Abstract
Background
Because myocardial infarction in young adults is rare, there has been limited research on the condition in this patient group. Very few data are available regarding the long-term outcomes of patients under 40 years of age with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing invasive treatments. The prognostic value of uric acid (UA) in young patients with NSTEMI who undergo percutaneous coronary intervention (PCI) has also not been studied. The purpose of this study was to evaluate the long-term clinical outcomes of this specific subset of young patients. In addition, we aimed to identify the role of serum UA in predicting the long-term prognosis of young patients with NSTEMI who have undergone PCI.
Methods
We performed a retrospective analysis of 213 young adult patients (≤40 years old) with NSTEMI who underwent PCI during their hospitalization at our tertiary referral center.
Results
The mean age of the 213 patients was 36.8 ± 3.3 years (range, 21–40 years). The median follow-up was 930 days. Our patients were predominantly male (88.3%) and the most frequent traditional cardiovascular risk factors were smoking and dyslipidemia. Baseline TIMI flow 0–1, estimated glomerular filtration rate (eGFR), and UA were found to be independently correlated with long-term major adverse cardiovascular events (MACEs) in multivariate Cox regression analysis.
Conclusion
In the present study, baseline TIMI flow 0–1, admission eGFR, and UA levels were correlated with MACEs during long-term follow-up in young patients with NSTEMI.
Zusammenfassung
Hintergrund
Da ein Herzinfarkt bei jungen Erwachsenen selten ist, gibt es Untersuchungen dazu in dieser Patientengruppe nur in begrenztem Umfang. Zu den Langzeitergebnissen von Patienten unter 40 Jahren mit Nicht-ST-Strecken-Hebungs-Infarkt (NSTEMI), bei denen eine invasive Therapie durchgeführt wurde, gibt es nur sehr wenige Daten. Der prognostische Wert der Harnsäurewerte bei jungen Patienten mit NSTEMI, bei denen eine perkutane Koronarintervention (PCI) erfolgt, ist bisher ebenfalls nicht untersucht worden. Ziel der vorliegenden Studie war es, die klinischen Langzeitergebnisse dieser speziellen Untergruppe junger Patienten zu untersuchen. Darüber hinaus war es Ziel der Autoren, die Bedeutung der Harnsäure im Serum für die Vorhersage der Langzeitprognose junger Patienten mit NSTEMI zu ermitteln, bei denen eine PCI durchgeführt wurde.
Methoden
Retrospektiv wurden die Daten von 213 jungen erwachsenen Patienten (≤40 Jahre) mit NSTEMI ausgewertet, bei denen während ihres stationären Aufenthalts in dem Tertiärversorgungszentrum der Autoren eine PCI erfolgte.
Ergebnisse
Das durchschnittliche Alter der 213 Patienten betrug 36,8 ± 3,3 Jahre (Spanne: 21–40 Jahre). Im Mittel dauerte die Nachbeobachtungsphase 930 Tage. Die hier untersuchten Patienten waren in der Mehrzahl Männer (88,3 %), und die häufigsten üblichen kardiovaskulären Risikofaktoren waren Rauchen und Fettstoffwechselstörungen. Ein TIMI-Fluss (Thrombolysis-in-myocardial-Infarction-Klassifikation) zu Studienbeginn von 0–1, die geschätzte glomeruläre Filtrationsrate (eGFR) und die Harnsäurewerte stellten sich in der multivariaten Cox-Regressionsanalyse auf lange Sicht als unabhängig mit schweren ungünstigen kardiovaskulären Ereignissen (MACE) korreliert heraus.
