Abstract
Southern pine beetle (Dendroctonus frontalis Zimmermann) and black turpentine beetle (Dendroctonus terebrans Olivier) are two sympatric bark beetle pests of the southeastern United States of America that adversely affect pine (Pinus spp.) health. Successful host tree colonization and reproduction is dependent on a chemical communication system that includes compounds produced by both the beetles and their host trees. To better understand the role of host volatiles in the ecology of these species, we (1) used coupled gas chromatography-electroantennographic detection (GC-EAD) to analyze olfactory sensitivity of D. frontalis and D. terebrans to volatile constituents of host resin, and (2) investigated olfactory stimulants for behavioral effects on both pest species and a major predator, Thanasimus dubius Fabricius (Coleoptera: Cleridae) in field trapping studies. In GC-EAD analyses of the headspace of fresh host resin, antenna of both D. frontalis and D. terebrans produced strongest responses to alpha-pinene, beta-pinene, myrcene, and 4-allylanisole. Field tests indicated that alpha-pinene, beta-pinene, and 4-allylanisole significantly enhanced attraction of D. frontalis, D. terebrans, and T. dubius to traps baited with attractive pheromone components of both bark beetle species, and myrcene diminished this response for D. frontalis. The observed attractive synergism of 4-allylanisole contrasts with previously reported repellency of this compound for D. frontalis and instead suggests this semiochemical may have multiple ecological roles for this species. Lures used for monitoring D. frontalis may be enhanced in sensitivity by adjusting the composition of their host odor components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Dendroctonus is a group of economically and ecologically important bark beetles (Coleoptera: Curculionidae) characterized by a life-history spent primarily in the phloem and cambium of host pines, where they excavate feeding and reproductive galleries (Six and Bracewell 2015). Colonization can result in tree death due to the mining activity of the beetle (which causes girdling of the phloem) and possibly also the effects of weakly pathogenic fungi introduced by the beetles (Nebeker et al. 1993; Six and Wingfield 2011). Conifers have evolved an elaborate chemical defense against biotic invaders that includes the production of toxic oleoresin components and phenolics (Brignolas et al. 1995; Raffa and Smalley 1995; Tisdale et al. 2003; Franceschi et al. 2005; Chiu et al. 2017; Huang et al. 2020). Nevertheless, host trees have strongly co-evolved with Dendroctonus and other bark beetles, and monoterpenes present in host oleoresin for defensive purposes can also be attractive and aid in host location and selection (Byers 1992; Seybold et al. 2006; Miller and Rabaglia 2009).
Within the Dendroctonus genus, D. frontalis and Dendroctonus terebrans are the only two species present in the southeastern United States of America (U.S.), and D. frontalis also occurs in parts of northeastern and southwestern U.S., Mexico, and Central America (Cognato 2011; Dodds et al. 2018). Both beetles colonize all species of pines within their range, but in the southeastern U.S., D. frontalis infests predominantly loblolly (Pinus taeda L.) and shortleaf (P. echinata Mill) pines (Thatcher and Barry 1997; Clarke and Nowak 2009). Typically, these beetle species colonize trees weakened by other agencies such as lightning strikes, other insects, or diseases (Paine et al. 1981; Coulson et al. 1986; Flamm et al. 1993; Sullivan et al. 2003a). Both species may colonize the same host trees and often occur in combination with other bark beetles (Thatcher and Pickard 1966; Flamm et al. 1993; Nebeker 2011). Dendroctonus frontalis is considered a more aggressive and destructive pest of pines than D. terebrans, although both are capable of being primary agents of tree mortality at high population densities (Hopkins 1909; Smith and Lee 1972; Merkel 1981; Thatcher and Barry 1997; Gan 2004). D. frontalis must mass attack to deplete host defenses sufficiently to colonize healthy trees, but D. terebrans can colonize trees that continue to produce a defensive response. Both species use semiochemicals in host finding, mate location, and aggregation, and these may include host odors as well as pheromones of conspecific and heterospecific bark beetles [topics reviewed in Sullivan (2016) and Munro et al. (2019)].
Dendroctonus frontalis releases an aggregation pheromone attractive to both sexes that is strongly synergized by odors from the host tree, and this semiochemical combination stimulates and sustains mass attacks on individual trees (Sullivan 2016). The aggregation pheromone of D. frontalis consists primarily of the female-produced component frontalin (1,5-dimethyl-6,8-dioxabicyclo[3.2.1]octane) and male-produced endo-brevicomin (endo-7-ethyl-5-methyl-6,8-dioxabicyclo[3.2.1]octane) (Renwick and Vité 1969; Vité et al. 1985; Sullivan et al. 2007). Frontalin is the only semiochemical attractive to flying D. frontalis in the absence of other semiochemicals (Payne et al. 1978), although this attractiveness is weak and dramatically increased by the presence of other pheromone components and host odors (Billings 1985; Sullivan et al. 2007). Although host odors (including odors arising from damaged pine tissues, pine resin, and resin distillates) can strongly increase the attractiveness of the aggregation pheromone, host odors alone have not demonstrated attractiveness to flying D. frontalis (Sullivan 2016), and it is unclear how “pioneer” (those first to attack) female beetles locate suitable hosts. At least three non-exclusive mechanisms have been proposed to mediate host selection by pioneer D. frontalis: (1) chance landing of beetles on suitable hosts, (2) pheromones of sympatric pine bark beetles, and (3) visual or olfactory stimuli from host trees (Person 1931; Gara et al. 1965; Heikkenen 1977; Payne 1986; Payne et al. 1987; Hain et al. 2011). Although there is no evidence of long-range attraction to uninfested trees by host odors in D. frontalis, some evidence suggests host odors could influence host selection at short range by stimulating arrestment (Payne 1986; Payne et al. 1987).
In contrast to D. frontalis, D. terebrans is strongly attracted to host odors in the absence of pheromone components (Fatzinger 1985; Siegfried et al. 1986), and they apparently use these to locate suitable hosts prior to bark beetle attacks. Hence, D. terebrans utilizes a “primary” host attractant, a trait that is typical of Dendroctonus species with parasitic relationships with their hosts (Six and Bracewell 2015). As in D. frontalis, female D. terebrans produce the pheromone components frontalin, and males produce brevicomin (the exo- rather than the endo-isomer). It is hypothesized that D. terebrans pheromone components function primarily in mediating interactions between the sexes rather than in host finding or overcoming host defenses (Payne et al. 1987; Phillips et al. 1989).
Limited study has been devoted to the chemical composition of the host-generated semiochemicals for these two species. Numerous studies have demonstrated that distillates of the volatile components of pine resin (i.e., turpentine) are either attractive or synergize attraction for both species [discussed in Sullivan (2016) and Munro et al. (2019)]. However, the volatile composition of pine resin is highly complex, and can differ by tree species, geographic location, and site characteristics (Mirov 1961; Zavarin et al. 1969; Smith 1977, 2000). The volatile components of the resin of preferred host species for both D. frontalis and D. terebrans are similar and dominated by monoterpenes (in particular, alpha-pinene, beta-pinene, beta-phellandrene, camphene, myrcene, and limonene) and the phenylpropanoid 4-allylanisole (Mirov 1961). Lower levels (typically <1%) of alpha-humulene, alpha-phellandrene, alpha-terpinene, beta-caryophyllene, gamma-terpinene, para-cymene, sabinene, terpinolene, and tricyclene may also occur (Hodges et al. 1979; Smith 2000; Sullivan et al. 2003b; Turner et al. 2018; Bookwalter et al. 2019). The enantiomeric composition of chiral host monoterpenes may also vary (Mirov 1961; Phillips et al. 1999; Marques et al. 2012). To date, alpha-pinene (typically the dominant volatile constituent of resin of host pines) is the only component demonstrated to be an attractant synergist for D. frontalis (Staeben et al. 2015), whereas 4-allylanisole can reduce attraction (Hayes et al. 1994a). D. frontalis has previously shown a preference for (+)-alpha-pinene over (−)-alpha-pinene but shows no preference for (+)-alpha-pinene over racemic mixtures (Staeben et al. 2015). Although turpentine is attractive to flying D. terebrans, none of its major constituents (alpha-pinene, beta-pinene, camphene, myrcene, limonene, and beta-phellandrene) were significantly attractive alone (Siegfried et al. 1986). Further, when combined in proportions matching those in the turpentine, these monoterpenes were less attractive than the turpentine itself, implying that the turpentine contained additional attractive constituents. Electroantennogram studies of olfactory sensitivity have indicated that antennae of both species can respond to several individual host odor components (Payne 1975; Dickens and Payne 1977; Delorme and Payne 1990; Niño-Domínguez et al. 2015, 2018).
