Abstract
Objectives
Aim of this study was to develop a finite element model of the forces that patients with rapid maxillary expansion bear and to validate it by a mechanical test.
Methods
Computer-aided design models of the metallic screw and polymeric splint were modelled and discretized. Two forces were generated and considered independently: F1 at the temporary molar (2.5 N) and F2 at the permanent molar (2.5 N). The results of the finite element analysis were used to define the strain values which the anterior and posterior arms of the rapid maxillary expansion appliance bore as a linear function of F1–F2 by calculating the strain–force coefficient δ ij . Two strain gauge rosettes were attached to an appliance which was placed in an XY motorized stage to reproduce the same forces used in the finite element analysis. Once the system was validated, the matrix was inverted to determine forces F1 and F2 that a group of 40 patients underwent (median age 8.33 years, standard deviation 1.86 years) for 75 days, using their strain values. The parents of the patients activated a quarter turn (0.20 mm) twice a day until 50% transversal overcorrection was achieved.
Results
Finite element analysis showed that the effects of the forces on stress at the location of the arms were notably different. There was a satisfactory correlation between finite element analysis predictions and in vitro values. Dissipation of F1 and F2 in patients was predicted to be 62.5 and 80%, respectively, after 75 days of retention.
Conclusion
These results back the finite element analysis model for force prediction.
Zusammenfassung
Ziele
Ziel der vorliegenden Untersuchung war die Entwicklung eines Finite-Elemente-Modells (FEM) zur Bestimmung der Kräfte die bei apparativer forcierter Gaumennahterweiterung (GNE) auftreten. Die FEM-Ergebnisse sollten mit einem mechanischen in-vitro-Test validiert werden.
Methoden
CAD (“computer-aided design”)-Modelle der Metallschraube und des Polymersplints wurden erstellt und diskretisiert. Zwei Kräfte wurden generiert und unabhängig voneinander berücksichtigt: F1 am Milchzahnmolaren (2,5 N) und F2 am permanenten Molaren (2,5 N). Die Ergebnisse der Finite-Elemente-Analyse (FEA) wurden verwendet, um die Dehnungswerte an den anterioren bzw. posterioren Armen der GNE-Apparaturen, als lineare Funktion von F1–F2 durch Berechnung des Dehnung-Kraft-Koeffizienten δ ij zu definieren. Nach Befestigung von 2 Dehnungsmessrosetten an der Apparatur wurde diese in ein “XY motorized stage” eingebracht, um die Kräfte zu reproduzieren, die in der FEA verwendet worden waren. Nach Validierung des Systems wurde die Matrix invertiert, um anhand der Dehnungswerte die Kräfte F1 und F2 zu bestimmen, denen eine Gruppe von Patienten (Median 8,33, Standardabweichung 1,86 Jahre) 75 Tage ausgesetzt war. Die Schrauben wurden von den Eltern der Patienten zwei Mal täglich um eine Vierteldrehung (0,20 mm) bis zum Erreichen einer 50%igen transversalen Überkorrektur aktiviert.
Ergebnisse
Mittels FEA konnten deutliche Unterschiede hinsichtlich der auf die Apparaturarme einwirkenden Kräfte nachgewiesen werden. Zwischen der Vorhersagbarkeit von FEA und In-vitro-Werten zeigte sich eine zufriedenstellende Korrelation. Die Vorhersagbarkeit von F1 und F2 am Patienten lag bei 62,5 bzw. 80% nach 75 Tagen Retention.
Schlussfolgerung
Die dargestellten Ergebnisse bestätigen, dass sich FEA für die Vorhersage von Kräften eignet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discrepancies of the transverse size of the jaw are corrected with rapid maxillary expansion (RME). The objective of RME is to disarticulate the midpalatal suture, providing a stable maxillary width increase [16]. Understanding how the craniofacial complex responds to this technique has been the subject of numerous studies. Aspects such as the anatomic pattern of mineralization in the midpalatal suture [5] or dentoskeletal shift in young patients [7, 20] and adult patients were studied using two-dimensional slice images [8]. The recent addition of computed tomography has allowed us to analyze changes in anatomic volume [18, 22] and animal tests, such as those of Kanekawa and Shimizu [14], have allowed for describing short-term histomorphometric findings in the median suture. Clinical importance has been attached to protocol activation and retention. Thus, Ramuglu and Sari [24] suggest that RME and semirapid maxillary expansion (SRME) have similar effects on dentofacial structures in three planes of space and other authors like Hicks [11] confirmed that during 2–3 months of fixed retention, relapse can decrease between 10 and 23%.