Schlussfolgerung
Ein TIMI-Fluss von 0–1, die eGFR bei stationärer Aufnahme und die Harnsäurewerte waren in der vorliegenden Studie mit MACE während der Langzeitnachbeobachtung bei jungen Patienten mit NSTEMI korreliert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many investigators have observed a decline in cardiovascular mortality rates in the general population throughout the industrialized world. Conversely, cardiovascular events have increased in young adults especially in low- and middle-income regions over the past 20 years. Acute myocardial infarction (AMI) is an uncommon disease in young individuals and its incidence varies between 2 and 10% [1]. Among young adult patients with acute coronary syndrome (ACS), the most frequent presentation is ST-segment-elevation myocardial infarction (STEMI; [2]). However, there are remarkably few published reports on the characteristics, risk profile, and prognosis of young patients with non-STEMI. Although smoking, obesity, dyslipidemia, and family history of coronary artery disease (CAD) are the predominant risk factors, the other traditional risk factors such as hypertension (HT) and type 2 diabetes mellitus (DM) are becoming increasingly prevalent in young adults. In addition, cocaine and cannabis have been more frequently implicated as triggers of AMI in younger patients [3, 4]. Unfortunately, the current risk scores fail to identify young individuals at risk of experiencing their first MI; consequently, these young adults rarely receive the recommended preventive medications and lifestyle modifications that reduce the incidence of heart diseases. With the increasing rates of traditional cardiovascular risk factors and the inadequacy of preventive measures, rates of cardiovascular events are on the rise among young adults.
Uric acid (UA), an end product of purine metabolism, has been shown to be an independent predictor of the short- or long-term prognosis of patients with AMI [5]. In addition, high serum UA levels have been associated with critical CAD in young patients with AMI [6]. However, there is a paucity of data on the value of UA as an independent predictor of long-term prognosis in young patients with NSTEMI.
The purpose of this study was to evaluate the long-term clinical outcomes of this specific subset of young patients. Moreover, we sought to determine the role of UA in predicting long-term outcomes of young patients with NSTEMI who underwent percutaneous coronary intervention (PCI).
Methods
Patients
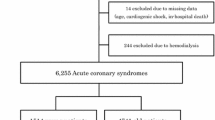
The database of our tertiary referral center was analyzed for patients aged 40 years or younger who underwent PCI between 2010 and 2015 and a total of 697 patients were identified. Initially, 349 (50.1%) patients with the diagnosis of STEMI and 135 (19.3%) patients with stable CAD were excluded. We then performed a retrospective analysis of 213 (30.5%) young adult patients (≤40 years old) with NSTEMI who underwent PCI during hospitalization. All of these patients were included in the analysis. Demographic, clinical, angiographic, and laboratory data were collected from hospital records. Data on outcomes after discharge were assessed from the medical records of patients who were followed up at our institution and their first cardiovascular event was noted. Missing data about the latest clinical status were obtained from the patients or their families by telephone interview. The study was carried out in accordance with the Declaration of Helsinki, and approval from a local ethics committee was obtained.
Definitions
We defined NSTEMI according to the European Society of Cardiology (ESC) guidelines for ACS without ST-segment elevation [7]. Hypertension was defined as the use of antihypertensive drugs or systolic blood pressure (SBP) greater than or equal to 140 mm Hg and/or diastolic blood pressure greater than or equal to 90 mm Hg. Diabetes mellitus was diagnosed on the basis of fasting blood glucose levels of ≥126 mg/dl, blood glucose levels of >200 mg/dl at any time, or a history of DM. The diagnosis of hypercholesterolemia was made on the basis of total cholesterol values of ≥200 mg/dl and/or low-density lipoprotein cholesterol of ≥140 mg/dl or use of cholesterol-lowering agents. Family history was considered significant if a first-degree relative younger than 55 years (men) or 65 years (women) had CAD. Renal failure was defined as a glomerular filtration rate less than 60 ml/min/1.73m2, which was assessed via the estimated glomerular filtration rate (eGFR) using the CKD-EPI formula [8]. Hyperuricemia was defined as serum UA values of >6.0 mg/dl in women and >7.0 mg/dl in men. Multivessel coronary disease was defined as the presence of ≥50% luminal diameter stenosis involving at least two major epicardial coronary arteries. A successful procedure was defined as an infarct-related artery (IRA) stenosis of <20% associated with Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow. A TIMI thrombus score of ≥4 was defined as high thrombus burden, which was assessed according to the TIMI thrombus grading scale [9]. The patient was considered to exhibit a no-reflow phenomenon if blood flow in the IRA was graded as TIMI ≤2 flow despite successful dilatation and absence of mechanical complications after completion of the procedure [10].