Our study investigated the semiochemistry of host resin volatiles with D. frontalis and D. terebrans to a depth not addressed in previous research. To detect candidate semiochemicals possibly not identified in prior studies, we applied coupled gas chromatography-electroantennographic detection (GC-EAD) to (1) screen for olfactory stimulants in fresh host resin and (2) assess beetles’ relative olfactory sensitivities to these volatiles, others reported in the resin of host species, and two suspected degradation products of resin monoterpenes encountered by the authors in association with aging resin, distillates, and pine tissue. Semiochemical status for olfactory stimulants was then investigated with trapping assays that released these compounds in combination with pheromone components. Our hypothesis was that, since D. terebrans displays strong attraction to host odors in the absence of their pheromone whereas D. frontalis does not, this disparity could be reflected in differences in both olfactory and behavioral responses to volatile constituents of resin. In-depth knowledge of host-produced semiochemicals for these species may be used to (1) enhance lures for pest detection and monitoring, (2) identify semiochemicals for use in tree and stand protection (e.g., as repellants or attraction disruptants), (3) understand the role of semiochemistry in host discrimination and selection by bark beetles, and (4) elucidate semiochemical interactions between these two species during joint colonization of hosts. Additionally, we examined behavioral responses of the common bark beetle predator, Thanasimus dubius Fabricius (Coleoptera: Cleridae), to the experimental lures, since the same resin-associated semiochemicals may influence predator efficiency in locating prey and thereby affect predation rates for pest management (Erbilgin and Raffa 2001a).
Methods
Electrophysiological response
Dendroctonus frontalis and D. terebrans used for GC-EAD analyses were reared from logs cut from naturally infested pines in the Homochitto National Forest, southwestern Mississippi, U.S. (approximately 31.4°N, 91.0°W). Additionally, due to difficulties in obtaining sufficient numbers of D. terebrans by this method alone, some experimental insects were obtained from pine logs artificially infested in the laboratory (with parents from the aforementioned field-collected logs), and others were collected as callow adults directly under the bark of infested trees at the same location and allowed to melanize in Petri dishes (with moistened filter paper held at room temperature). Beetles used for antennal preparations had emerged or melanized less than 3 weeks earlier, and during this interval were housed at 5 °C in plastic containers with moistened paper wipes. D. frontalis and D. terebrans were sexed using characters in Wood (1982) and Godbee and Franklin (1978), respectively.
GC-EAD apparatus and antennal preparation methods (Asaro et al. 2004, Shepherd and Sullivan 2013) have been described in detail previously. Briefly, the effluent from the GC (Hewlett-Packard model 5890, Palo Alto, California, U.S.) was split with half delivered to a flame ionization detector (FID) and the remainder conveyed to an antennal preparation via a stream of charcoal-filtered, humidified air. The antennal preparation consisted of a pair of glass capillary electrodes (containing Beadle–Ephrussi ringer’s solution and AgCl2-coated silver wires) either inserted into the insect’s excised head or placed in contact with one side of the antennal club. Voltage changes across the electrodes were conditioned with a Syntech (Buchenbach, Germany) Auto-Spike 2/3 IDAC and recorded with an SRI Instruments (Torrance, California, U.S.) model 202 analog–digital converter interfaced with a PC operating with Peak Simple software (SRI Instruments). The GC had an HP-INNOWax column (60 m × 0.25 mm × 0.25 μm film; Agilent Technologies, Wilmington, Delaware, U.S.), and used helium as the carrier gas (30.5 PSI constant pressure). The temperature program was 40 °C for 1 min, ramped 16 °C/min to 80 °C, then 7 °C/min to 230 °C held 8 min. GC-EAD analyses were performed with (1) headspace of fresh resin combined from three major host species (P. taeda, P. echinata, and Pinus elliottii Engelm.) of D. frontalis and D. terebrans and (2) a dose-series (three concentrations) of commercially obtained compounds associated with host pine resin and its distillates.
For resin headspace GC-EAD analysis, resin was tapped from mature trees in Pineville, Louisiana, U.S. (31.36°N, 92.43°W). Glass capillaries (3–5 cm × 1.5 mm i.d.) were inserted into nail-produced holes at 1.5–2 m height on the bole; these penetrated the bark to sapwood depth. Capillaries (one per tree) were left in place until they were at least partially filled with resin (typically 1–2 h). Afterwards, they were removed and immediately placed together (3–6 capillaries) into a 20 ml-capacity amber glass vial with a Teflon-lined septum closure. Although a general effort was made to balance the representation of pine species in the vial headspace, variability in resin production by the individual trees caused disproportions among species. Since the purpose of the test was a broad screening for olfactory stimulants in fresh host resin, we considered this sampling scheme to be adequate. A new headspace vial was prepared fresh each day. The capped vial was shaken to cause resin to exit the capillaries and then left undisturbed for >0.5 h at room temperature prior to headspace sampling. Headspace air (500 µl) from the interior of the vial was sampled at room temperature with a clean gas-tight syringe and after three syringe pumps was injected directly into the GC inlet (200 °C) in split mode (1:20 split ratio). Six males and six females were analyzed for each species. Composition of the headspace samples was analyzed by coupled gas chromatography–mass spectrometry (GC–MS) (model 6890 GC coupled to a 5973-mass selective detector; electron impact mode and a quadrupole ion trap; Hewlett Packard, Palo Alto, California, U.S.) running with identical oven program and column as the GC-EAD; carrier gas was helium at a constant 1 ml/min. Headspace odors were identified by mass spectral data and retention time matches to commercial standards (beta-phellandrene was identified from dipentene, Millenium, Hunt Valley, Maryland, U.S.). Quantitative proportions of compounds in the headspace mixtures were calculated relative to the dominant compound (alpha-pinene) from relative FID integration areas corrected with response factors calculated from commercially obtained standards. EAD spikes were considered evidence of an olfactory response if the voltage amplitude at the given retention time was greater than the 90th percentile of the background noise amplitude for 4 or more of the 12 sampled beetles of each species (sexes were pooled to provide better sensitivity; binomial probabilities test, α = 0.05).
For GC-EAD dose-series analyses, two synthetic mixtures each with nine different compounds identified from resin of hosts of D. frontalis and D. terebrans (Mirov 1961; Sullivan 1997; Sullivan et al. 1997; Bookwalter et al. 2019) were prepared as sources of antennal stimuli (Table 1). Monoterpene alcohols, ketones, and aldehydes (which typically appear in minor quantities in pine resin and extracts) were not included since these compounds are often produced by the beetles (i.e., they may have additional origins than the host) and include pheromone components. beta-Phellandrene was not available to us in sufficient purity to be included in the tests. Although two monoterpene ethers, 4-cineole and eucalyptol, have only occasionally been detected by the authors in odors of pines of the southeastern U.S., they were included in the GC-EAD test mixtures because of their presence in substantial quantities in some commercial turpentines as well as commercial host odor lures for D. frontalis (BTS, unpublished data). Several of the compounds were chiral, and the included enantiomeric composition varied among compounds (Table 1). The enantiomeric ratios (in samples from tissues, volatiles, and resin) reported for alpha-pinene, camphene, and limonene, vary considerably among major host species for the two bark beetles (Mirov 1961; Phillips et al. 1999; Marques et al. 2012), and they were therefore included as 1:1 blends of manufacturer-labeled (+) and (−)-products. [Enantiomeric excess (EE) was indicated only on the alpha-pinene products, with both being >96%.] beta-Pinene in hosts is reported in these same references to be >80% (−); hence, only (−)-labelled commercial product was used in the dilutions. For the remaining chiral compounds, no enantiomeric composition data were available for host species, and enantiomeric composition of the included component was that of the commercial sources available to us.
Compounds were assigned to each mixture to maximize antenna recovery time between exposure to consecutively eluting compounds (minimum 13 s retention time difference). Mixtures were tenfold diluted in solvent (hexane) twice to produce three concentrations, and an identical amount of an olfactory stimulant (endo-brevicomin) was added to each dilution as an internal standard. Mixtures were prepared by adding standards at a fixed volume using calibrated microcapillaries, and approximate (i.e., uncorrected for density or purity) concentrations of compounds in tested dilutions were 0.04, 0.4, and 4 mg/ml (0.04 mg/ml endo-brevicomin in all). Since analyses were run with a 1:1 split ratio between the FID and EAD, a 1:20 split ratio at the GC inlet, and an injection volume of 2 µl, rough approximations of quantities of each compound delivered to the antenna were 0.002, 0.02, and 0.2 µg (all with 0.002 µg endo-brevicomin). At the beginning and end of each GC-EAD run, the antenna was exposed to a standard odor stimulus puffed from a Pasteur pipette (10 µl of a 0.5 mg/ml mineral oil solution of frontalin on a strip of filter paper) into the airstream flowing over the antennal preparation. Three to four each of the male and female beetles of each species were assayed with each concentration of either standard. Olfactory response amplitude to test compounds was normalized relative to response amplitude to the endo-brevicomin internal standard. This latter value was adjusted to compensate for a decline in antenna responsiveness during the run (due to loss in preparation vigor over time) by presuming a linear trend in antennal response voltage with an x-intercept calculated from the change in response amplitudes to the odor puffs at the beginning and end of the run (Sullivan et al. 2007). A genuine olfactory response was recognized at a given retention time if in three or more runs the EAD peak amplitude exceeded the 90th percentile of background amplitude (binomial probabilities test, α = 0.05).