In terms of biomechanics, in the last century, strain gauges were used to measure the tension produced during the expansion phase and the retention of the expansion appliance [5, 29]. With the introduction of the finite element model (FEM), it is possible to simulate the force generated after RME and its effects on the skull, detecting the highest concentration of stress at the pterygoid plates of the sphenoid bone in the region close to the cranial base [13]. Furthermore, by applying viscoelastic material properties to the bone, a newly proposed model gives a realistic simulation of RME as Ludwig et al. indicated [19]. Recent studies in vitro have interrelated strain, force, and displacement generated in circumaxillary sutures by combining stress analysis and FEM [4]. However, there are few studies that reflect the behavior of the apparatus after undergoing RME forces.
The aim of this study is to develop and validate a set of FE computer models to be able to assess the behavior of resistant forces received in the skeleton of different patients treated under the same activation protocol and compared by vertical growth pattern.
Methods and subjects
In vivo tests
A group of 40 patients (18 boys and 22 girls, mean age 8.48 years, range 4–12 years) who showed signs of maxillary compression were treated with RME in the Dental Hospital of the University of Barcelona. Lateral radiographies of the cranium were carried out on the patients and used to calculate the VERT coefficient of variation to classify patients by their vertical growth pattern [25]. The children were divided into three groups:
-
Mesocephalic, described as a proportioned growth pattern,
-
Brachycephalic, described as a horizontal growth pattern, and
-
Dolichocephalic, described as a vertical growth pattern.
The expansion appliance was adhered to the temporary upper molars and first permanent molars. Two strain gauges (SG) (EA-06-015DJ-120/LE, Measurement Group, Texas Instruments Inc., Raleigh, NC, USA) were used to measure the strain’s value which accumulated with the opening of the appliance during treatment. These strain gauges have been used in other studies [5, 26]. The strain gauge temperature self-compensation coefficient and the low variability of the oral temperature allowed us to estimate an upper limit for strain inaccuracy due to temperature variations of ±1% of the readout. The strain gauges were bonded with an adhesive kit (M-bond 200 adhesive and 200 Catalyst/Adhesive Kit & Micro Measurements SR-4®) to the right anterior and posterior arms of a type “S” Hyrax split palatal screw made of AISI 303 and AISI 301 stainless steel (167-1326, Forestadent, Pforzheim, Germany) and the gauges were coated with orthodontic wax (Fig. 1). A uniaxial gauge was used to measure the normal deformation in the longitudinal direction of the arm.
a Rapid maxillary expansion appliance. The red arrow points to the area were the gauge is to be placed. b Side view of the strain gauge bonded to the right posterior arm of a Hyrax screw
Abb. 1 (a) Apparatur zur forcierten Gaumennahterweiterung. Der rote Pfeil zeigt auf den Bereich, in dem der Messstreifen platziert wird. (b) Seitenansicht des auf dem rechten hinteren Arm einer Hyrax-Schraube befestigten Dehnungsmessstreifens
To record the strain, the Model D4 Data Acquisition Conditioner® (Micro-Measurements Raleigh, NC, USA, version 1.32) was used. Channel 1 and channel 2 were used for the anterior arm and the posterior arm, respectively, in all patients. Once installed, the first measurement was taken as 0. Once said data was obtained with the gauge, it was forwarded to the two channels of the Model D4 via a RJ-45 cable and the microstrain values (µS = 10−6) were internally transformed in µV/V. The Model D4 was operated via commands sent over a USB connection. Application software was provided to control the D4 with a MS Windows-based personal computer. During the expansion phase, the parents of the patients activated a quarter turn (0.20 mm) twice a day. Once the overexpansion (50% transversal overcorrection) was obtained, the screw was stabilized with a 0.014-inch double thread ligature for 6 months. Strain values were obtained during the activation phase (T1–2) and the retention phase (T3–7).
The Ethics Committee for Clinical Research of the Dental Hospital of the University of Barcelona, adopted the case–control study (reference number 11/2011) and the parents of all the patients signed a human informed consent form with a waiver containing 21 guidelines based on the International Ethical Guidelines agreed upon in Geneva in 2002.
Finite element model and experimental validation
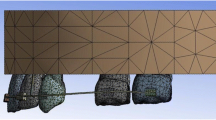
The stress/strain state of the palatal screw and acrylic splint was analyzed by finite element analysis (ANSYS Multiphysics, ANSYS Inc., Canonsburg, PA, USA). Computer-aided design (CAD) models of the metallic screw and polymeric splint were modelled and discretized (the appliance volume partition into elements and nodes). The appliance mesh included 225,728 tetrahedral elements and 332,234 nodes, and the splint mesh included 151,279 tetrahedral elements and 217,313 nodes. The mesh was generated using curvature control. The mechanical properties assigned to the stainless steel appliance were E = 193 GPa (Young’s modulus, isotropic, linear behavior) and ν = 0.25 (Poisson’s ratio). In the acrylic splint, the assigned properties were E = 3.0 GPa and ν = 0.35. Since the objective of the study is not to analyze what is happening inside the molars, their modeling is ancillary (E = 90 GPa, ν = 0.25; Fig. 2a).
