Abstract
Background
Angiopoietin-2 (Angpt2) mediates endothelial dysfunction (ED) following coronary artery bypass grafting (CABG). Its triggers are, however, poorly understood.
Methods
We examined the time course of ED beyond the early phase of postoperative recovery in 75 patients following CABG with a special focus on different cardiopulmonary bypass (CPB) modes as potential triggers of Angpt2 release.
Results
Nine patients (12.0%) underwent off-pump coronary artery bypass (OPCAB), 31 patients (41.3%) received minimized extracorporeal circulation (MECC), and 35 patients (46.6%) were operated on with (conventional) CPB. Angpt2 levels steadily increased across the observation period (1.7 [1.4–2.1] to 3.4 [2.5–6.1] ng/ml, p < 0.001). Angpt2 levels did not differ between the MECC and CPB groups (p = 0.564). There was no difference between MECC and CPB patients regarding net fluid balance (p = 0.821) and other surrogate markers of postoperative ED. The magnitude of Angpt-2 increase correlated more strongly with baseline C‑reactive protein (r = 0.459, p < 0.001) than with any other parameter. Hospital length of stay correlated more strongly with baseline Angpt2 levels (r = 0.512, p = 0.005) than with follow-up Angpt2 levels and appeared not to be influenced by CPB mode (p = 0.428).
Conclusion
CABG is associated with prolonged ED, which is determined by the patient’s preoperative inflammatory state rather than by CPB modifications.
Zusammenfassung
Hintergrund
Angiopoietin-2 (Angpt2) führt zur endothelialen Dysfunktion (ED) nach Koronararterien-Bypass-Operation (CABG). Über seine Trigger ist jedoch bislang nur wenig bekannt.
Methoden
Die Autoren untersuchten den Zeitverlauf der ED jenseits der Frühphase der postoperativen Genesung bei 75 Patienten nach CABG mit speziellem Fokus auf den verschiedenen Arten des kardiopulmonalen Bypass (CPB) als potenziellem Trigger der Angpt2-Freisetzung.
Ergebnisse
Bei 9 Patienten (12,0 %) erfolgte eine CABG ohne Herz-Lungen-Maschine („off-pump coronary artery bypass“, OPCAB), bei 31 Patienten (41,3 %) unter Einsatz der minimierten extrakorporalen Zirkulation („minimized extracorporeal circulation“, MECC) und bei 35 Patienten (46,6 %) mit (konventionellem) CPB. Die Angpt2-Werte stiegen während der Beobachtungsphase ständig an (von 1,7 [1,4–2,1] bis 3,4 [2,5–6,1] ng/ml, p < 0,001). Dabei unterschieden sich die Angpt2-Werte nicht zwischen der MECC- und der CPB-Gruppe (p = 0,564). Auch gab es keinen Unterschied zwischen den Patienten mit MECC und denen mit CPB hinsichtlich der Nettoflüssigkeitsbilanz (p = 0,821) und anderer Surrogatmarker der postoperativen ED. Die Größenordnung des Angpt-2-Anstiegs war stärker mit dem Ausgangswert für C‑reaktives Protein korreliert (r = 0,459; p < 0,001) als mit einem der anderen Parameter. Die Verweildauer im Krankenhaus war stärker mit dem Ausgangswert für Angpt2 korreliert (r = 0,512; p = 0,005) als mit den Folgewerten für Angpt2 und schien nicht durch die Art des CPB beeinflusst zu werden (p = 0,428).