Procedures
Venous blood samples were obtained from all patients on admission. Blood counts were studied with an autoanalyzer (Cell-dyn 3700; Abbott, Wiesbaden, Germany) within 30 min of blood sampling. Troponin I was measured on the Elecsys 2010 analyzer (Roche Diagnostics GmbH, Mannheim, Germany) and the test result was considered positive at a cut-off value of >0.03 ng/ml. C‑reactive protein (CRP) measurements were conducted on a Cobas Integra analyzer (Roche Diagnostics, Istanbul, Turkey) using the turbidimetric method. The plasma UA concentration was determined with an enzymatic colorimetric test on a Cobas Integra analyzer. Left ventricular systolic function was assessed via with two-dimensional echocardiography (Vivid 3 system; General Electric Company, Milwaukee, WI, USA) using the modified biplane Simpson method [11].
All of the patients underwent invasive treatment within 72 h of their first hospital admission. The exact timing of the invasive procedure was based on the clinical judgment of the physician. Coronary angiography was performed with the Judkins technique using the femoral artery. Procedural decisions, including device selection and adjunctive pharmacotherapy, such as glycoprotein IIb/IIIa inhibitors, were made at the discretion of the operator. All patients received a loading dose of 600 mg clopidogrel and 300 mg acetyl salicylic acid at admission. Unless contraindicated, beta-blockers, angiotensin-converting enzyme inhibitors, and statin therapy were administered to all patients during hospitalization. Also, 75 mg clopidogrel and 100 mg acetyl salicylic acid were given in combination once daily.
Major adverse cardiovascular events (MACE) were defined as death, reinfarction, or target vessel revascularization after hospital discharge and beyond 30 days. Reinfarction was diagnosed on the basis of the presence of electrocardiographic changes and new elevations of cardiac biomarkers with the recurrence of symptoms. Target vessel revascularization was defined as any repeat percutaneous intervention or surgical bypass of any segment of the target vessel. The target vessel was defined as the entire major coronary vessel proximal and distal to the target lesion that includes upstream and downstream branches and the target lesion itself.
Statistical analysis
All data are presented as a mean ± SD or a median [interquartile range] for parametric variables and as percentages for categorical variables. Continuous variables were checked for the normal distribution assumption using Kolmogorov–Smirnov statistics. Differences between MACE(+) and MACE(−) were evaluated using two-sample t tests. Categorical variables were tested with Pearson’s chi-squared test and Fisher’s exact test. Correlation between two continuous variables was calculated with the Pearson coefficient correlation. Receiver operating characteristic (ROC) curves were generated to define the cut-off values of UA for MACE. Using these cut-off values of UA levels, Kaplan–Meier estimates and curves were generated, and groups were compared using log-rank tests. Cox regression analyses were used to investigate the univariable and multivariable predictors of MACE during the study period. Forward stepwise multivariable regression models using parameters with p < 0.10 were created in Cox regression analyses. All p values were two-sided, and values less than 0.05 were considered statistically significant. All statistical studies were carried out using the Statistical Package for Social Sciences software (SPSS 22.0 for Windows, SPSS, Inc., Chicago, IL, USA). The ROC curves of the models were compared using with MEDCALC software program (Software bvba 13, Ostend, Belgium).
Results
The mean age of the 213 patients was 36.8 ± 3.3 years (range, 21–40 years). Median follow-up was 930 days (interquartile range: 32–3700 days). Our patients were predominantly male (88.3%) and the most frequent traditional cardiovascular risk factors were smoking and dyslipidemia (66.7 and 40.8%, respectively). Demographic, angiographic, and laboratory characteristics of the patients are summarized in Tables 1 and 2. In all, 25% of the study participants had multivessel disease. The left anterior descending (LAD) artery was the most common culprit artery (45.5%). Procedural success was achieved in 196 (92%) patients. Hyperuricemia was present in 47 (22.1%) patients. Serum creatinine and UA levels as well as and mean platelet volume as continuous variables were significantly higher in the MACE+ group. Hypertension and hyperuricemia as categorical variables were also significantly more frequent in the MACE+ group. The eGFR was significantly lower in the MACE+ patient group and there were only two patients with eGFR <60 ml/min/1.73 m2 from the whole patient cohort. No patient presented with cardiopulmonary arrest, cardiogenic shock, and Killip classes III–IV. In-hospital outcomes were excellent without any morbidity and mortality. During long-term follow-up, MACEs (CV death, reinfarction, target vessel revascularization) were observed in 46 patients (21.6%). The leading cause of MACE was reinfarction (38 out of 46 patients). Only two patients died during the follow-up period.