Computer-aided design models of the metallic screw and polymeric splint. (a) Appliance–splint mesh. (b) The two considered forces are applied at midcrown of the temporary molars and the permanent molar (55 and 16, as classified by FDI). (c) The insets show the mesh refinement around the location of the strain gauges A–B
Abb. 2 CAD (“computer-aided design)-Modelle der Metallschraube und des Polymersplints. (a) Apparatur-Splint-Mesh. (b) Die beiden berücksichtigten Kräfte werden auf mittlerer Kronenhöhe auf den Milchmolaren und dem bleibenden Molaren aufgebracht (55 und 16 nach FDI-Einteilung). (c) Ausschnitte zur Darstellung des verfeinerten Drahtgeflechts im Bereich der Dehnungsmessstreifen A–B
The followed approach considered a symmetric appliance; therefore, a fully constrained surface was introduced into the midsagittal plane (plane of symmetry). The bonded interfaces between the appliance and the acrylic splint were modelled as completely rigid connections. It was found that there was no displacement or slip page between the acrylic resin and the metal arms of the appliance for manual loading of the appliances removed after treatment, such as loading the appliance in the experimental validation. Model analysis with another nonrigid connection was discarded because it would give unrealistic results. Auxiliary teeth in contact with the splint were modeled to apply forces perpendicular to the appliance plane of symmetry and located 3 mm below the tooth crown surface. Two forces, from 2.5 N to 55 N were considered independently, F1 at the temporary molar and F2 at the permanent molar (55 and 16, as classified by FDI, Fédération Dentaire International; Fig. 2b). In order to validate the model with the mechanical test, the same areas of the RME appliance which best expressed deformation when undergoing forces were compared. For the anterior arm of the screw, gauge A was established and for the posterior arm, gauge B (Fig. 2c). The size of the metal gauge is 0.51/0.38 mm. FEM model in a control zone with these dimensions is defined (for the simulated reading); furthermore, the mesh is refined in an area of 1.5 mm around the area of the gauge, for accuracy of the simulation. The strains produced were determined for different values of the applied forces at the gauge locations. The results of the FEA were used to define strain gauge values A and B as a linear function of forces F1 and F2, by calculating the strain–force coefficients δ ij (Eq. 1).
In order to validate the FEM model, mechanical test measurements with known forces were compared with the FEM model. Mechanical tests were performed on the appliance–splint assembly, displacements were produced by a XY-motorized stage 531.PD (resolution 0.5 µm, repeatability 0.5 µm, Physik Instrumente, Palmbach, Germany), and applied forces were measured by load cell transducers (range 0–100 N, accuracy 0.05%, repeatability 0.02%, Vishay S-STC). During the test, the signals of the strain gauges were recorded, in addition to displacement at different monitoring points. The auxiliary mechanical parts required to apply forces were added to the FEM in the validating configuration.
Prediction of the force received by the RME appliance
Once the FEM was validated, the strain values T2 (end of activation phase) and T7 (end of retention phase) of the patients were used with an inverted matrix (Eq. 1) in order to calculate forces F1 and F2. In this way, the forces to which the RME appliances of the patients of the study were subjected could be predicted.
In the scheme shown in Fig. 3, the steps for determining the forces generated on the patient are illustrated. The mechanical validation is performed once to trust the model, as can been seen in the scheme. Subsequently, once it validated the model, this can be adapted to future, specific geometries and undefined numbers of patients.
