Abstract
The Chinese white pine beetle, Dendroctonus armandi Tsai and Li, is one of the most destructive insects in natural and managed forests of Chinese white pine (Pinus armandii Franch.) in China. Female-produced volatiles, as aggregation pheromone candidates, and volatiles released from host pine trees, as possible kairomones, were collected via aeration and hindgut solvent extraction techniques, analyzed by gas chromatography–mass spectrometry (GC–MS), and assayed by field-trapping experiments in Shaanxi and Hubei provinces, China. GC–MS analyses showed that monoterpenes are the major volatile compounds from all aeration samples of P. armandii logs, with or without D. armandi attacks, accounting for >95 % of total volatiles. α-Pinene (63.18 %), Δ3-carene (14.4 %), β-pinene (7.9 %), limonene (6.2 %) and β-myrcene (3.85 %) were the major monoterpenes, while (+)-longifolene (1.1 %) was the most dominant sesquiterpene. Four bark beetle-related volatile compounds, trans-verbenol (tV; major component), exo-brevicomin (EBV), seudenol (SD), and 1-methyl-2-cyclohexen-1-ol (MCOL) were identified from both the aeration extracts of P. armandii logs with D. armandi females and their hindgut extracts. Our field bioassays showed that all four female-produced individual pheromone candidates and their possible combinations (binary, ternary and quaternary) were not significantly attractive to D. armandi. Two pheromone candidate blends [one ternary (tV/SD/EBV) and one quaternary (tV/SD/EBV/MCOL)], and a mixture of host terpene kairomone candidates were also inactive when tested alone. However, when the five major host monoterpenes and one sesquiterpene (mimicking the natural ratio) were combined with the pheromone ternary or quaternary blend, D. armandi trap catches were significantly (4–10 times) higher than captures in the blank control traps or traps baited with the individual blends, indicating a strong synergistic effect. These synergistically attractive semiochemicals are potentially of great utility for monitoring and mass-trapping this serious forest pest of Chinese white pine in western China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Chinese white pine beetle, Dendroctonus armandi Tsai and Li, is one of the most destructive pest insects in natural and managed forests of Chinese white pine (Pinus armandii Franch.) in China (Yin et al. 1984). It is the only Dendroctonus species that is solely distributed in China, including Shaanxi, Sichuan, Gansu, Hubei and Henan provinces. Unlike many other bark beetle species that mainly feed on weakened or unhealthy trees, this native bark beetle species primarily attacks healthy Chinese white pine trees of 30 years or older (Chen and Tang 2007). Serious outbreaks frequently occur in its range, especially in the Qinling and Bashan Mountains, and have resulted in significant tree mortality (about 3 × 108 m3 of pine trees were killed) in the Chinese white pine forests since the 1970s (Xie and Lv 2012). In recent years, through a field survey in Miaotaizi Forest Farm, Liuba, Shaanxi province, it was found that D. armandi is also capable of attacking/killing much younger P. armandii trees (10–30 years) as well as another pine species, P. tabuliformis Carr. (G. Chen, General Station of Forest Pest Management, SFA, China, unpubl data).
The basic biology and ecology of this primary, aggressive bark beetle species have been intensively studied over the past 50 years mainly in the Qinling Mountains (Yin et al. 1984; Chen and Tang 2007; Pu and Chen 2007; Wang and Wang 2010; Wang et al. 2010, 2014). Since 2005, efforts to identify semiochemicals involved in the host tree selection and aggregation behavior and mass attacks of D. armandi have been conducted by several Chinese forest entomologists (Chen et al. 2007; Xie and Ding 2010; Zhang et al. 2010, 2011; Wang et al. 2011a, b; Wu et al. 2012; Xie and Lv 2012). D. armandi females initiate attacks on healthy P. armandii tree trunks, excavate nuptial chambers/galleries in the phloem (Yin et al. 1984; Wang et al. 2014), from which both female-produced pheromone components and pine bark volatiles are released to attract both males and females for mass attacks. Bark volatile compounds from host pine trees (P. armandii), mainly the monoterpenes, were tested on D. armandi in various electroantennogram (EAG) experiments and Y-tube bioassays in the laboratory; significant EAG and positive walking responses were reported to all the major host monoterpenes tested, with females being more responsive than males in most cases (Zhang et al. 2010; Wang et al. 2011a, b). However, neither potential aggregation pheromone components nor EAG-active host volatiles have been adequately identified or field-tested [but see below on problems with Xie and Ding (2010) and Xie and Lv (2012)].