In univariate correlation analysis, plasma UA levels were positively correlated with creatinine levels (r = 0.19, p < 0.01) and negatively correlated with eGFR measurements (r = −0.16, p = 0.01). The tolerance and variance inflation factor (VIF) were determined to assess multicollinearity for the parameters in the model. Because all values of tolerance were >0.1 and VIF values were <10, we can conclude that there was no multicollinearity between each of the parameters in the model (Table 3).
In univariate regression analysis, age, hypertension, the culprit lesion in the LAD, baseline TIMI flow 0–1, admission UA levels, and eGFR measurements were found to be correlated with MACE in the study population. When applying these parameters to multivariate Cox regression analysis, baseline TIMI flow 0–1 (Hazard ratio [HR]: 2.76, 95% CI: 1.24–6.16, p = 0.01), eGFR (HR: 0.97, 95% CI: 0.96–0.99, p = 0.02), and UA (HR: 1.24, 95% CI: 1.00–1.55, p = 0.04) were found to be independently correlated with MACE in the study population (Table 4).
In ROC analysis, UA levels of >5.2 mg/dl predicted MACE with a sensitivity of 84.7% and a specificity of 43.7% (AUC: 0.68, p < 0.001; Fig. 1). Patients with UA levels of >5.2 mg/dl had a 2.9-fold increased risk for MACE compared with patients with UA levels of <5.2 mg/dl (HR: 2.93, 95% CI 1.30–6.61, p = 0.01 for UA level >5.2 vs. <5.2).
We also performed a ROC analysis of the regression model with and without UA separately. When the dependent variable was MACE, the AUC of the model without the inclusion of UA was 0.547. When we included UA in the model, the AUC was 0.591. The inclusion of UA into the multivariate model was associated with a significant improvement in the discriminatory power of the model in terms of MACE (p = 0.01).
In Kaplan–Meier curves, patients with UA levels above the cut-off value had a significantly higher risk for MACE (log-rank p < 0.01; Fig. 2). According to the survival table, Kaplan–Meier estimates of 1‑year MACE rates between both groups (UA level >5.2 and <5.2) were 9.9% vs. 3.8%, respectively.
Discussion
The main findings of our study were as follows: (1) Admission UA levels, baseline TIMI 0–1 flow, and lower eGFR were independent predictors of poor long-term prognosis in young NSTEMI patients (≤40 years old) who underwent PCI. (2) In the present study, in-hospital MACE-free survival rates for young NSTEMI patients were excellent; in addition, long-term MACE rates were relatively high and comparable with those in previous studies involving NSTEMI patients from non-age-restricted populations. (3) In ROC analysis, the cut-off value for UA to predict MACE was 5.2 mg/dl and this value was far below the limits used to define hyperuricemia. (4) Patients with UA levels of >5.2 had a 2.9-fold increased risk for MACE during long-term follow-up. (5) In addition, the inclusion of UA into the multivariate model significantly improved its performance.
Acute MI is rarely seen in patients aged 40 years or younger and STEMI is the most common clinical manifestation of ACS in young adults. The available data were mainly obtained from the entire spectrum of ACS, ranging from STEMI to unstable angina with a wider age range of participants up to 55 years old [12,13,14,15,16]. Consequently, there are few reports with a very limited number of patients in the literature investigating outcomes of young patients who underwent PCI during their hospitalization with the diagnosis of NSTEMI [17, 18]. Further, STEMI and NSTEMI are two distinct pathophysiological entities in terms of diagnostic criteria, therapeutic approaches, timing of the revascularization, and short- or long-term outcomes. For this reason, we tried to establish a homogeneous population with regard to the admission diagnosis so as to improve the predictive accuracy of our findings. Following cardiac catheterization, patients with NSTEMI have a higher risk of long-term mortality than patients with STEMI. Young adults with ACS have a favorable outcome in terms of mortality but reinfarction and revascularization rates are similar to those of the older counterparts in the long-term follow-up [19]. Recently, researchers have found that the percentage of admissions for STEMI decreased, while that of admissions for NSTEMI increased significantly over the years in a recent multicenter, prospective study that included 1639 young patients with the entire spectrum of ACS [20]. In addition, in a previous study involving adults without age restriction, NSTEMI patients were found to have worse long-term survival after 6 months than their STEMI counterparts [21]. In light of these previous studies, our findings are particularly important for the prediction of long-term adverse events that pose serious quality-of-life issues owing to the young age of the patients and their long life expectancy.