Schlussfolgerung
Eine CABG ist mit prolongierter ED assoziiert, die eher durch den präoperativen Entzündungsstatus des Patienten bestimmt wird als durch Modifikationen des CPB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
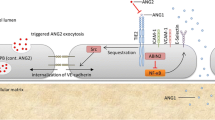
Hemodynamic instability due to reduced effective arterial volume following coronary artery bypass grafting (CABG) performed with the help of cardiopulmonary bypass (CPB) is a very common scenario. Endothelial activation has emerged as a key mechanism among the various causes of early postoperative hemodynamic compromise. The endothelial-specific angiopoietin-Tie2 ligand-receptor system serves as an important booster of endothelial disarray [1, 2]. Triggers such as thrombin or hypoxia can cause angiopoietin-2 (Angpt2) to be released from Weibel–Palade bodies in endothelial cells and disrupt constitutive angiopoietin-1/Tie2 signaling by preventing angiopoietin-1 from binding to the receptor [3, 4]. Disruption of endothelial tight junctions, net extravasation of fluid, a decrease in vascular tone, and collapse of the microcirculation are the clinical consequences of this Tie2 signaling loss [5, 6], which could be demonstrated in critically ill patients with acute kidney injury [7, 8], acute liver failure [9], and more recently in patients undergoing CPB for various cardiac surgery procedures. Clajus et al. prospectively included 25 patients receiving valve replacement or CABG. Angpt2 levels increased within the first postoperative 24 h and were associated with the duration of CPB, fluid balance, and disease severity measures. In immunofluorescence and confocal microscopy in vitro studies, Angpt2 was found to be associated with the disruption of endothelial integrity [10]. A very recent study confirmed this association between CPB and higher Angpt2 plasma levels, although the patient cohort was small [11]. Minimized extracorporeal circulation (MECC) has been introduced to reduce such side effects of the extracorporeal circulation and has been associated with lower rates of blood transfusion, major perioperative adverse events, and in-hospital mortality [12, 13].
Based on these observations, our study had two aims: (a) to describe the time course of Angpt2 release and postoperative net fluid balance as surrogate markers of vascular permeability in a larger, more homogeneous patient cohort; and (b) to examine the role of patient-inherent factors and the CPB mode as potential triggers of Angpt2 release.
Patients and methods
Inclusion and exclusion criteria
Prospective consecutive screening of patients 18 years of age or older who had been scheduled for isolated CABG and consented to participate in the study was performed at our institution between September 2015 and September 2016. Patients with rheumatic or inflammatory diseases, patients on immunosuppressive or antineoplastic medications, patients who had undergone any major operative procedure within the past 3 months, patients with chronic kidney injury of grade 3 (i. e., an estimated glomerular filtration rate <60 ml/min), as well as patients with acute coronary syndromes undergoing nonelective surgery were excluded.
Operative procedure
Operations were performed according to in-house standard operating procedures. General anesthesia was induced by intravenous application of sufentanil (0.7–1.0 ng/kg BW) followed by propofol (1.0 mg/kg BW) and rocuronium (0.6–0.9 mg/kg BW). Furthermore, 8 mg dexamethasone was used as an antiemetic and anti-inflammatory agent as well as standard antibiotic. Patients were given 1–2 mg lorazepam as premedication before transfer to the operating theater. After surgical preparation of the left internal mammary artery, heparin was administered intravenously (400 IU/kg BW) to raise the activated clotting time (ACT) over 400 s before initiating CPB. Heparinization was antagonized by protamine sulfate after termination of CPB with 1 g/100 IU heparin to lower the ACT back to baseline level. All patients underwent routine median sternotomy. By definition, off-pump CABG was performed without extracorporeal circulation. Conventional CPB was performed with the help of a heparin-coated circuit (Dideco C23221/04, LivaNova, London, UK), a venous blood reservoir (Dideco Synthesis R, LivaNova, London, UK), the Stöckert S5 CP5 as centrifugal pump (Sorin Deutschland GmbH, München, Germany), and Quadrox‑i HMO 71000 (Maquet Deutschland GmbH, Rastatt, Germany) as oxygenator. MECC, which is characterized by lower priming volumes, reduced foreign surface areas [14] and the inability to replenish the extracorporeal circuit with blood suctioned from the operating field, but salvaging and purging blood losses by a separate cell saver device was performed with the help of a heparin-coated circuit (Terumo CX-ROCFX25, Terumo Deutschland GmbH, Eschborn, Germany), the Jostra HL20 (Maquet Deutschland GmbH, Rastatt, Germany) as centrifugal pump, and Capiox FX25 (Terumo Deutschland GmbH, Eschborn, Germany) as oxygenator. The left internal mammary artery served as a standard graft, complemented by free radial artery or saphenous vein grafts at the discretion of the operator.