To better understand the semiochemical-based olfactory communication mechanisms underlying successful mass attacks of D. armandi on healthy pine trees, we studied D. armandi female-produced volatiles as aggregation pheromone candidates, host tree volatiles as possible kairomones, and the interactions of these insect- and plant-derived volatiles in Shaanxi and Hubei provinces, China.
Materials and methods
Volatile collections from P. armandii logs with or without D. armandi attacks
Aerations of P. armandii logs (9–13 cm diameter, 20–50 cm long; active aeration length ~20–30 cm long) with or without D. armandi attacks (naturally or caged) using Turkey bags (Toppits®) and Porapak-Q absorbent (Supelco; 100 mg in glass tube) through mini vacuum pumps (Atmospheric Sampling Instrument, QC-1S, Beijing Labor Protection Institute, China) at 100 ml/min air-flow rate were carried out during 27–28 October 2012 for 4–8 h under laboratory conditions. The volatiles trapped on the Porapak-Q absorbent were extracted with 2 ml of hexane for each aeration sample.
Aeration sample #1 A P. armandii log (13 cm diameter, 50 cm long) taken from a tree naturally attacked by D. armandi for about 1–2 weeks, from Liuba, Shaanxi province, was aerated for 5 h in the laboratory. More than 10 D. armandi galleries with mated pairs within the aeration area of the log were recorded. Aeration sample #2 A P. armandii control log (9 cm diameter, 20 cm long) taken from an uninfested tree from the same forest stand as sample #1 at Liuba, Shaanxi province was aerated for 5 h in laboratory. Aeration sample #3 An uninfested P. armandii log (9.5 cm diameter, 25 cm long; cut from the same tree as for sample #2) was artificially infested with 30 D. armandi females for 3 days via pre-drilled holes kept in netting cage in the laboratory. This artificially infested log was aerated for 8 h. These D. armandi females were collected from newly attacked P. armandii logs/trees from Liuba, Shaanxi province, on 20 October 2012. Most females were from new and short galleries containing one female and a male, thus the mating status of these beetles was unknown. Aeration sample #4 An uninfested P. armandii log (9.5 cm diameter, 25 cm long; cut from the same tree as for samples #2 and #3) was artificially infested with 30 D. armandi males for 3 days via pre-drilled holes kept in a netting cage in the laboratory. This artificially infested log was aerated for 4 h. These D. armandi males were collected from newly attacked P. armandii logs/trees from Liuba, Shaanxi province, on 20 October 2012. Most beetles were from new, short galleries with one female and a male; thus, their mating status was unknown. The collected males and females were kept in plastic bottles with pine bark and moistened tissue paper, and kept in 4 °C before using for artificial attacks. Sex of the beetles was determined by acoustic differences (males stridulate) before placing in the test cages.
Hindgut extracts of D. armandi females
Two hindgut extracts of D. armandi females were each sampled with 1 ml of hexane in 2-ml glass vials set on dry ice. The 1st hindgut extract (#1) was prepared from 10 live D. armandi females collected from the aeration sample #3 log (after 3-day feeding and aeration sampling) on 27 October 2012 by pulling out hindguts through abdominal tips using a sharp forceps under a stereomicroscope. The second hindgut extract (#2) was prepared in the same way as the first extract, but from 15 live D. armandi females (mostly mated, some freshly mated and some unmated females in the galleries) that were taken from a naturally infested P. armandii log, on 8–9 August 2013, at Miaotaizi Forest Farm, Liuba, Shaanxi province.