In our study, cardiovascular risk factors were relatively low except for dyslipidemia and smoking, procedural success rates were high, and single-vessel disease was more frequently detected in concordance with the results of similar previous studies conducted with young ACS patients [12, 19, 22, 23]. Approximately 20% of the patients had baseline TIMI flow 0–1 before the revascularization procedure and this was the strongest independent predictor of poor long-term prognosis in the present study. Current guidelines recommend immediate, early, or delayed invasive strategies according to the estimated risk in NSTEMI patients. Sometimes, young patients who have baseline TIMI flow 0–1 do not meet the criteria for immediate invasive strategies and may not benefit from the favorable long-term effects of early revascularization. The long duration of low baseline TIMI flow poses a higher risk to NSTEMI patients during the short- and long-term period. Timely revascularization of younger patients with NSTEMI is particularly important to improve the long-term outcomes because the clinical benefits tend to emerge late rather than early.
Our research data indicate that increased serum UA levels are associated with long-term adverse cardiovascular outcome in young NSTEMI patients treated with PCI. Uric acid, the end product of endogenous and dietary purine metabolism, is produced via the enzymatic activity of xanthine oxidase. Xanthine oxidase activity generates free radicals and other oxidant reactive species that play a significant role in increased vascular oxidative stress [24]. Oxidative stress is closely related to endothelial dysfunction and vascular inflammation. Reactive oxygen species are key mediators of signaling pathways that underlie vascular inflammation in atherogenesis, starting from the initiation of fatty streak development, through lesion progression, to ultimate plaque rupture. In addition, UA induces endothelial dysfunction owing to its detrimental effects on nitric oxide synthesis [25]. In addition, a high UA level causes insulin resistance and is associated with increased lipid peroxidation and platelet activation [26,27,28].
Previous studies have shown the relationship between UA and different disorders such as HT [29], DM [30], metabolic syndrome [31], renal failure [32] and CAD [33]. High UA has been found as an independent marker of impaired prognosis in patients with moderate to severe congestive heart failure (CHF) [34]. Although, the role of UA as an independent prognostic factor in patients with ACS has been a matter of debate for years, recent studies have reported the independent association of elevated UA with long-term mortality and MACE in ACS patients undergoing PCI [35,36,37]. A community-based, prospective observational study by Culleton and colleagues which was conducted in Framingham Heart Study participants claimed that UA does not have a causal role in the development of CAD and outcomes, instead, these outcomes might be due to the association of UA level with other risk factors [38]. In furtherance of this study, Pineda et al. have found that hyperuricemia was independently associated with medium/long-term mortality and major cardiovascular events in ACS patients, however, this significant relationship disappeared when non-diabetic patients were analyzed separately [39]. Consequently, high UA level is closely associated with age, HT, DM, dyslipidemia, renal function, and previous diuretic use. Although the independent association between UA and mortality persists after adjustment for confounding factors in many trials that found UA as a prognostic factor in ACS patients, there may still be residual confounding variables such as hyperinsulinemia or diuretic use. The strength of UA as an independent prognostic marker may increase when the prevalence of associated cardiovascular risk factors is decreased in the analyzed population. In our opinion, the relatively low cardiovascular risk profile of our participants and the presence of a few patients with a history of CHF and CKD strengthens the prognostic value of UA in our study.
Furthermore, ROC analysis showed >5.2 as the best cut-off value for UA to predict MACE in our young patients with NSTEMI. This cut-off value was much lower than previous studies had detected, which investigated the role of UA as an independent predictor of cardiovascular events and mortality [37, 40]. In addition, the UA value was independently associated with MACEs, while hyperuricemia, as a categorical variable, was not found to be an independent predictor of MACEs in the present study. These findings should be emphasized because the UA level, even within normal limits, may be a reliable marker for predicting the prognosis of young patients with NSTEMI.
Limitations
Our study has a number of limitations, including its retrospective nature, a small sample size, and the fact that it was conducted at a single institution. The small sample size affects the power and significance of the findings. Although we adjusted our Cox proportional hazards model for known confounding variables, other unknown confounders might have affected the outcome. We restricted the selection to young patients to analyze a homogeneous population and included only the patients who underwent PCI with the diagnosis of NSTEMI. However, considering the retrospective nature of this study, the timing of the intervention was left to the discretion of the physician resulting in inappropriately delayed invasive therapy in some cases, which might have an impact on outcomes. Finally, we were unable to obtain data for all inflammatory markers, including the levels of interleukins, tumor necrosis factor, and myeloperoxidase, which have been shown to be associated with long-term outcomes in patients with ACS.