Data acquisition, serum sampling, and Angpt2 quantification
All perioperative laboratory and clinical data, including the Sequential Organ Failure Assessment (SOFA) score 24 h after ICU admission [15], were obtained prospectively via electronic chart records (Imeso®). Obesity was classified as recommended by the World Health Organization [16]. Sampling of Angpt2 was performed at baseline, directly before, directly after, and 24 h as well as 72 h following the completion of CPB (before sternal re-adaptation following off-pump coronary artery bypass [OPCAB]). Serum samples were immediately centrifuged at 3000 g for 10 min and stored at −20 °C. Angpt2 was quantified in a blinded fashion by in-house enzyme-linked immunosorbent assay as described by our group previously [7, 8].
Statistical analysis
Statistical analysis was performed with the help of IBM SPSS Statistics version 22. Data are presented as medians (with 25th and 75th percentiles). Differences between the various CPM modes were compared univariately using the Mann–Whitney U test (comparison of two groups), Kruskal–Wallis test (comparison of three groups), or exact Fisher’s test, as appropriate. Bivariate correlations between Angpt2 levels and continuous clinical or laboratory parameters were tested according to Spearman. All analyses were two-tailed, and the null hypothesis µ0 (assuming equality between the different CPB modes or Angpt2 samples) was rejected on the basis of a type-1 error rate of <5%.
Results
Patient baseline characteristics and postoperative clinical course
The study included 75 patients who had been scheduled for elective CABG and had gone through the study protocol (Fig. 1). Ten out of 75 patients (13.3%) were female, and the median age was 66 years (60–74). The majority of patients presented with pre-obesity (body mass index [BMI]: 29.4 [25.8–32.7]). The median number of coronary anastomoses performed was 3 (2–4). Nine patients (12.0%) underwent OPCAB, 31 patients (41.3%) received MECC, and 35 patients (46.6%) were operated on with CPB support. The OPCAB, MECC, and CPB groups were well matched regarding their baseline clinical values. CPB times were 90 min (75–109) in the MECC group vs. 83.5 min (71.5–101) in the CPB group (p = 0.310). The SOFA score 24 h after ICU admission was 8 (7–9.5). The ICU length of stay (ICU LOS) was short (1.3 days [1.0–3.1]) with a small, but nonsignificant trend towards longer ICU LOS in the OPCAB and MECC groups (p = 0.364). All patients were discharged from hospital after 8 days (7–9), irrespectively of CPB mode used (log rank p = 0.428, Table 1).
Time course of postoperative Angpt2 level increase
Angpt2 levels steadily increased across the observation period from 1.7 ng/ml (1.4–2.1) preoperatively to 3.4 ng/ml (2.5–6.1) 72 h postoperatively (p < 0.001). Of note, Angpt2 levels continued to rise 72 h after surgery, i. e., beyond the time of clinical recovery, indicated by the transition from the ICU to the surgical ward (Table 1, Fig. 2).
Endothelial activation and inflammatory response according to CPB modes
Angpt2 levels were higher in patients scheduled for MECC or conventional CPB compared with patients undergoing OPCAB (p < 0.02); the levels subsequently dropped to reach their trough directly before CPB was established, and increased thereafter (p < 0.001). However, the use of MECC did not prevent the increase of Angpt2 levels compared with conventional CPB (p = 0.564, Table 1, Fig. 2). In contrast to the OPCAB group, patients operated on with the help of extracorporeal support experienced a continuous, but not statistically significant, decline in platelet counts (p = 0.643). However, there was no difference between MECC and CPB patients regarding platelet counts at 48 h (174 [143–217] vs. 182 103/µl [152–211], p = 0.763), lactate levels at 24 h (1.5 [1.0–1.7] vs. 1.6 mmol/l [1.1–2.1], p = 0.580), and net fluid balance at 48 h (638 [260–996] vs. 649 ml [375–956], p = 0.821). Although patients in the MECC group had slightly higher C‑reactive protein levels at 24 h (34.0 [20.3–50.0] vs. 25.4 mg/l [16.5–38.4], p = 0.018), the pattern of overall inflammatory postoperative response was similar between the MECC and CPB groups (Table 1, Fig. 3).