Beetle behavioral response
Compounds eliciting an exceptionally strong olfactory response in D. frontalis and D. terebrans were field tested in two consecutive trapping studies to determine if these compounds could influence beetle attraction when pheromone components were also present. Experiment 1 assessed the capacity of each selected olfactory stimulant alone to influence responses of both beetle species to their attractive pheromone components. Test compounds that did not enhance beetle attraction in Experiment 1 were further assessed in Experiment 2, in which their effects were observed when released simultaneously with the pheromone and a demonstrated host-produced attractant synergist (alpha-pinene). In the absence of a host odor synergist, attraction of both species to their pheromone components is minimal (Phillips et al. 1989; Sullivan 2016); hence detection of possible inhibitory effects required that the lure common to all traps include an attractive host odor component.
Lines of traps were established at four sites in the Oconee Ranger District (ORD), Chattahoochee National Forest, Greene County, Georgia, U.S (center coordinate; site 1: 33.41°N, 83.21°W; site 2: 33.40°N, 83.21°W; site 3: 33.40°N, 83.20°W; site 4: 33.40°N, 83.19°W). The sites were at a sufficient distance from each other to ensure that traps at any two sites were >350 m apart. D. frontalis populations were at endemic (i.e., non-outbreak) levels during experiments, as no infestations had been detected that year in this portion of ORD. Sites were comprised of mixed pines (primarily mature P. taeda and P. echinata Miller) and hardwoods [principally flowering dogwood (Cornus florida L.), hickory (Carya spp.), oaks (Quercus spp.), red maple (Acer rubrum L.), sweetgum (Liquidambar styraciflua L.), and tulip poplar (Liriodendron tulipifera L.)].
Both experiments employed black cross-vane panel traps (IPM Technologies, Portland, Oregon, U.S.) the tops of which were suspended from the metal poles at approximately 2.5 m above the ground. Traps within sites were located >150 m apart to minimize interactions among semiochemical lures and >9 m from the nearest pine to reduce the risk of inducing a beetle attack. Each site had traps equal in number to the treatments, and treatments were assigned randomly to each trap at each site. Treatment positions were re-randomized without replacement every time catches were collected, with collections equal in number to treatments. Hence, our design was a multiple Latin square (to control both temporal and spatial variation within site) that treated sites as squares, and, within each square, traps as rows and collection dates as columns. Trap collection cups were partially filled with dilute propylene glycol (Prestone® Low Tox® Antifreeze/Coolant, Prestone Products Corporation, Danbury, Connecticut, U.S.) to preserve captured insects, and catches were collected every 4 days. Traps in both experiments were consistently baited with frontalin and both endo- and exo-brevicomin, as this ternary combination included the major attractive pheromone components of each respective species [frontalin for both species; endo-brevicomin for D. frontalis and exo-brevicomin for D. terebrans (Phillips et al. 1989; Phillips et al. 1990; Sullivan 2016)]. At low release rates (similar to those produced by the devices chosen for this study) both isomers of brevicomin have demonstrated attraction-enhancing effects for both species (Phillips et al. 1990; Pureswaran et al. 2008). Relative release rates and enantiomeric ratios of individual host-associated compounds in lures broadly reflected the relative concentrations in the odor blends associated with host resin or commercial turpentine, with the major components (alpha-pinene, beta-pinene, and myrcene) released at 3–4 g/day @ ~23 °C, and minor components (4-allylanisole, 1,4-cineole, and eucalyptol) tested at 1–2 orders of magnitude lower release rates (Table 2). The rates used for the major monoterpenes reflected those in host-odor component baits used operationally for monitoring D. frontalis population levels (Sullivan 2016). 1,4-Cineole was tested at two different rates (approximately 0.01 and 0.1 g/day) to detect possible dose-dependent variation in the behavioral activity of this compound, as suggested by the very low olfactory response thresholds of both species to it. Release devices were all attached adjacent to one another at the center of each panel trap.
Experiment 1: Response to host volatiles plus frontalin and brevicomin
Eight panel traps were established at each site (32 traps total; 256 samples) in April 2018. Treatments (i.e., additions to the ternary pheromone lure) were (1) no additional semiochemical (control), (2) alpha-pinene, (3) beta-pinene, (4) myrcene, (5) 1,4-cineole (lower rate), (6) 1,4-cineole (higher rate), (7) eucalyptol, and (8) 4-allylanisole (Table 2).
Experiment 2: Response to host volatiles plus frontalin, brevicomin, and alpha-pinene
Five panel traps were established at each site (20 traps total; 100 samples) in May 2018. In addition to the ternary pheromone blend, the standard lure for experiment 2 included the alpha-pinene device utilized in experiment 1. Treatments (i.e., additions to the standard lure) were: (1) no additional semiochemical (control), (2) myrcene, (3) 1,4-cineole (lower rate), (4) 1,4-cineole (higher rate), and (5) eucalyptol. For both experiments, collected adult D. frontalis, D. terebrans, and T. dubius predators were identified, and voucher specimens were deposited at the Insect Collection at the Natural History Museum, University of Georgia, Athens, Georgia, U.S.
Statistical analyses
Trap catch data were non-normal (Shapiro–Wilk’s test) and over-dispersed (Cameron and Trivedi 1990); therefore, the main effects of the lure treatment on insect captures (i.e., response variable) were determined using negative binomial generalized linear models (GLMs) with fixed effects:
where τ represents the ith treatment, δ represents the jth site, γ represents the kth date, and ρ represents the lth trap. Sum-to-zero contrasts were used for site and trap, so these terms represent differences from the grand mean for each parameter. For all three species, interactions were found to be non-significant and were removed from the final model.
Post hoc Dunn’s tests (i.e., nonparametric pairwise multiple-comparison procedure) with a Holm stepwise adjustment were performed to detect significant differences between lure treatments. Due to low trap catches for D. terebrans in experiment 1, trap catches were summed within site (32 samples; four per treatment), and the log-transformed data (which met parametric assumptions) were analyzed assuming normally distributed errors as a complete block design with site as a block (i.e., model with main effects treatment and site) (Eq. 2).
Since D. terebrans in experiment 1 met parametric assumptions, Post hoc Tukey’s HSD range tests were performed when a significant main effect for treatment was detected. All analyses were completed using R statistical software version 3.6.2 (R Core Team 2019) and RStudio (RStudio Team 2016) using the packages FSA (Ogle et al. 2019), ggplot2 (Wickham 2009), lattice (Sarkar 2008), MASS (Venables and Ripley 2002), multcomp (Hothorn et al. 2008), plyr (Wickham 2011), and rcompanion (Mangiafico 2018). All tests used an alpha level of 0.05.
Results
Electrophysiological response
In the GC-EAD tests with headspace of fresh pine resin (Fig. 1), significant olfactory responses were registered from both species at the retention times of five FID peaks (followed by their quantitative contribution to the odor composition in the same headspace): alpha-pinene (66–73%), beta-pinene (22–28%), myrcene (0.90–2.25%), limonene (0.72–4.7%), and 4-allyanisole (0.10–0.72%). Significant responses also were recorded at the retention times of tricyclene (0.33–0.43%) in D. terebrans and beta-phellandrene (0.30–1.2%) in D. frontalis. For both species, strong responses were observed consistently only at the retention times of alpha-pinene, beta-pinene, myrcene, and 4-allylanisole (Fig. 1).
Coupled gas chromatograph-electroantennographic detector (GC-EAD) recordings of the antenna of bark beetles Dendroctonus frontalis (a) and D. terebrans (b) responding to the headspace of freshly tapped resin of host pines. Both the flame ionization detector (FID) and electroantennographic detector (EAD) traces represent composites (averaging of voltages at identical retention times) of multiple analyses that had low EAD noise levels (for D. frontalis, four analyses of each sex; for D. terebrans, analyses of two males and four females). Responses for males and females were similar within species and thus combined. Peak labels are applicable to both traces
All compounds in the two synthetic blends elicited an electrophysiological response in D. frontalis and/or D. terebrans at least with the highest concentration of exposure (Figs. 2, 3). D. frontalis and D. terebrans both displayed particular sensitivity (indicated by a low response threshold and generally higher response amplitudes) to alpha-pinene, beta-pinene, myrcene, 1,4-cineole, eucalyptol, alpha-terpinene, and 4-allylanisole. The two species differed conspicuously only in sensitivity to the sesquiterpene alpha-humulene which produced a response in D. frontalis at all three concentrations but did not generate a response in D. terebrans.