Conclusion
Despite the increasing prevalence of young patients with myocardial infarction (MI) in recent years, there is a paucity of information concerning the long-term prognosis of young adults with non-ST-elevation myocardial infarction (NSTEMI). Baseline Thrombolysis in Myocardial Infarction flow grade 0–1, serum uric acid (UA) levels, and estimated glomerular filtration rate (eGFR) have been shown to be independent predictors of major adverse cardiac events (MACE) in young patients with NSTEMI who underwent percutaneous coronary intervention during their hospitalization.
The most important finding in the current study is the independent association of increased plasma UA levels and MACE during the long-term follow-up of our young patients. Uric acid is easy to measure, inexpensive, and a reliable parameter that can be used to identify high-risk patients. Despite the contradictory evidence in the literature with regard to the role of UA as a prognostic marker in acute coronary syndrome, our results may provide additional evidence in favor of the independent predictive value of UA. An association between UA and MACE is shown for the first time in such a young population with a relatively low cardiovascular risk profile. Further research is needed with a larger sample size to evaluate the role of UA as a risk marker in young patients.
References
Zimmerman FH, Cameron A, Fisher LD, Ng G (1995) Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry). J Am Coll Cardiol 26:654–661
Schoenenberger AW, Radovanovic D, Stauffer JC et al (2011) Acute coronary syndromes in young patients: Presentation, treatment and outcome. International Journal of Cardiology 148:300–304
Canga Y, Osmonov D, Karataş MB et al (2011) Cannabis: a rare trigger of premature myocardial infarction. Anadolu Kardiyol Dergisi 11:271–275
Qureshi AI, Suri MF, Guterman LR, Hopkins LN (2001) Cocaine use and the likelihood of nonfatal myocardial infarction and stroke: data from the third national health and nutrition examination survey. Circulation 103:502–506
Trkulja V, Car S (2012) On-admission serum uric acid predicts outcomes after acute myocardial infarction: systematic review and meta-analysis of prognostic studies. Croat Med J 53(2):162–172
Zand S, Shafiee A, Boroumand M, Jalali A, Nozari Y (2013) Serum uric acid is not an independent risk factor for premature coronary artery disease. Cardiorenal Med 3(4):246–253
Roffi M, Patrono C, Collet JP et al (2015) ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev Esp Cardiol (engl Ed) 68(2015):1125
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Sianos G, Papafaklis MI, Serruys PW (2010) Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol 22(10 suppl B):6B–14B
Kirma C, Izgi A, Dundar C, Tanalp AC, Oduncu V, Aung SM et al (2008) Clinical and prodedural predictors of no-reflow phenomenon after primary percutaneous coronary interventions: experience at a single center. Circ J 72:716–721
Cheitlin MD, Armstrong WF, Aurigemma GP et al (2003) ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography-summary article: a report of the American college of cardiology/American heart association task force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography). J Am Coll Cardiol 42(5):954–970
de Soeiro AM, Fernandes FL, Soeiro MC et al (2015) Clinical characteristics and long-term progression of young patients with acute coronary syndrome in Brazil. Einstein (São Paulo) 13(3):370–375
Matsis K, Holley A, Al-Sinan A et al (2017) Differing clinical characteristics between young and older patients presenting with myocardial infarction. Heart Lung Circ 26(6):566–571
Pelletier R, Choi J, Winters N et al (2016) Sex differences in clinical outcomes after premature acute coronary syndrome. Can J Cardiol 32(12):1447–1453
Sabbag A, Matetzky S, Porter A et al (2017) Sex differences in the management and 5‑year outcome of young patients (〈55 years) with acute coronary syndromes. Am J Med 130(11):1324.e15–1324.e22
Ma Q, Wang J, Jin J et al (2017) Clinical characteristics and prognosis of acute coronary syndrome in young women and men: a systematic review and meta-analysis of prospective studies. Int J Cardiol 228:837–843