Role of baseline circulating Angpt2 levels and inflammatory markers in outcome prediction
Baseline Angpt2 levels correlated well with BMI and were more often and more strongly associated with follow-up markers of inflammation and endothelial dysfunction than were follow-up Angpt2 levels. The strength of early Angpt2 levels in predicting inflammation and hospital LOS was more pronounced in patients receiving MECC (r = 0.512, p = 0.005) than in those receiving conventional CPB. The magnitude of Angpt2 increase correlated more strongly with baseline C‑reactive protein levels (r = 0.459, p < 0.001) than with any other (patient-inherent or procedural) parameter. Interestingly, CPB time correlated more closely with baseline Angpt2 than with post CPB Angpt2 levels (Fig. 4, Supplementary Table 1).
Correlation of baseline Angpt2 with clinical and laboratory endpoints: a BMI, b CPB time, c baseline CRP, d net fluid balance at 48 h, e WBC at 24 h, and f hospital length of stay. Angpt2 angiopoietin-2, BMI body mass index, CPB cardiopulmonary bypass, CRP C-reactive protein, WBC white blood cell count
Discussion
To the best of our knowledge, this study is the largest to characterize Angpt2, a marker of endothelial dysfunction, in the post-CABG setting and the first to compare OPCAB, MECC, and conventional CPB regarding their role in Tie2 system imbalance.
Clajus et al. were the first to show that the endothelial-specific angiopoietin-Tie2 ligand-receptor system mediates endothelial activation following cardiac surgery. Their study was, however, limited by the number of patients included (n = 25), the mixture of surgical procedures performed (only 36% of patients underwent isolated CABG), as well as the short Angpt2 observation period of 24 h [10]. Using propensity matching, Jongman et al. demonstrated that Angpt2 levels increase beyond 48 h in patients after CABG and that Angpt2 levels show a particular rise in patients with postoperative acute kidney injury [17]. Charbonney et al. included 41 patients undergoing cardiac surgery (n = 20 with CABG), again limiting their sampling period to 24 h postoperatively [18]. These studies found circulating Angpt2 levels to correlate with the duration of CPB, thereby suggesting a pathogenic potential of intraoperative extracorporeal circulation. However, a prospective randomized trial including 60 patients following on-pump vs. off-pump CABG suggested that CPB was not associated with an increased Angpt2 release [19]. Accordingly, in a very recent prospective trial, CABG patients randomized to the off-pump arm had no benefit over patients in the on-pump arm regarding survival, stroke, myocardial infarction, renal failure, or repeat revascularization [20].
A main finding of our study is that on-pump CABG was associated with higher Angpt2 levels than off-pump CABG was. Our data also confirm earlier results that the duration of CPB is associated with Angpt2 levels. Of note, our findings suggest that a miniaturized CPB (MECC) had no advantage over a conventional CPB in terms of reducing endothelial activation and adverse outcomes. This finding is surprising because the use of conventional CPB is sometimes thought to be accompanied by a pronounced low output state and increased third-space fluid losses due to endothelial barrier dysfunction postoperatively, and MECC has been applied in an attempt to ameliorate these side effects. One should keep in mind, however, that a potential advantage of MECC regarding endothelial activation might have been masked by the use of modern centrifugal pump systems in the conventional CPB group and a (nonsignificant) trend toward higher CPB times in the MECC group. When using modern centrifugal pumps and heparin-coated circuits, the time on CPB might be more important than other factors (circuit surface, addition of suctioned blood to the circuit in conventional CPB settings) in terms of endothelial barrier dysfunction. Additionally, patient-related factors might have biased our results, as the allocation to CPB modes was not randomized.
