamada S (2008) Antinociceptive effects of St. John’s wort, harpagophytum procumbens extract and grape seed proanthocyanidins extract in mice. Biol Pharm Bull 31:240–245
Ahmed HH, Mannaa F, Elmegeed GA, Doss SH (2005) Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin-induced cardiotoxicity. Bioorg Med Chem 13:1847–1857
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.Ş. Adıyaman, Ö.A. Adıyaman, A.F. Dağlı, M.Z. Karahan, İ. Kaya and M.N. Dağlı declare that they have no competing interests.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study protocol was approved by the Institutional Ethics Committee of Firat University for Experimental Animals.
Rights and permissions
About this article
Cite this article
Adıyaman, M.Ş., Adıyaman, Ö.A., Dağlı, A.F. et al. Effects of grapeseed extract on doxorubicin-induced cardiotoxicity in rats. Herz 46 (Suppl 1), 103–108 (2021). https://doi.org/10.1007/s00059-019-04888-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-019-04888-w