Schematic of the steps to estimate forces produced during the trial. In vivo test: model D4 is used to record the strain and values are divided into three vertical growth patterns. Experimental finite element model: obtaining a computer-aided design (CAD) model of appliance RME. The finite element analysis (FEA) model is performed with known forces (2.5 N → 55 N) and a tension is obtained in the anterior and posterior region of the screw by calculating the strain–force coefficients δ ij (equation). Experimental validation: the appliance is subjected to the same force that has been done on FEA and the strain is recorded. This tension is validated with the values obtained from FEA (high coefficient of determination, R 2). Once validated, FEM values of experimental strain (in vivo test) and FEM can be related. In this manner, the forces that each appliance was subjected to in each patient can be estimated
Abb. 3 Schematische Darstellung der Schritte zur Abschätzung der bei den Versuchen auftretenden Kräfte. In-vivo-Testung: Anhand des Modells D4 werden Dehnungen ermittelt, und die Werte werden 3 vertikalen Wachstumsmustern zugeordnet. Experimentelles Finite-Elemente-Modell: Erhalten eines CAD(“computer-aided design”)-Modells für die apparative GNE. Das FEA(Finite-Elemente-Analyse)-Modell wird mit bekannten Kräften (2,5 N → 55 N) durchgeführt, die Dehnung wird ermittelt in der anterioren wie posterioren Schraubenregion durch Berechnung der Belastung-Kraft-Koeffizienten δij (Gleichung). Experimentelle Validierung: Die Apparatur wird derselben Kraft ausgesetzt wie das FEA-Modell, die Belastung wird dokumentiert. Diese Dehnung wird validiert mit den bei der FEA erhaltenen Werten (hoher Determinationskoeffizient, R2). Sodann lassen sich die experimentellen Werte des In-vivo-Tests mit denen des FEM in Beziehung setzen. Auf diese Weise werden die Kräfte ermittelt, denen jede Apparatur bei jedem Patienten ausgesetzt war
Statistical analysis of the predictive values of the forces obtained from the validation was undertaken using the SPSS software program, version 11.5 (SPSS, Chicago, IL, USA). The Kruskal–Wallis test was used to correlate independent variables F1 and F2 with the T1–T2 time difference and growth pattern. A value of P = 0.05 was considered to be statistically significant.
Results
Strain–stress finite element analysis
Figure 2a shows the mesh of the appliance–splint assembly that was employed during finite element analysis (FEA). In order to obtain representative results at the strain gauge location on the 1.5 mm diameter arm, the mesh element size was reduced to 0.1 mm. The component of deformation which is determined by the simulation is the normal strain in the direction of the gauge. Greater refinement produced no differences in results and increased computation time without improving the accuracy. By contrast, a mesh (e.g., 0.3 mm) would produce erroneous predictions because it would average many points of the arm beyond the position of the gauge. As a result, the output values from analysis were precisely extracted from the zones where the strain gauges were located (Fig. 2c).
Figure 4a shows the von Mises stress in the appliance and splint which underwent a 2.5 N force, F1, at the midcrown of the temporary molars, while Fig. 4b shows the same graph when force F2 is applied at the midcrown of the permanent molar. It can be observed that, given the two forces F1 and F2, the effects of force on stress are notably different depending on the arm’s location. A force exerted by the temporary molars does stress the anterior arm more than the posterior arm; on the contrary, a force exerted by the permanent molar produces similar stress in both arms. In other words, under the considered appliance–splint geometry, the strain of the anterior gauge is similarly determined by both forces, while the strain at the posterior arm predominantly depends on the force at the permanent molar. The plots depicted in Fig. 5 show the predicted average normal strain at the gauge locations for F1 and F2 forces when applied independently. The predicted correlation between force and strains was expressed as a matrix in accordance with Eq. (1).
Strain predictions (normal strain in the arm direction) at the location of the strain gauges for a force normal to the palatal symmetry plane: (a) load applied at temporary molar 55; (b) load applied at permanent molar 16
Abb. 5 Vorhersage der Dehnungen (normale Dehnung in Richtung Arm) am Ort der Dehnungsmesstreifen: (a) Belastung auf den Milchmolaren 55; (b) Belastung auf den permanenten Molaren 16
Validation of the FEA model
The results of model validation are summarized in Fig. 6. The experimental set-up and the model, including auxiliary mechanical elements, are shown in Fig. 6a, b. Experimental strain gauge measurements and FEA predictions for forces exerted at the anterior side and posterior side of the splint are displayed in Fig. 6c, d, respectively. The FEA predictions are observed to satisfactorily match the experimental values. Minor deviations of the applied forces between the predictions and the measured values, said deviations being observed at high values, are attributed to the nonlinear behavior of the stainless steel real stress–strain curve. The coefficient of determination R 2 backed the FEA model for force prediction from experimental strain values.
Validation of the finite element analysis model: (a) experimental set-up; (b) model with auxiliary elements for load application and the corresponding mesh; (c) and (d) simulation predicted and experimental measurements for F1 and F2
Abb. 6 Validierung des Finite-Elemente-Analyse-Modells: (a) experimentelles Set-up, (b) Modell mit Hilfselementen für die Lastapplikation und korrespondierendem Drahtgeflecht; (c) und (d) Simulation vorhergesagter und experimenteller Messungen für F1 und F2
Coefficient of determination R 2
-
F1 at splint anterior side: strain located arm A: 0.97; strain located arm B: 0.84
-
F2 at splint posterior side: strain located arm A: 0.86; strain located arm B: 0.93
Predicted force evolution during the clinical trial based on experimental values A and B
The average residual strain remaining at T2 and T7 in the anterior and posterior arm and the average strain dissipation (experimental values) were classified by growth patterns (Table 1a); thus, in this study, the forces were predicted and were classified in the same way. Subsequently, the equation was inverted to calculate the matrix that allows for the prediction of the forces at the anterior and posterior molars from the strain gauges’ experimental values A and B (Eq. 2).