To precisely characterize the triggers of Angpt2 release, sampling was undertaken at five different pre-, intra-, and postoperative time points. Interestingly, Angpt2 levels dropped from the time of hospital admission on the afternoon before surgery to the time of CPB institution, although sternotomy and cannulation of central vessels had already been performed. The fact that OPCAB patients presented with the lowest baseline and follow-up Angpt2 levels, received fewer bypass anastomoses, and presented with lower lactate levels at 24 h and lower fluid balance at 48 h after surgery might indicate a lower disease and inflammatory burden in this patient group; however, this hypothesis is not supported by the other inflammatory markers or the SOFA score.
Previous studies in the field have attributed endothelial dysfunction to the surgical intervention, central vessel cannulation, and/or the harmful effects of CPB [10]. This hypothesis has been challenged, however, by the noninferiority of on-pump versus off-pump surgery in a recent randomized controlled trial regarding robust endpoints [20]. Using a well-established, specific marker of endothelial injury, we showed that (a) reducing the invasiveness of CPB (MECC) neither affected Angpt2 temporal kinetics nor reduced hospital LOS, that (b) baseline instead of post-CPB Angpt2 levels were associated with hospital LOS, and that (c) baseline CRP most strongly correlated with the magnitude of Angpt2 increase. It is therefore tempting to speculate that the baseline inflammatory state of the CABG candidate has a greater influence on clinical outcomes than procedural factors do. This has already been demonstrated for post-CABG neurocognitive outcomes, which had been thought to be amenable to procedural modifications but later appeared to be more closely linked to the degree of preoperative cerebrovascular disease than to the perioperative management itself [21,22,23]. It remains speculative, but preventive measures to improve the preoperative inflammatory state of the patient, e. g., by postponing the operation date or by preoperatively administrating anti-microbial or anti-inflammatory drugs, might receive more attention aiming to minimize endothelial activation in the future.
It is of clinical importance that the state of increased systemic inflammation and endothelial activation with associated endothelial barrier dysfunction and third-space volume shifts following CABG seems to outweigh the phase of acute illness. In our study, the majority of patients were transferred from the ICU to the ward at a time when 24-h fluid balance was still positive and Angpt2 continued to rise, indicating that barrier dysfunction and effective arterial volume had not yet normalized.
In comparison to recent studies in the field, our prospective study is characterized by a large and homogeneous patient cohort treated at a center with significant experience with MECC (41% of CABG procedures performed). The exclusion of combined coronary valve and emergency procedures probably led to lower Angpt2 baseline levels (2.0 vs. 2.6 ng/ml), shorter CPB times (80 vs. 117 min), much shorter mechanical ventilation times (7 vs. 41 h), shorter ICU stays (1.3 vs. 4 days), and a more favorable hospital discharge rate (100% vs. 84%) compared with recent data from our group [10].
Limitations
The limitations of this study are: (a) an imbalance regarding the patient numbers in the OPCAB vs. MECC/conventional CPB groups, (b) a probable underestimation of real-world disease severity owing to exclusion of patients with higher-grade chronic kidney injury, (c) the possibility that the application of MECC circuit components other than those used here might have led to different results, and (d) the fact that Angpt2 levels were not measured beyond the 72-h observation period.
Conclusion
In conclusion, endothelial barrier dysfunction continues beyond the phase of overt acute clinical illness and is mainly determined by the patient’s baseline inflammatory state. On-pump CABG is associated with higher Angpt2 levels than off-pump CABG. When compared with modern centrifugal-pump conventional CPB systems, MECCs seem to offer no advantage in terms of reduced endothelial activation. Further study is warranted to clarify whether postcardiotomy endothelial activation can be targeted by preoperative anti-inflammatory therapeutic strategies or a more vigorous prolonged fluid repletion.