Responses (±SE) of Dendroctonus frontalis antenna exposed to three dose levels of two different mixtures (1 and 2; Table 1) of selected pine-associated volatiles in a GC-EAD. Data are the combined responses of three females and three males tested at each concentration. Y axis values are amplitude of voltage response to the indicated compound divided by the response to an olfactory stimulant internal standard (endo-brevicomin) included in identical concentrations in both mixtures. An asterisk above a bar indicates that a genuine olfactory response was detected (i.e., the signal amplitude was consistently greater than background noise; see text)
Beetle behavioral response
Experiment 1: response to host resin volatiles plus frontalin and brevicomin
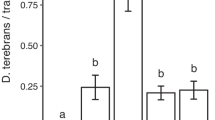
D. frontalis was the most abundant beetle (12,541 adults) trapped followed by T. dubius (7679), and D. terebrans (168). Presence of either alpha-pinene (z = 16.1; p < 0.001), beta-pinene (z = 10.2; p < 0.001), or 4-allylanisole (z = 14.5; p < 0.001) increased catches of D. frontalis by the pheromone blend alone (Fig. 4a). Both alpha-pinene and 4-allylanisole increased average catches approximately 50-fold (albeit with a high degree of variance). The two treatments did not differ in their effect, and both produced greater enhancement than the beta-pinene treatment. In contrast, a high-rate of 1,4-cineole (z = −2.52; p = 0.016) and myrcene (z = −2.17; p = 0.040) reduced D. frontalis captures by threefold, while eucalyptol and low 1,4-cineole had no effect on catches. Although D. terebrans was trapped in low numbers, their catches were increased by all lure treatments other than eucalyptol (F8,23 = 5.74; p < 0.001) (Fig. 4b). Mean catches of T. dubius by the pheromone blend were increased greater than fivefold by alpha-pinene (z = 11.4; p < 0.001), beta-pinene (z = 11.8; p < 0.001), and 4-allylanisole (z = 7.39; p < 0.001) (Fig. 4c). The increase caused by 4-allylanisole was significantly less than that of either alpha- or beta-pinene. Low 1,4-cineole also increased catches of T. dubius (z = 11.4; p < 0.001); however, we saw less than a doubling in numbers. High 1,4-cineole, eucalyptol, and myrcene did not affect trap catches of T. dubius (Fig. 4c).
Mean number (±SE) of bark beetles a Dendroctonus frontalis, b D. terebrans, and c clerid predator Thanasimus dubius trapped with different host-associated olfactory stimulants for both species a and b in combination with a blend of pheromone components for both species (frontalin, endo-brevicomin, and exo-brevicomin). Lure composition and release rates are in Table 2. The control had pheromone but lacked a host odor lure. Bars with the same letters were not significantly different [Dunn’s test; for D. terebrans, Tukey HSD; (α = 0.05)]
Experiment 2: response to host resin volatiles plus frontalin, brevicomin, and alpha-pinene
Dendroctonus frontalis was the most abundant beetle species trapped (5976 adults) followed by T. dubius (3083), and D. terebrans (166). Only myrcene altered (reduced) trap catches by the standard attractant composed of pheromone components plus alpha-pinene (z = −2.08; p = 0.037) for D. frontalis (Fig. 5a). All other host odor treatments did not alter responses: low 1,4-cineole, high 1,4-cineole, and eucalyptol. For D. terebrans, catches by the pheromone and alpha-pinene combination were altered by low (z = 1.74; p = 0.04) and high (z = 2.88; p = 0.004) 1,4-cineole and eucalyptol (z = 2.68; p = 0.007) (Fig. 5b). All three lure treatments increased mean catches by around threefold. Addition of myrcene did not affect trap catches of D. terebrans. Lure treatment did not have a significant effect on catches of T. dubius (Fig. 5c).
Mean number (±SE) of bark beetles a Dendroctonus frontalis, b D. terebrans, and c clerid predator Thanasimus dubius trapped with different host-associated olfactory stimulants for both species a and b in combination with a blend of pheromone components for both species (frontalin, endo-brevicomin, and exo-brevicomin) and the attractive synergist alpha-pinene. Lure composition and release rates are in Table 2. The control had pheromone and alpha-pinene. Bars with the same letters were not significantly different (Dunn’s test; α = 0.05)
Discussion
In our GC-EAD studies, D. frontalis and D. terebrans exhibited similar olfactory response profiles to volatiles associated with resin of their hosts. The odors in the direct headspace samples of fresh resin presumably reflected the same compounds and relative proportions that are encountered in the air by a bark beetle responding to constitutive resin released from a potential host tree as a result of penetration of the bark (as might result from a beetle attack or mechanical damage to the tree). Thus, olfactory stimulants detected in these analyses are candidates as mediators of host finding, selection, and acceptance. The five compounds in resin headspace that generated an olfactory response in both beetle species (alpha-pinene, beta-pinene, myrcene, limonene, and 4-allylanisole) are the predominant components of resin of their shared host species (Mirov 1961; Cook and Hain 1986; Strom et al. 2002; Bookwalter et al. 2019). These compounds were not the only olfactory stimulants in the resin volatiles, as some stimulants identified in the dose–response study such as sabinene were found in very small amounts by GC–MS in the resin headspace; however, the concentrations were likely beneath the threshold of antennal response at the assayed concentration of the headspace. In general, both beetle species exhibited greater sensitivity (Figs. 2, 3) to the more abundant hydrocarbon monoterpenes (e.g., alpha-pinene, beta-pinene) associated with the resin of their hosts. Evident exceptions to this trend were camphene (often an abundant host resin component but not a strong olfactory stimulant) and alpha-terpinene (a strong olfactory stimulant but typically present in minute quantities in resin). This general resemblance of olfactory response profile with the odor composition of the host’s constitutive resin is consistent with adaptations by these species to their host taxa (Becerra 1997; Bruce et al. 2005). Further, the similarity of the olfactory response profiles between these two bark beetle species suggests that their semiochemical-mediated host interactions are governed by many of the same compounds, and that both species—despite conspicuous differences in life-history strategies and host use—are using olfaction to derive similar information about their hosts. Olfactory sensitivity by D. frontalis to alpha- and beta-pinene, and 3-carene (Smith et al. 1993; Niño-Domínguez et al. 2015), and by D. terebrans to alpha- and beta-pinene (Delorme and Payne 1990) has been demonstrated in previous research.
Furthermore, the four compounds in the headspace of host resin coinciding with the largest EAD response—alpha-pinene, beta-pinene, myrcene, and 4-allylanisole—were shown to influence the attraction of both D. frontalis and D. terebrans to traps baited with their pheromone components (Fig. 4a, b). alpha-Pinene and beta-pinene are the predominant two components of the volatile fraction of oleoresin in the major host species of both D. frontalis and D. terebrans (Mirov 1961; Wood 1982; Turner et al. 2018; Bookwalter et al. 2019), typically composing >75% of this fraction. Both alpha- and beta-pinene significantly enhanced attraction of both bark beetle species to their combined pheromone components. Attractant synergism or attraction by alpha-pinene has been demonstrated previously for both D. frontalis and D. terebrans (Renwick and Vité 1969; Miller and Rabaglia 2009; Staeben 2014), although the limited published research on the behavioral activity of beta-pinene with these species failed to discover a response (Renwick and Vité 1969; Siegfried et al. 1986). Additionally, both alpha-pinene and beta-pinene have been identified as attractants or attractant synergists for numerous conifer-infesting beetles (Schroeder 1988; Volz 1988; Schroeder and Lindelow 1989; Hofstetter et al. 2008; Miller and Rabaglia 2009) and are hypothesized to function as a general indicator of host suitability and susceptibility for these insects.