The average values for force predictions by growth pattern are collected in Table 1b. Due to the dissimilar influence of forces on the appliance arms at the anterior and posterior molars, the predicted forces did not follow the patterns previously analyzed for the strains, and further analysis is required for these. Focusing on forces produced at the end of the retention phase, force F1 at the anterior molar was detected to be higher than force F2 at the posterior molar in both mesocephalic and brachycephalic growth patterns. These finding are reversed in the dolichocephalic pattern. The average of the maximum values of F1 was produced at T2, at 75 days’ retention (33.97 N), while the average of the minimum values was at T2, at the end of the expansion phase (1.106413 N). In 62.5% of the patients, force decreased before time T2. However, the average of the minimum values of F2 was at T7 (37.663118) (regardless of T1).
Table 2 shows the descriptive statistics of the periods in which there was residual force dissipation (force A–B) by growth pattern. The percentage of estimated forces which began their dissipation, by biotype, and according to the total sample, was assessed. In 80% of the patients, force decreased before time T7. This prediction indicates that patients received a resistant force in the skull at the end of the expansion phase which was larger in the posterior area than in the anterior area and it dissipated faster in the posterior area. In the average values of dissipated force, a reverse pattern was present; that is to say, the dissipated force was greater in F2 in strong patterns (brachycephalic), decreased in balanced patterns (mesocephalic), and the minimum force was expressed in patterns of vertical growth (dolichocephalic). For F1, dissipation occurred in a contrary manner, being higher in the vertical pattern and lower in stronger horizontal patterns. Inferential statistics show that there is no statistically significant difference in force changes between the end of the expansion phase and the end of the retention phase according to facial biotype (=0.175 PF1–PF2 = 0.130). Furthermore, in Table 3, the evolution of force 1–2 from the first activation (T1) to the end of retention (T7) is given. It shows that there were statistically significant differences in the median force 2 by growth pattern for day 60 (T6) of P = 0.027.
If the ratio of the two forces is analyzed during treatment (at the end of the expansion phase, at the maximum force in retention, and at the end of retention treatment; Fig. 7), it is observed that the posterior molar force in dolichocephalic patterns is accentuated. Thus, it can be concluded that this pattern is related to force transference to the posterior molar and can be seen in force evolution during retention treatment in dolichocephalic growth pattern patients (Fig. 8a). On the contrary, the behavior observed in mesocephalic pattern patients is that the balance of forces after expansion is preserved during retention. Therefore, no transference of forces between the anterior and posterior molar is seen in mesocephalic patients. This behavior can be observed through the force evolution during retention treatment of mesocephalic pattern patients, depicted in Fig. 8b. A third different evolution can be observed in brachycephalic pattern patients, where the transference of force during retention is produced from the posterior molar toward the temporal molar. Such behavior is illustrated in Fig. 8c, which shows different force evolution patterns during the retention phase in brachycephalic pattern patients; a reduction of posterior molar force and an increase in temporal molar force can be observed. It can be understood that when two curves do not intersect, this may be because strain is dissipated. By contrast, when they do intersect, this could mean that a higher initial retention force in the anterior/posterior area is replaced by a higher final retention force in the posterior/anterior zone, respectively.
Force state as a function of the growth pattern: (a) end of expansion treatment; (b) maximum during retention treatment; (c) residual forces at the end of the retention treatment
Abb. 7 Kraftzustand als eine Funktion des Wachstumsmusters: (a) Ende der Expansionsbehandlung; (b) Maximum während der Retentionsphase; (c) am Ende der Retentionsphase verbleibende Kräfte
Representative curves showing force extinction and no force transference during retention treatment. Higher force is preferentially located at the permanent molar for this pattern on dolichocephalic pattern (a), no preference is observed for the location of the higher force between tooth 55 and permanent molar on mesocephalic pattern (b), and higher force is located at tooth 55 on brachycephalic pattern (c)
Abb. 8 Repräsentative grafische Darstellung der Kraftelimination und der fehlenden Übertragung während der Retentionsbehandlung. Für dieses Muster auf dem dolichozephalen Muster wird bevorzugt eine größere Kraft am permanten Molaren lokalisiert (a). Für die Lokalisierung der größeren Kraft zwischen Zahn 55 und dem permanenten Molaren beim mesozephaen Muster lässt sich keine Präferenz beobachten (b), beim brachyozephalen Muster liegt die größere Kraft am Zahn 55 (c)
Discussion
This study analyzed how the arms of hyrax screws respond to the application of independent forces in different facial biotypes. The combination of (in vivo test) clinical trials with FEA and experimental validation provides information (forces exerted on the circuit breaker during treatment) can not be obtained through clinical trials alone, or by FEM simulation alone. Moreover, the findings were compared with the clinical results indicating the strain state and deformation of the RME appliances of 40 patients. For the construction of the FEM of the maxillary–palatal split screw complex, a hyrax screw with acrylic splints was used. The geometry of the appliance was obtained using the CAD technique. In literature based on FEM models, how RME has influenced zygomatic–maxillary sutures at the level of the zygomatic arch [23], downward displacement and backward rotation of the maxilla [9], and the base of the sphenoid bone and the frontal process of the zygomatic bone and lateral bending of the free ends of the pterygoid plates have been discussed [2]. However, no study in the literature has examined the influence of independent forces on the hyrax screw.