Andronescu AM, Delcea C, Enache V, Stamate CS, Dorobantu M (2014) Mean platelet volume variability in young patients with non-ST elevation acute myocardial infarction. J Med Life 7(Spec No. 3):107–113
Özlü MF, Öztürk S, Ayhan SS et al (2013) Predictive value of mean platelet volume in young patients with non-ST-segment elevation acute coronary syndromes: a retrospective observational study. Anadolu Kardiyol Derg 13(1):57–61
Tini G, Proietti G, Casenghi M et al (2017) Long-term outcome of acute coronary syndromes in young patients. High Blood Press Cardiovasc Prev 24(1):77–84
Trzeciak P, Gierlotka M, Poloński L, Gasior M (2017) Treatment and outcomes of patients under 40 years of age with acute myocardial infarction in Poland in 2009–2013: an analysis from the PL-ACS registry. Pol Arch Intern Med 127(10):666–673
Ren L, Ye H, Wang P, Cui Y, Cao S, Lv S (2014) Comparison of long-term mortality of acute ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome patients after percutaneous coronary intervention. Int J Clin Exp Med 7(12):5588–5592
Maroszyńska-Dmoch EM, Wożakowska-Kapłon B (2016) Clinical and angiographic characteristics of coronary artery disease in young adults: a single centre study. Kardiol Pol 74(4):314–321
Andrade PB, Rinaldi FS, Bienert IGC et al (2015) Clinical and angiographic profile of young patients undergoing primary percutaneous coronary intervention. Rev Bras Cardiol Invasiva 23(2):91–95
Landmesser U, Spiekermann S, Dikalov S et al (2002) Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106:3073–3078
Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ (2005) Hyperuricemia induces endothelial dysfunction. Kidney Int 67:1739–1742
Baldwin W, McRae S, Marek G et al (2011) Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60:1258–1269
De Scheerder IK, van de Kraay AM, Lamers JM et al (1991) Myocardial malondialdehyde and uric acid release after short-lasting coronary occlusions during coronary angioplasty: potential mechanisms for free radical generation. Am J Cardiol 68:392–395
Alderman M, Aiyer KJ (2004) Uric acid: role in cardiovascular disease and effects of losartan. Curr Med Res Opin 20:369–379
Perlstein TS, Gumieniak O, Williams GH et al (2006) Uric acid and the development of hypertension: the normative aging study. Hypertension 48(6):1031–1036
Kodama S, Saito K, Yachi Y et al (2009) Association between serum uric acid and development of type 2 diabetes. Diabetes Care 32(9):1737–1742
Johnson RJ, Nakagawa T, Sanchez-Lozada LG et al (2013) Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62:3307–3315
Soltani Z, Rasheed K, Kapusta DR, Reisin E (2013) Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep 15(3):175–181
Bickel C, Rupprecht HJ, Blankenberg S et al (2002) Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. Am J Cardiol 89(1):12–17
Anker SD, Doehner W, Rauchhaus M, Sharma R et al (2003) Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 107(15):1991–1997
Tscharre M, Herman R, Rohla M et al (2018) Uric acid is associated with long-term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis 270:173–179
Ndrepepa G, Braun S, Haase HU et al (2012) Prognostic value of uric acid in patients with acute coronary syndromes. Am J Cardiol 109(9):1260–1265
Timóteo AT, Lousinha A, Labandeiro J et al (2013) Serum uric acid: a forgotten prognostic marker in acute coronary syndromes? Eur Heart J Acute Cardiovasc Care 2(1):44–52
Culleton BF, Larson MG, Kannel WB, Levy D (1999) Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 131(1):7–13
Lopez-Pineda A, Cordero A, Carratala-Munuera C et al (2018) Association analysis between hyperuricemia and long term mortality after acute coronary syndrome in three subgroups of patients. Data in Brief 17(5):885–889 (Feb)
Kojima S, Sakamoto T, Ishihara M et al (2005) Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am J Cardiol 96:489–495
Funding
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Çanga, A. Emre, M.B. Karataş, A.N. Çalık, N.S. Yelgeç, D. İnan, G. Yüksel, and S. Terzi declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Çanga, Y., Emre, A., Karataş, M.B. et al. Prognostic value of serum uric acid levels in patients with non-STEMI undergoing percutaneous coronary intervention. Herz 45, 389–396 (2020). https://doi.org/10.1007/s00059-019-04849-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-019-04849-3