Abbreviations
- Angpt2:
-
angiopoietin-2
- BMI:
-
body mass index
- CABG:
-
coronary artery bypass grafting
- CPB:
-
cardiopulmonary bypass
- CRP:
-
C-reactive protein
- ICU:
-
intensive care unit
- LOS:
-
length of stay
- MECC:
-
minimized extracorporeal circulation
- OPCAB:
-
off-pump coronary artery bypass
- SOFA:
-
sequential organ failure assessment
- WBC:
-
white blood cell count
References
Augustin HG, Koh GY, Thurston G et al (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10:165–177
Fiedler U, Reiss Y, Scharpfenecker M et al (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12:235–239
Fiedler U, Scharpfenecker M, Koidl S et al (2004) The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103:4150–4156
Fiedler U, Krissl T, Koidl S et al (2003) Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem 278:1721–1727
Parikh SM, Mammoto T, Schultz A et al (2006) Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. Plos Med 3:356–370
Roviezzo F, Tsigkos S, Kotanidou A et al (2005) Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 314:738–744
Kümpers P, Lukasz A, David S et al (2008) Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 12:R147
Kümpers P, Hafer C, David S et al (2010) Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med 36:462–470
Hadem J, Bockmeyer CL, Lukasz A et al (2012) Angiopoietin-2 in acute liver failure. Crit Care Med 40:1499–1505
Clajus C, Lukasz A, David S et al (2012) Angiopoietin- is a potential mediator of endothelial barrir dysfunction following cardiopulmonary bypass. Cytokine 60:352–359
Brettner F, Chappell D, Schwartz L et al (2017) Vascular endothelial dysfunction during cardiac surgery: on-pump versus off-pump coronary surgery. Eur Surg Res 58:354–368
Puehler T, Haneya A, Philipp A et al (2009) Minimal extracorporeal circulation: an alternative for on-pump and off-pump coronary revascularization. Ann Thorac Surg 87:766–772
El-Essawi A, Hajek T, Skorpil J et al (2011) Are minimized perfusion circuits the better heart lung machines? Final results of a prospective randomized multicentre trial. Perfusion 26:470–478
Kutschka I, Skorpil J, El Essawi A et al (2009) Beneficial effects of modern perfusion concepts in aortic valve and aortic root surgery. Perfusion 24:37–44
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
WHO (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO technical report series 894. World Health Organization, Geneva
Jongman RM, van Klarenbosch J, Molema G et al (2015) Angiopoietin/Tie2 dysbalance is associated with acute kidney injury after cardiac surgery assisted by cardiopulmonary bypass. PLoS ONE 10:e136205
Charbonney E, Wilcox E, Shan Y et al (2017) Systemic angiopoietin-1/2 dysregulation following cardiopulmonay bypass in adults. Future Sci Oa 3:FSO166
Jongman RM, Zijlstra JG, Ko KWF et al (2014) Off-pump CABG surgery reduces systemic inflammation compared with on-pump surgery but does not change systemic endothelial responses: a prospective randomized study. Shock 42:121–128
Lamy A, Devereaux PJ, Prabhakaran D et al (2016) Five-year outcomes after off-pump or on-pump coronary-artery bypass grafting. N Engl J Med 375:2359–2368
Shroyer AL, Grover FL, Hattler B et al (2009) On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 361:1827–1837
Selnes OA, Gottesmann RF, Grega MA et al (2012) Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med 366:250–257
Selnes OA, Grega MA, Baily MM et al (2009) Do management strategies for coronary artery disease influence 6‑year cognitive outcomes? Ann Thorac Surg 88:445–454
Acknowledgements
We are grateful for the help of Jan Reck, Tom Kreft, and Christian Ulrich, who assisted in the acquisition of serum samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Hadem, R. Rossnick, B. Hesse, M. Herr, M. Hansen, A. Bergmann, G. Kensah, C. Maess, H. Baraki, P. Kümpers, A. Lukasz, and I. Kutschka declare that they have no competing interests.
The study adhered to the ethical guidelines of the 1975 Declaration of Helsinki, was approved by the local ethics committee (approval number 51/15), and was registered at the German Clinical Trials Register (DRKS study number 00008855). All patients provided informed consent prior to study entry.
Caption Electronic Supplementary Material
59_2018_4708_MOESM1_ESM.docx
Listing of significant correlations (Spearman) of Angiopoietin-2 and hospital length of stay with selected patient-inheritant, inflammatory and endothelial markers
Rights and permissions
About this article
Cite this article
Hadem, J., Rossnick, R., Hesse, B. et al. Endothelial dysfunction following coronary artery bypass grafting. Herz 45, 86–94 (2020). https://doi.org/10.1007/s00059-018-4708-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-018-4708-0