The screw that was used in this study was studied by Camporesi et al. [6] and the force generated by the screw after being activated on 20 occasions (1 activation = 0.2 mm) was discussed. It was concluded that, with more activations, more force is generated, and, if the arms of the appliance are more rigid, force is better transmitted to the bone structure. The height of the screw determines the angle of the anterior and posterior arms and, therefore, deformation. The greater the length of the arms, the less rigid the appliance will be. It was decided that the screw be placed to 3 mm to palatal mucosa cranially on the teeth, based on a location similar to clinical location and following the conclusions of Araugio et al. [1]. In their study, tooth tipping was assessed using FEM after RME, positioning the screw 10 mm below the maxillary first molars’ center of resistance, at the same level as the maxillary first molars’ center of resistance, and 10 mm above the maxillary first molars’ center of resistance, whereby the latter one generated less dental tipping. However, according Muchitsch et al. [21], during the prepubertal period, the rigidity of the retention arms is not as important as it is in the subsequent periods of increasing interdigitation and ossification of the median palatal suture. So, rigidity is of vital importance in the design of RME appliances for older patients, but is not a problem in our young study group.
At the time when the interdigitating suture separates after RME, Krebs [15] described that a triangular-based opening pattern occurs in the anterior area of the suture and, according Bishara and Staley [3], in the posterior area the main cause of resistance opening can be explained by the decrease of the elasticity of the pterygoid process as the patients progress through adolescence. Provatidis et al. [23] found similar results after analyzing RME by FEM, from which it was concluded that maximum displacement developed in the maxilla below the hard palate, this being higher in the central incisors and decreasing with distance from the latter.
Given that the force applied at the F1 point (close to the anterior zone of the suture) and F2 point (near the posterior zone of the suture) responds to the level of resistance in this area, it is understood that the accumulated strain or stress in the anterior region, as confirmed clinically by our results [25], is higher due to the stress of F1 and F2 being accumulated, while in the posterior zone it is mainly expressed by force F2. That is to say, if similar forces are generated in the temporary and permanent molars, the anterior gauge would give a greater result than the posterior. When the reading of the gauge is greater in the posterior area, the processed results (F1 and F2) would indicate a negative F1, i.e., there would only be resistance in the posterior area. Therefore, the question may arise whether it would be possible that, during activation, upon exerting force on the posterior area, the anterior area could also expand, leaving expansion in the anterior area without resistance. If separation of the suture means that the anterior area can be separated more easily than the posterior one, then this can explain the effect. As shown in the averages of F1 and F2 obtained in Table 1 from the FEA, it can be seen that, at the end of the expansion phase and, thus, time that greatest suture separation is reached, in all growth patterns, F2 is greater than F1 and, therefore, greater distance between hemimaxilla areas and areas of less force can be associated. Mesocephalic and brachycephalic biotypes at the end of the retention force are greater in F1 (anterior) by the sum of the two forces.
The first factor is the existence of a force/strain which is greater in the anterior area of the bone component; the second factor is the geometry/material of the RME appliance. The two factors are combined. The RME appliance’s effect is to divert a small part of the force/strain of the anterior bone via the posterior arm, while a part of the force/strain of the posterior bone is also deflected to the anterior area. The appliance acts as a source of interference which blurs the anterior arm–anterior bone relationship and the posterior arm–posterior bone relationship, and mixes them lightly. That is to say, if the reading of the gauge indicates that the anterior area is only slightly greater, it may be that the force is approximately the same in front as behind. But if the reading of the gauge is much greater in the front, the force will also be higher in the anterior bone. FEA helps to remove this interference and find out exactly (with the hypothesis of two forces) the value in the bone. Thus, when F1 is greater than F2 at the end of retention phase for mesocephalic and brachycephalic patients, as shown in Fig. 4a, force is more noticeable in the anterior area and, hence, in the posterior area dissipation force is greater. By contrast, in dolichocephalic biotypes, F2 is greater than F1, and, although the strain is expressed equally in the anterior area and in the posterior area (Fig. 4b), a dissipated force of 107 N in the anterior area explains the importance of F1. That is to say, at the time that F1 does not increase, as in mesocephalic–brachycephalic patterns or decreased dolichocephalic patterns, force dissipation exists; as the resistance is much lower (Table 1). In addition, it is found that the experimental behavior of the appliance is linear for loads greater than or equal to 60 N. That is, for the usual values that a patient can receive, a linear elastic model that works successfully was demonstrated.