The phenylpropanoid 4-allylanisole was a particularly potent olfactory stimulant and significantly enhanced attraction of both D. frontalis and D. terebrans to pheromone components. This increase was similar to that generated by an approximately 50-fold higher release rate of (racemic) alpha-pinene, previously the only verified host-produced synergist for D. frontalis attraction. Our result is surprising since 4-allylanisole had been shown to be an attraction inhibitor of D. frontalis (Hayes et al. 1994a; Strom et al. 1999), and as such it was explored considerably for use as a tree protectant (Hayes et al. 1996; Strom et al. 2004). It was registered with the Environmental Protection Agency, U.S. as a biorational pesticide against this and other conifer pests (PC Code 062150). However, in the only fully replicated study examining 4-allylanisole as a tree protectant against D. frontalis (Strom et al. 2004), the release of 4-allylanisole from “challenged” trees (i.e., treated with D. frontalis aggregation attractant or weakened with microbiocide) neither decreased nor increased their rate of mortality. Evidence had suggested that 4-allylanisole might serve as an indicator of host susceptibility for D. frontalis, as its quantity declined in pines whose susceptibility was increased artificially with herbicide treatment (Hayes et al. 1994b), with similar results observed in other bark beetle–host systems (Hobson 1995). Avoidance of 4-allylanisole could potentially be beneficial to D. frontalis, as it has been shown to deter the growth of this species’ fungal symbionts and thus potentially interfere with brood development (Bridges 1987). However, other research has not found an association between elevated 4-allylanisole and low host susceptibility: progeny of D. frontalis “escape” trees (pines that survived within infestations and thus, were ostensibly less susceptible to attack) had a lower 4-allylanisole content in their resin than trees in the general population (Strom et al. 2002). In other Scolytinae, 4-allylanisole has been shown either to be an attractant (Rappaport et al. 2000; Joseph et al. 2001) or inhibitor of attraction (Werner 1995; Hobson 1995; Joseph et al. 2001). To our knowledge, there are no prior reports of behavioral activity of 4-allylanisole with D. terebrans; however, Joseph et al. (2001) found it to be an attractant at low release rates (19.2 mg/day at 21 °C) for D. valens, a sibling species to D. terebrans.
There are several conceivable explanations for the contrasting results between our study and previous work regarding response of D. frontalis to 4-allylanisole. It is possible that 4-allylanisole is a “multifunctional”-type semiochemical that enhances attraction at low release rates but inhibits attraction at high rates (Rudinsky 1973; Borden 1997). Bark beetles have displayed multifunctional-type dose responses to certain host odors (Bakke 1983; Erbilgin et al. 2003; Gallego et al. 2008). The earlier research which showed that 4-allylanisole reduced response by D. frontalis to attract-baited traps utilized higher release rates of this compound (160 mg/day) (Hayes et al. 1994a, b) than our study (~50 mg/day). Other differences between the current and past experiments that presumably could have influenced the outcome include (1) differences in the composition of the lures used for the standard attractant (i.e., the earlier studies did not include brevicomin in the lure), (2) trap design (funnel versus panel traps), and (3) positioning of traps within active D. frontalis infestations (earlier studies) or not (our study).
Myrcene was the only olfactory stimulant in host resin found to inhibit attraction of D. frontalis to its aggregation pheromone, either alone or with an attraction-enhancing host odor component present (Figs. 4a, 5a). This diminished response was not observed in either D. terebrans or the predator, T. dubius. Myrcene has been found associated with pines within D. frontalis infestations that had escaped mortality, and thus it was hypothesized to be an indicator of an unsuitable host (Gollob 1980).
Although the monoterpene ethers 1,4-cineole and eucalyptol have rarely been reported as pine-associated odors (Pettersson et al. 2000; Amin et al. 2013; Kiliç and Koçak 2014), they were included in the GC-EAD dose–response study because they have been detected in association with host odor lures used for trapping conifer-infesting beetles (BTS, unpublished data). Although, neither was detected either by GC-EAD or GC–MS in our headspace of pine resin, both compounds generated particularly strong olfactory responses in the dose–response GC-EAD study. Both produced some behavioral activity at the relatively low release rates used in our trapping tests (7–105 mg/day), including enhancement of attraction of D. terebrans by both compounds (Figs. 4b, 5b) and reduction of D. frontalis response to the pheromone component lure by the higher release rate of 1,4-cineole (Fig. 4a). Eucalyptol previously was identified by GC-EAD as a D. frontalis olfactory stimulant present in volatiles of leaves and bark of non-host Carya alba (L.) (Shepherd and Sullivan 2013). Neither compound has previously been reported to have behavioral activity with Dendroctonus; however, eucalyptol has been shown to strongly inhibit response by spruce bark beetle, Ips typographus L., to its pheromone (Andersson et al. 2010). There may be semiochemicals produced by tissues of the host pine but absent in fresh host resin (Sullivan et al. 2000); additionally, semiochemicals produced by non-hosts may play a role in host discrimination (Zhang and Schlyter 2004). Our research did not consider such odors, and it is possible that these also may influence host location and selection in both Dendroctonus species.
Thanasimus dubius is considered a generalist predator of conifer-infesting bark beetles and is possibly an important mortality agent of D. frontalis (Turchin et al. 1999; Erbilgin and Raffa 2001b). It has displayed attraction to a range of semiochemicals produced by both their prey and their prey’s host trees (Herms et al. 1991; Costa and Reeve 2011). Natural selection should favor predators whose attractive cues closely resemble those of their prey (Greenstone and Dickens 2005), and this may be reflected in the general similarities in behavioral responses by T. dubius and D. frontalis to the bioassayed host-associated odors. Strongest attraction enhancement was observed to the same three compounds (alpha-pinene, beta-pinene, and 4-allylanisole); however, preferences among these compounds differed between predator and prey. Unlike D. frontalis, T. dubius was less responsive to 4-allylanisole than alpha-pinene at the tested release rates and showed a similar level of response to both alpha- and beta-pinene (D. frontalis was much more attracted to alpha- than beta-pinene). Prior studies showed similar T. dubius responses to alpha-pinene and beta-pinene (Mizell et al. 1984). Conversely, although the present study found 4-allylanisole to be an attractant synergist for T. dubius, previous research found that 4-allylanisole had no effect on the attraction of T. dubius (Hayes et al. 1994a, b).
Due to a shared pheromone component (frontalin) and cross-attractiveness of non-shared pheromone components (endo- and exo-brevicomin), a significant amount of cross-attraction between D. frontalis and D. terebrans can be presumed to occur during host colonization (Sullivan 2016; Munro et al. 2019). Our data indicate that similar olfactory sensitivities and behavioral responses to host-associated odors should additionally enhance cross-attraction during host colonization. This phenomenon is particularly interesting since it has been hypothesized that D. frontalis may exploit the pheromones from D. terebrans attacks as a means of locating suitable hosts (Payne et al. 1987; Munro et al. 2019), and thus there may be positive feedback between the two species for optimal host location.
Three important considerations should be made in interpreting results of this study: (1) It was not intended as an exhaustive or comprehensive survey of host-generated volatiles that might influence insect–host interactions by the two bark beetles; rather, our studies focused on volatiles associated with constitutive host resin. As previously discussed, numerous field experiments have demonstrated that host resin contains semiochemicals for both species (see reviews Sullivan 2016 and Munro et al. 2019), and, therefore, volatiles associated with constitutive resin are an appropriate initial focus for studies of host-produced semiochemicals for these two pine bark beetles. However, additional odors or differing proportions may be associated with the whole undamaged tree, tree tissues, or induced defensive responses to insect or fungal colonization (Paine et al. 1987, Delorme and Lieutier 1990, Harley et al. 1998, Sullivan et al. 2000, Semiz et al. 2012). Such odors may have distinct influences on beetle behavior and play their own role in insect–host semiochemical interactions, and thus deserve future, additional study. (2) We examined behavioral responses of D. frontalis and D. terebrans to host compounds in the presence of the bark beetles’ pheromones, and thus our behavioral experiments may be relevant to semiochemistry of resin odors only in the context of location of a host tree after beetles have released secondary attractants. Both behavioral tests included pheromone lures because our primary interest was understanding the role that host odors play in cross-attraction between species. It is possible that behavioral responses of D. terebrans to odors present in host resin might be different in the absence of con- or heterospecific pheromones, since in this instance host odors would mediate a different biological function, namely, initial location of an uninfested host. We would not expect an attractive response by D. frontalis to any of the tested host odors in the absence of pheromone since no such response has been reported to resin or distillates (Sullivan 2016). (3) Electroantennogram and trapping experiments included just a single enantiomeric blend of chiral compounds, and this could have influenced the observed activity levels. D. frontalis displays a small but statistically significant difference in both its olfactory and behavioral responses to the enantiomers of alpha-pinene (Staeben 2014), although the racemate does not differ significantly in attractive synergism from the more active (+)-enantiomer. Enantiomeric discrimination may occur also with the other tested chiral compounds of the present study.