Furthermore, the magnitude of the forces estimated for patients can generate a controversial issue. While Sun et al. [27] demonstrated with the use of strain gauges that, even if residual strain exists in the circumaxillary sutures, disarticulation does not occur, Wang et al. [28] and Leonardi et al. [17] succeeded in suture separation, demonstrating that circumaxillary disarticulation is greater when the expander appliance is nearer. Boryor et al. [4], with their ex vivo study of an adult skull, assures it takes relatively light force (90 N) to disarticulate the jaws. Although this can not extrapolate to a young patient with mixed dentition, it suggests that the force required to perform a disarticulation has to vary among individuals. The estimated forces found in our system are based on the facial biotype and exceed said amount in the retention phase significantly in the anterior arm of brachycephalic and mesocephalic patterns and in the posterior arm of dolichocephalic patterns. Considering the fact that this force produces residual tension beyond the 75 days’ retention, it can be asked whether the excess accumulated residual strain to which the individual is exposed for prolonged periods of time is necessary. From a clinical point of view, one should consider if it is convenient to accumulate so much residual strain or if it would be better to adapt the range of activations depending on the strain dissipation so that the force is not more than necessary. As Haas [10] mentioned, the median palatal suture is restored during 3–6 months of retention. Our results show that residual force should be a minimum at the end of this period. However, a small percentage of patients showed that the force did not decrease after 75 days. Future investigations should be carried out to analyze longitudinal studies over a longer term and try to find the reason that these forces did not relapsed. FEA of the RME appliance allows for the disposal of more relevant information (force on molars) than the information offered by center screw rotation, even if the hypothesis of two forces does not fully conform to reality.
The validation was carried out in this study in an age group which includes mixed dentition. However, FEM analysis as performed by Holberg [12] shows that age associated with bone elasticity is an important factor in the distribution of stress in deep skeletal structures of the viscero and neurocranium. The study results illustrate that the screw must be activated according to the required expansion and the generated force, as the same activation may result in different forces on the molars for different patients. Moreover, knowing the received force variability, new studies are recommended with the objective to understand the applied forces on the dental effects and its relapse (relate the applied forces with the dental effects and its relapse). Therefore, future studies which validate the forces generated after RME in adolescents and adult patients are required.
Conclusions
Prediction of forces indicates that, at the end of the retention phase, resistance to the expansion of the skull should decrease by 62.5% in the anterior zone and 80% in the posterior. A force (F1) exerted by the temporary molars does stress the anterior arm more than the posterior arm; on the contrary, a force (F2) exerted by the permanent molar produces similar stress in both arms. Force dissipation is greater in the anterior area as the vertical component of the growth pattern increases; however, force dissipation is greater in the posterior area as the horizontal component of the growth pattern increases. The retention time of 6 months is adequate according our results. Nevertheless, in accordance with force prediction, certain patients undergo high forces during the retention phase. Thus, orthodontist should think about clinical implications in these patients. These results back the FEA model for force prediction from experimental strain values.