The demonstrated significance of semiochemicals in the ecology of bark beetles has inspired extensive research into the use of semiochemicals in monitoring and management of pest species, and our results are particularly relevant to semiochemical management tools for D. frontalis. The host odor component in the lure currently deployed for D. frontalis population monitoring and forecasting consists of a polyethylene enclosure releasing approximately 70% alpha-pinene and 30% beta-pinene (Billings 2011). Our study provides the first experimental evidence indicating beta-pinene to be a synergist for D. frontalis pheromone components, although the effect of combining alpha- and beta-pinene requires investigation. Further, our results indicate that 4-allylanisole should be investigated as a possible lure adjuvant for use in D. frontalis monitoring and management. A more potent attractive lure may aid in the early detection of invasive D. frontalis populations, such as those evidently expanding their range northward in the eastern U.S. in response to climate change (Lesk et al. 2017; Dodds et al. 2018). Our results illustrate how a semiochemical that shows potential as a tree protectant (4-allylanisole) may possibly produce unanticipated and undesirable outcomes. Since 4-allylanisole applications have the potential to increase the attraction of D. frontalis, they presumably could have counter-productive effects such as increased risk of attack on treated trees or enhanced growth of treated infestations. Future research may examine this host volatile more thoroughly to understand the variability of its activity and further investigate the possibly complex role of host volatiles in general in the chemical ecology of D. frontalis.
Change history
14 October 2020
The title of the article the word ���of��� has been deleted.
References
Amin HS, Russo RS, Sive B, Hoebeke ER, Dodson C, McCubbin IB, Hallar AG, Hartz KEH (2013) Monoterpene emissions from bark beetle infested Engelmann spruce trees. Atmos Environ 72:130–133
Andersson MN, Larsson MC, Blaženec M, Jakuš R, Zhang QH, Schlyter F (2010) Peripheral modulation of pheromone response by inhibitory host compound in a beetle. J Exp Biol 213:3332–3339
Asaro C, Sullivan BT, Dalusky MJ, Berisford CW (2004) Volatiles associated with preferred and nonpreferred hosts of the Nantucket pine tip moth, Rhyacionia frustrana. J Chem Ecol 30:977–990
Bakke A (1983) Dosage response of the ambrosia beetle Trypodendron lineatum (Oliver) (Coleoptera, Scolytidae) to semiochemicals. Zeitschrift für angewandte Entomologie 95:158–161
Becerra JX (1997) Insects on plants: macroevolutionary chemical trends in host use. Science 276:253–256
Billings RF (1985) Southern pine bark beetles and associated insects: effects of rapidly-released host volatiles on response to aggregation pheromones. J Appl Entomol 99:483–491
Billings RF (2011) Aerial detection, ground evaluation, and monitoring of the southern pine beetle: state perspectives. In: Coulson RN, Klepzig KD (eds) Southern pine beetle II. US Forest Service, Southern Research Station, General Tech. Rpt. SRS-140, Asheville, pp 245–261
Bookwalter JD, Riggins JJ, Dean JFD, Mastro VC, Schimleck LR, Sullivan BT, Gandhi KJK (2019) Colonization and development of Sirex noctilio (Hymenoptera: Siricidae) in bolts of a native pine host and six species of pine grown in the southeastern United States. J Entomol Sci 54(1):1–18
Borden JH (1997) Disruption of semiochemical-mediated aggregation in bark beetles. In: Cardé RT, Minks AK (eds) Insect pheromone research: new directions. Chapman and Hall, New York, pp 421–438
Bridges JR (1987) Effects of terpenoid compounds on growth of symbiotic fungi associated with the southern pine beetle. Phytopathology 77(1):83–85
Brignolas F, Lacroix B, Lieutier F, Sauvard D, Drouet A, Claudot AC, Yart A, Berryman AA, Christiansen E (1995) Induced responses in phenolic metabolism in two Norway spruce clones after wounding and inoculations with Ophiostoma polonicum, a bark beetle-associated fungus. Plant Physiol 109(3):821–827
Bruce TJ, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Byers JA (1992) Attraction of bark beetles, Tomicus piniperda, Hylurgops palliatus, and Trypodendron domesticum and other insects to short-chain alcohols and monoterpenes. J Chem Ecol 18(12):2385–2402
Cameron AC, Trivedi PK (1990) Regression-based tests for overdispersion in the Poisson model. J Econom. 46(3):347–364
Chiu CC, Keeling CI, Bohlmann J (2017) Toxicity of pine monoterpenes to mountain pine beetle. Sci Rep-UK 7(1):8858
Clarke SR, Nowak JT (2009) The southern pine beetle. U.S. Department of Agriculture Forest Service, Forest Insect & Disease Leaflet, vol 49. pp 1–8
Cognato AI (2011) A review of Dendroctonus frontalis Zimmermann systematics. In: Coulson RN, Klepzig KD (eds) Southern pine beetle II. Gen. Tech. Rep. SRS-140, vol 140. US Department of Agriculture Forest Service, Southern Research Station, Asheville, pp 7–12
Cook SP, Hain FP (1986) Defensive mechanisms of loblolly and shortleaf pine against attack by southern pine beetle, Dendroctonus frontalis Zimmermann, and its fungal associate, Ceratocystis minor (Hedgecock) hunt. J Chem Ecol 12(6):1397–1406
Costa A, Reeve JD (2011) Olfactory experience modifies semiochemical responses in a bark beetle predator. J Chem Ecol 37(11):1166–1176
Coulson RN, Flamm RO, Pulley PE, Payne TL, Rykiel EJ, Wagner TL (1986) Response of the southern pine bark beetle guild to host disturbance. Environ Entomol 15:859–868
Delorme L, Lieutier F (1990) Monoterpene composition of the preformed and induced resins of Scots pine, and their effect on bark beetles and associated fungi. Eur J For Pathol 20:304–316
Delorme JD, Payne TL (1990) Antennal olfactory responses of black turpentine beetle, Dendroctonus terebrans (Olivier), to bark beetle pheromones and host terpenes. J Chem Ecol 16:1321–1329
Dickens JC, Payne TL (1977) Bark beetle olfaction: pheromone receptor system in Dendroctonus frontalis. J Insect Physiol 23:481–489
Dodds KJ, Aoki CF, Arango-Velez A, Cancelliere AW, DiGirolomo MF, Rabaglia RJ (2018) Expansion of southern pine beetle into northeastern forests: management and impact of a primary bark beetle in a new region. J For 116:444–453
Erbilgin N, Raffa KF (2001a) Modulation of predator attraction to pheromones of two prey species by stereochemistry of plant volatiles. Oecologia 127:444–453
Erbilgin N, Raffa KF (2001b) Kairomonal range of generalist predators in specialized habitats: responses to multiple phloeophagous species emitting pheromones vs. host odors. Entomol Exp Appl 99:205–210
Erbilgin N, Powell JS, Raffa KF (2003) Effect of varying monoterpene concentrations on the response of Ips pini (Coleoptera: Scolytidae) to its aggregation pheromone: Implications for pest management and ecology of bark beetles. Agric For Entomol 5:269–274
Fatzinger CW (1985) Attraction of the black turpentine beetle (Coleoptera: Scolytidae) and other forest Coleoptera to turpentine-baited traps. Environ Entomol 14:768–775
Flamm RO, Pulley PE, Coulson RN (1993) Colonization of disturbed trees by the southern pine bark beetle guild (Coleoptera: Scolytidae). Environ Entomol 22(1):62–70
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–375
Gallego D, Galián J, Diez J, Pajares J (2008) Kairomonal responses of Tomicus destruens (Col., Scolytidae) to host volatiles α-pinene and ethanol. J Appl Entomol 132:654–662
Gan J (2004) Risk and damage of southern pine beetle outbreaks under global climate change. For Ecol Manag 191(1–3):61–71
Gara RI, Vité JP, Cramer HH (1965) Manipulation of Dendroctonus frontalis by use of a population aggregating pheromone. Contrib Boyce Thompson Inst 23:55–66
Godbee JF, Franklin RT (1978) Sexing and rearing the black turpentine beetle (Coleoptera: Scolytidae). Can Entomol 110:1087–1089
Gollob L (1980) Monoterpene composition in bark beetle resistant loblolly pine. Naturwissenschaften 67:409–410
Greenstone MH, Dickens JC (2005) The production and appropriation of chemical signals among plants, herbivores and predators. In: Barbosa P, Castellano I (eds) Ecology of predator-prey interactions. Oxford University Press, Oxford, pp 139–165
Hain FP, Duehl AJ, Gardner MJ, Payne TL (2011) Natural history of the southern pine beetle. In: Coulson RN, Klepzig KD (eds) Southern pine beetle II. US Forest Service, Southern Research Station, General Tech. Rpt. SRS-140, Asheville, pp 13–24
Harley P, Fridd-Stroud V, Greenberg J, Guenther A, Vasconcellos P (1998) Emission of 2-methyl-3-buten-2-ol by pines: a potentially large natural source of reactive carbon to the atmosphere. J Geophys Res Atmos 103:25479–25486
Hayes JL, Strom BL, Roton LM, Ingram LL (1994a) Repellent properties of the host compound 4-allylanisole to the southern pine beetle. J Chem Ecol 20(7):1595–1615
Hayes JL, Ingram LL, Strom BL, Roton LM, Boyette MW, Walsh MT (1994b) Identification of a host compound and its practical application: 4-allylanisole as a bark beetle repellent. In: JA Vozzo (ed) Research and applications of chemical sciences in forestry. Proceedings of the 4th Southern Station Chemical Sciences Meeting. U.S. For. Servo Gen. Tech. Rep. SO-104, pp 69–80
Hayes JL, Meeker JR, Foltz JL, Strom BL (1996) Suppression of bark beetles and protection of pines in the urban environment: a case study. J Arboricult 22:67–74
Heikkenen HJ (1977) Southern pine beetle: a hypothesis regarding its primary attractant. J For 75:412–413
Herms DA, Haack RA, Ayres BD (1991) Variation in semiochemical-mediated prey-predator interaction: Ips pini (Scolytidae) and Thanasimus dubius (Cleridae). J Chem Ecol 17(8):1705–1714
Hobson KR (1995) Host compounds as semiochemicals for bark beetles. In: Salom SM, Hobson KR (eds) Proceedings of an informal conference on Applications of Semiochemicals for Management of Bark Beetle Infestations; December 12-16, 1993. USDA, For. Serv. Gen. Tech, Rep. INT-GTR-318, Indianapolis, pp 48–51
Hodges JD, Elam WW, Watson WF, Nebeker TE (1979) Oleoresin characteristics and susceptibility of four southern pines to southern pine beetle (Coleoptera: Scolytidae) attacks. Can Entomol 111(8):889–896
Hofstetter RW, Chen Z, Gaylord ML, McMillin JD, Wagner MR (2008) Synergistic effects of alpha-pinene and exo-brevicomin on pine bark beetles and associated insects in Arizona. J Appl Entomol 132:387–397
Hopkins AD (1909) Contributions toward a monograph of the scolytid beetles: the genus Dendroctonus (No. 17). US Government Printing Office, US Bureau of Entomology Technical Series
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Huang J, Kautz M, Trowbridge AM, Hammerbacher A, Raffa KF, Adams HD, Goodsman DW, Xu C, Meddens AJH, Kandasamy D, Gershenzon J, Seidl R, Gershenzon J (2020) Tree defence and bark beetles in a drying world: carbon partitioning, functioning and modelling. New Phytol 225(1):26–36
Joseph G, Kelsey RG, Peck RW, Niwa CG (2001) Response of some scolytids and their predators to ethanol and 4-allylanisole in pine forests of central Oregon. J Chem Ecol 27(4):697–715
Kiliç Ö, Koçak A (2014) Essential oil composition of six Pinus L. taxa (Pinaceae) from Canada and their chemotaxonomy. J Agr Sci Tech-Iran B 4:67
Lesk C, Coffel E, D’Amato AW, Dodds K, Horton R (2017) Threats to North American forests from southern pine beetle with warming winters. Nat Clim Change 7:713
Mangiafico S (2018) rcompanion: functions to support extension education program evaluation. R package version 1.13.0. https://CRAN.R-project.org/package=rcompanion
Marques FAG, Frensch G, Zaleski SRM, Nagata N, Sales Maia BHLN, Lazzari SMN, Lenz CA, Corrêa AG (2012) Differentiation of five pine species cultivated in Brazil based on chemometric analysis of their volatiles identified by gas chromatography-mass spectrometry. J Braz Chem Soc 23:1756–1761
Merkel EP (1981) Control of the black turpentine beetle. Georgia Forest Research Paper 15. Georgia Forestry Commission, Macon
Miller DR, Rabaglia RJ (2009) Ethanol and (–)-alpha-Pinene: attractant kairomones for bark and ambrosia beetles in the southeastern US. J Chem Ecol 35:435–448
Mirov NT (1961) Composition of gum turpentines of pines. USDA Forest Service Pacific Southwest Forest and Range Experiment Station, pp 25
Mizell RF, Frazier JL, Nebeker TE (1984) Response of the clerid predator Thanasimus dubius (F.) to bark beetle pheromones and tree volatiles in a wind tunnel. J Chem Ecol 10(1):177–187
Munro HL, Sullivan BT, Villari C, Gandhi KJK (2019) A Review of the ecology and management of black turpentine beetle (Coleoptera: Curculionidae). Environ Entomol 48:765–783
Nebeker TE (2011) Southern pine bark beetle guild. In: Coulson RN, Klepzig KD (eds) Southern pine beetle II. US Forest Service, Southern Research Station, General Tech. Rpt. SRS-140. US Forest Service, Asheville, pp 199–209
Nebeker TE, Hodges JD, Blanche CA (1993) Host reactions to colonization by bark beetles and associated pathogens. Interactions among Bark Beetles, Pathogens and Conifer in North American Forests. Academic, London, pp 157–173
Niño-Domínguez A, Sullivan BT, López-Urbina JH, Macías-Sámano JE (2015) Pheromone-mediated mate location and discrimination by two syntopic sibling species of Dendroctonus bark beetles in Chiapas, Mexico. J Chem Ecol 41:746–756
Niño-Domínguez A, Sullivan BT, López-Urbina JH, Macías-Sámano JE (2018) Discrimination of odors associated with conspecific and heterospecific frass by sibling species Dendroctonus frontalis (Coleoptera: Curculionidae: Scolytinae) and Dendroctonus mesoamericanus (Coleoptera: Curculionidae: Scolytinae). Environ Entomol 47:1532–1540
Ogle DH, Wheeler P, Dinno A (2019) FSA: fisheries stock analysis. R package version 0.8.25. https://github.com/droglenc/FSA
Paine TD, Birch MC, Švihra P (1981) Niche breadth and resource partitioning by four sympatric species of bark beetles (Coleoptera: Scolytidae). Oecologia 48(1):1–6
Paine TD, Blanche CA, Nebeker TE, Stephen FM (1987) Composition of loblolly pine resin defenses: comparison of monoterpenes from induced lesion and sapwood resin. Can J For Res 17:1202–1206
Payne TL (1975) Bark beetle olfaction. III. Antennal olfactory responsiveness of Dendroctonus frontalis Zimmermann and D. brevicomis Le Conte (Coleoptera: Scolytidae) to aggregation pheromones and host tree terpene hydrocarbons. J Chem Ecol 1:233–242
Payne TL (1986) Olfaction and vision in host finding by a bark beetle. In: Payne TL, Birch MC, Kennedy CE (eds) Mechanisms in insect olfaction. Clarendon Press, Oxford, pp 111–116
Payne TL, Coster JE, Richerson JV, Edson LJ, Hart ER (1978) Field response of the southern pine beetle to behavioral chemicals. Environ Entomol 7(4):578–582
Payne TL, Billings RF, Delorme JF, Andryszak NA, Bartels J, Francke W, Vité JP (1987) Kairomonal-pheromonal system in the black turpentine beetle, Dendroctonus terebrans (Ol.). J Appl Entomol 103(1):15–22
Person HL (1931) Theory in explanation of the selection of certain trees by the western pine beetle. J For 29(5):696–699
Pettersson EM, Sullivan BT, Anderson P, Berisford CW, Birgersson G (2000) Odor perception in the bark beetle parasitoid Roptrocerus xylophagorum exposed to host associated volatiles. J Chem Ecol 26:2507–2525
Phillips TW, Nation JL, Wilkinson RC, Foltz JL (1989) Secondary attraction and field activity of beetle-produced volatiles in Dendroctonus terebrans. J Chem Ecol 15:1513–1533
Phillips TW, Nation JL, Wilkinson RC, Foltz JL, Pierce HD Jr, Oehlschlager AC (1990) Response specificity of Dendroctonus terebrans (Coleoptera: Scolytidae) to enantiomers of its sex pheromones. Ann Entomol Soc Am 83:251–257
Phillips MA, Savage TJ, Croteau R (1999) Monoterpene synthases of loblolly pine (Pinus taeda) produce pinene isomers and enantiomers. Arch Biochem Biophys 372:197–204
Pureswaran DS, Hofstetter RW, Sullivan BT (2008) Attraction of the southern pine beetle, Dendroctonus frontalis, to pheromone components of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae: Scolytinae), in an allopatric zone. Environ Entomol 37:70–78
Raffa KF, Smalley EB (1995) Interaction of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle-fungal complexes. Oecologia 102(3):285–295
Rappaport NG, Stein JD, del Rio Mora AA, DeBarr G, de Groot P, Mori S (2000) Responses of Conophthorus spp. (Coleoptera: Scolytidae) to behavioral chemicals in field trials: a transcontinental perspective. Can Entomol 132:925–937
Renwick JAA, Vité JP (1969) Bark beetle attractants: mechanism of colonization by Dendroctonus frontalis. Nature 224:1222–1223
RStudio Team (2016) RStudio: integrated development for R. RStudio Inc, Boston
Rudinsky JA (1973) Multiple functions of the southern pine beetle pheromone verbenone. Environ Entomol 2(4):511–514