References
Araugio RM, Landre J Jr, de Silva LD et al (2013) Influence of the expansion screw height on the dental effects of the hyrax expander: a study with finite elements. Am J Orthod Dentofac Orthop 43:221–227
Baldawa RS, Bhad WA (2011) Stress distribution analysis during an intermaxillary dysjunction: A 3-D FEM study of an adult human skull. Ann Maxillofac Surg 1:19–25
Bishara SE, Staley RN (1987) Maxillary expansion: clinical implications. Am J Orthod Dentofac Orthop 91:3–14
Boryor A, Hohmann A, Wunderlich A et al (2010) In-vitro results of rapid maxillary expansion on adults compared with finite element simulations. J Biomech 43:1237–1242
Brosh T, Vardimon AD, Ergatudes C et al (1988) Rapid palatal expansion. Part 3: Strains developed during active and retention phases. Am J Orthod Dentofac Orthop 114:123–133
Camporesi M, Franchi L, Doldo T et al (2013) Evaluation of mechanical properties of three different screws for rapid maxillary expansion. BioMed Eng Online 12:128
Chung CH, Font B (2004) Skeletal and dental changes in the sagittal, vertical, and transverse dimensions after rapid palatal expansion. Am J Orthod Dentofac Orthop 126:569–575
Garib DG, Henriques JFC, Janson G et al (2005) Rapid maxillary expansion—tooth tissue-borne versus tooth-borne expanders: a computed tomography evaluation of dentoskeletal effects. Angle Orthod 75:548–557
Gautam P, Valiathan A, Adhikaric R (2007) Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: a finite element method study. Am J Orthod Dentofac Orthop 132:e1–11
Haas AJ (1961) Rapid expansion of the maxillary dental arch and nasal cavity by opening the mid-palatal sutures. Angle Orthod 31:73–90
Hicks EP (1978) Slow maxillary expansion: a clinical study of the skeletal versus dental response to low-magnitude force. Am J Orthod 73:121–141
Holberg C (2005) Effects of rapid maxillary expansion on the cranial base—an FEM-analysis. J Orofac Orthop 66:54–66
Iseri H, Tekkaya AE, Oztan O et al (1998) Biomechanical effects of rapid maxillary expansion on the craniofacial skeleton, studied by the finite element method. Eur J Orthod 20:347–356
Kanekawa M, Shimizu N (1999) Age-related changes on bone regeneration in midpalatal suture during maxillary expansion in the rat. Am J Orthod Dentofac Orthop 114:646–653
Krebs A (1964) Mid-palatal suture expansion studied by the implant method over a 7 year period. Rep Congr Eur Orthod Soc 40:131–142
Lagravere MO, Major PW, Flores-Mir C (2005) Long-term dental arch changes after rapid maxillary expansion treatment: a systematic review. Angle Orthod 75:155–161
Leonardi R, Sicurezza E, Cutrera A et al (2011) Early post-treatment changes of circumaxillary sutures in young patients treated with rapid maxillary expansion. Angle Orthod 81:36–41
Lione R, Ballanti F, Franchi L et al (2008) Treatment and posttreatment skeletal effects of rapid maxillary expansion studied with low-dose computed tomography in growing subjects. Am J Orthod Dentofac Orthop 134:389–392
Ludwig B, Baumgaertel S, Zorkun B, Bonitz L, Glasl B, Wilmes B et al (2013) Application of a new viscoelastic finite element method model and analysis of miniscrew-supported hybrid hyrax treatment. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Const Soc Am Board Orthod 143:426–435. doi:10.1016/j.ajodo.2012.07.019
McNamara JA Jr (2000) Maxillary transverse deficiency. Am J Orthod Dentofac Orthop 117:567–570
Muchitsch AP, Wendl B, Winsauer H (2011) Rapid maxillary expansion screws on the test bench—a pilot study. Eur J Orthod 33:256–262
Ong SC, Khambay BS, McDonald JP et al (2015) The novel use of three-dimensional surface models to quantify and visualise the immediate changes of the mid-facial skeleton following rapid maxillary expansion. Surgeon 13:132–138
Provatidis CG, Georgiopoulos B, Kotinas A (2008) Evaluation of craniofacial effects during rapid maxillary expansion through combined in vivo/in vitro and finite element studies. Eur J Orthod 30:437–448
Ramuglu SL, Sari Z (2010) Maxillary expansion in the mixed dentition: rapid or semi-rapid? Eur J Orthod 32:11–18
Ricketts RM (1960) A foundation for cephalometric communication. Am J Orthod Dentofac Orthop 46:330–357
Robling AG, Warden SJ, Shultz KL, Beamer WG, Turner CH (2007) Genetic effects on bone mechanotransduction in congenic mice harboring bone size and strength quantitative trait loci. J Bone Miner Res 22:984–991
Sun Z, Hueni S, Tee BC et al (2011) Mechanical strain at alveolar bone and circumaxillary sutures during acute rapid palatal expansion. Am J Orthod Dentofac Orthop 139:e219–e228
Wang D, Cheng L, Wang C et al (2009) Biomechanical analysis of rapid maxillary expansion in the UCLP patient. Med Eng Phys 31:409–417
Zimring JF, Isaacson RJ (1965) Forces produced by rapid maxillary expansion. Part III. Forces presented during retention. Angle Orthod 35:178–186
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
VJ. Garcia, R.L. Cancelos, A. Riveiro, R. Comesaña, J.M. Ustrell, K. Kasem, A. Badaoui, M.C. Manzanares-Céspedes, and P Carvalho-Lobato declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
García, V.J., López-Cancelos, R., Riveiro, A. et al. Determination of forces on a split palatal screw after rapid maxillary expansion. J Orofac Orthop 78, 371–384 (2017). https://doi.org/10.1007/s00056-017-0091-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-017-0091-3