Sarkar D (2008) Lattice: multivariate data visualization with R. Springer, New York
Schroeder LM (1988) Attraction of the bark beetle Tomicus piniperda and some other bark- and wood-living beetles to the host volatiles a-pinene and ethanol. Entomol Exp Appl 46:203–210
Schroeder LM, Lindelow A (1989) Attraction of scolytids and associated beetles by different absolute amounts and proportions of a-pinene and ethanol. J Chem Ecol 15:807–817
Semiz G, Blande J, Heijari J, Işık K, Niinemets Ü, Holopainen J (2012) Manipulation of VOC emissions with methyl jasmonate and carrageenan in the evergreen conifer Pinus sylvestris and evergreen broadleaf Quercus ilex. Plant Biol 14:57–65
Seybold SJ, Huber DPW, Lee JC, Graves AD (2006) Pine monoterpenes and pine bark beetles: a marriage of convenience for defense and chemical communication. Phytochem Rev 5:143–178
Shepherd WP, Sullivan BT (2013) Southern pine beetle, Dendroctonus frontalis, antennal and behavioral responses to nonhost leaf and bark volatiles. J Chem Ecol 39:481–493
Siegfried BD, Fatzinger CW, Wilkinson RC, Nation JL (1986) In-flight responses of the black turpentine beetle (Coleoptera: Scolytidae) to individual monoterpenes, turpentine, and paraquat-treated slash pines. Environ Entomol 15:710–714
Six DL, Bracewell R (2015) Dendroctonus. In: Vega FE, Hofstetter RW (eds) Bark beetles: biology and ecology of native and invasive species. Academic, Oxford, pp 305–350
Six DL, Wingfield MJ (2011) The role of phytopathogenicity in bark beetle-fungus symbioses: a challenge to the classic paradigm. Ann Rev Entomol 56:255–272
Smith RH (1977) Monoterpenes of ponderosa pine xylem resin in western United States. USDA For. Servo Tech. Bull. No. 1532
Smith R (2000) Xylem monoterpenes of pines: distribution, variation, genetics, function. Gen. Tech. Rep. PSW-GTR-177, vol 454. Pacific Southwest Research Station, Forest Service, US Department of Agriculture, Albany, p 177
Smith RH, Lee RE III (1972) Black turpentine beetle. USDA For Serv For Pest Leaflet 12:1–8
Smith MT, Salom SM, Payne TL (1993) The southern pine bark beetle guild: an historical review of the research on the semiochemical-based communication system of the five principal species. Bulletin 93-4. Virginia Agricultural Experiment Station, Virginia Polytechnic Institute and State University, Blacksburg, VA
Staeben JC (2014) Southern pine beetle (Dendroctonus frontalis Zimmermann): semiochemical ecology, relationship between outbreak populations and lightning strike, and ecological impacts of suppression and control techniques. Ph.D. Dissertation, University of Georgia, Athens, Georgia
Staeben JC, Sullivan BT, Nowak JT, Gandhi KJ (2015) Enantiospecific responses of southern pine beetle (Dendroctonus frontalis) and its clerid predator, Thanasimus dubius, to α-pinene. Chemoecology 25(2):73–83
Strom BL, Roton LM, Goyer RA, Meeker JR (1999) Visual and semiochemical disruption of host finding in the southern pine beetle. Ecol Appl 9:1028–1038
Strom BL, Goyer RA, Ingram LL Jr, Boyd GDL, Lott LH (2002) Oleoresin characteristics of progeny of loblolly pines that escaped attack by the southern pine beetle. For Ecol Manag 158:169–178
Strom BL, Clarke SR, Shea PJ (2004) Efficacy of 4-allylanisole-based products for protecting individual loblolly pines from Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). Can J For Res 34(3):659–665
Sullivan BT (1997) The chemical ecology of host habitat location by larval parasitoids of the southern pine beetle, Dendroctonus frontalis Zimmermann: Olfactory cues and their possible sources. PhD Dissertation, University of Georgia, Athens, Georgia
Sullivan BT (2016) Chapter four—semiochemicals in the natural history of southern pine beetle Dendroctonus frontalis Zimmermann and their role in pest management. Adv Insect Physiol 50:129–193
Sullivan BT, Berisford CW, Dalusky MJ (1997) Field response of southern pine beetle parasitoids to some natural attractants. J Chem Ecol 23:837–856
Sullivan BT, Pettersson EM, Seltmann KC, Berisford CW (2000) Attraction of the bark beetle parasitoid Roptrocerus xylophagorum (Hymenoptera: Pteromalidae) to host-associated olfactory cues. Environ Entomol 29:1138–1151
Sullivan BT, Dalusky MJ, Berisford CW (2003a) Interspecific variation in host-finding cues of parasitoids of the southern pine beetle (Coleoptera: Scolytidae). J Entomol Sci 38:631–643
Sullivan BT, Fettig CJ, Otrosina WJ, Dalusky MJ, Berisford CW (2003b) Association between severity of prescribed burns and subsequent activity of conifer-infesting beetles in stands of longleaf pine. For Ecol Manag 185:327–340
Sullivan BT, Shepherd WP, Pureswaran DS, Tashiro T, Mori K (2007) Evidence that (+)-endo-brevicomin is a male-produced component of the southern pine beetle aggregation pheromone. J Chem Ecol 33(8):1510–1527
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Thatcher RC, Barry PJ (1997) Southern pine beetle. USDA Forest Service, Washington, D.C. (Forest and Disease Leaflet No. 49)
Thatcher RC, Pickard LS (1966) The clerid beetle, Thanasimus dubius, as a predator of the southern pine beetle. J Econ Entomol 59:955–957
Tisdale RA, Nebeker TE, Hodges JD (2003) Role of oleoresin flow in initial colonization of loblolly pine by southern pine beetle (Coleoptera: Scolytidae). J Entomol Sci 38:576–582
Turchin P, Taylor AD, Reeve JD (1999) Dynamical role of predators in population cycles of a forest insect: an experimental test. Science 285:1068–1071
Turner GW, Parrish AN, Zager JJ, Fischedick JT, Lange BM (2018) Assessment of flux through oleoresin biosynthesis in epithelial cells of loblolly pine resin ducts. J Exp Bot 70:217–230
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York. ISBN 0-387-95457-0
Vité JP, Billings RF, Ware CW, Mori K (1985) Southern pine beetle: Enhancement or inhibition of aggregation response mediated by enantiomers of endo-brevicomin. Naturwissenschaften 72:99–100
Volz HA (1988) Monoterpenes governing host selection in the bark beetles Hylurgops palliatus and Tomicus piniperda. Entomol Exp Appl 47(1):31–35
Werner RA (1995) Toxicity and repellency of 4-allylanisole and monoterpenes from white spruce and tamarak to the spruce beetle and eastern larch beetle (Coleoptera: Scolytidae). Environ Entomol 24:372–379
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wickham H (2011) The split-apply-combine strategy for data analysis. J Stat Softw 40(1):1–29. http://www.jstatsoft.org/v40/i01/
Wood SL (1982) The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat Mem 6:1359
Zavarin E, Critchfield WB, Snajberk K (1969) Turpentine composition of Pinus contorta × Pinus banksiana hybrids and hybrid derivatives. Can J Bot 47(9):1443–1453
Zhang QH, Schlyter F (2004) Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric For Entomol 6:1–19
Acknowledgements
We thank Derek Robertson (D.B. Warnell School of Forestry and Natural Resources, University of Georgia), Thomas D. Whitney (D.B. Warnell School of Forestry and Natural Resources, University of Georgia), and JoAnne Barrett [United States Department of Agriculture (USDA), Forest Service, Southern Research Station, Pineville, Louisiana, United States of America] for field and lab assistance. We thank the anonymous reviewers for their suggestions, as these have greatly improved the manuscript. Funding was provided by the USDA Forest Service, Forest Health Protection; USDA Forest Service, Southern Research Station; and D.B. Warnell School of Forestry and Natural Resources, University of Georgia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conceptualization. GC-EAD methodology and analyses were performed by Holly L. Munro, Brian T. Sullivan, and William P. Shepherd. Trapping surveys were performed by Holly L. Munro and Brittany F. Barnes. Statistical analyses were performed by Holly L. Munro and checked by Cristian R. Montes. The first draft of the manuscript was written by Holly L. Munro and Brian T. Sullivan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Handling Editor: Marko Rohlfs.
Rights and permissions
About this article
Cite this article
Munro, H.L., Gandhi, K.J.K., Barnes, B.F. et al. Electrophysiological and behavioral responses Dendroctonus frontalis and D. terebrans (Coleoptera: Curculionidae) to resin odors of host pines (Pinus spp.). Chemoecology 30, 215–231 (2020). https://doi.org/10.1007/s00049-020-00311-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00311-7