All the aeration and hindgut extracts were kept in −20 °C before gas chromatography–mass spectrometry (GC–MS) analyses.
Gas chromatography–mass spectrometry analyses
The aeration and hindgut extracts were concentrated to 100 µl under N2, and analyzed by coupled GC–MS using an HP 6890 GC coupled to an HP 5973 mass selective detector using (for all the samples) a polar GC column (HP-INNOWAX; 30 m × 0.53 mm × 1.0 μm film thickness; Agilent Technologies, Wilmington, DE, USA), and on one occasion (for aeration sample #2) using a non-polar Agilent GC column (HP-5MS, 30 m × 0.25 mm × 0.25 μm film thickness). 2 µl of each concentrated sample was injected in splitless mode for each column. Helium was used as the carrier gas, and the injector and detector temperatures were 250 and 300 °C, respectively. Column temperature was programmed from 50 °C for 1 min, to 240 °C at 10 °C/min, with a final hold for 10 min. Ionization was electron impact at 70 eV. Compounds of interest were identified using Wiley7 N and pal600 k spectral libraries, and by comparison of retention times and mass spectra to those for authentic standards. In cases of partially over-lapping or co-eluting peaks, the beetle-produced compounds were identified based on MS after subtracting background noises or partially co-eluting peaks (from pine log) or with the extracted characteristic selected ions for the compounds of interest.
Chemical standards
All synthetic compounds, their sources, purities, release devices and release rates for field-trapping experiments are listed in Table 1.
Field-trapping experiments
Two field-trapping experiments were carried out during 2013 (in Shaanxi) and 2014 (in Hubei) summers using black cross-barrier type traps (Pherobio Technology Co., Ltd., Beijing, China). Experiment 1 tested all four female-produced volatile components, trans-verbenol (tV), (±)-seudenol (SD), (±)-exo-brevicomin (EBV) and (±)-1-methyl-2-cyclohexen-1-ol (MCOL), in a full factorial experimental design, i.e., all the individuals, binary, ternary and quaternary blends during 20 March to 18 April 2013, and 5 August to 10 September 2013, at Liuba county, Shaanxi, China. One set of 16 cross-barrier type traps during each testing period was set up along the edge of a P. armandii forest stand (33°41′N; 106°50′E; 1,350 m.a.s.l.; 70 years old; 15 m tall, 24 cm DBH) on eastern slope at Miaotaizi Forest Farm with ca. 10 m between traps within the trap set, and ca. 10 m from the nearest trees. Within the trap set, 15 traps were baited with individual or various blends of the four synthetic pheromone candidates, and a 16th trap was left unbaited as a blank control. Experiment 2 tested a ternary blend of pheromone candidate compounds (tV, SD and EBV), a quaternary blend of pheromone candidates (tV, SD, EBV and MCOL), a synthetic terpene mixture (five monoterpenes plus one sesquiterpene identified from the P. armandii log as major volatile kairomone candidate compounds), and their combinations, plus an unbaited blank control during 6–28 July 2014, Wenshui Forest Farm, Shengnongjia, Hubei, China. Four sets (blocks) of six cross-barrier type traps were set up along the edges of three P. armandii forest stands (31°33′N; 110°20′E; 2,000 m.a.s.l.; 30–50 years old; 8–12 m tall, 8–12 cm DBH) with >30 m between two trap sets, ca. 10 m between traps within each trap set, and ca. 10 m from the nearest trees. Each tested compound or blend was released separately from a polyethylene bag (with a substrate felt; and with different sizes and thickness). The dispenser types, loading and release rates of the tested semiochemicals are described in Table 1. The positions of traps together with dispensers within each set were assigned randomly, and were re-randomized after each collection replicate when 5–10 or more beetles were caught in the best trap(s) to minimize the positional effect (Byers 1993; Fettig et al. 2006). Each collection period (temporal replicate period) lasted from 1 day to 1 week depending on the weather and beetle flight activity.
Statistical analysis
Trap-catch data were log(x + 1) transformed to fit the assumption of homogeneity of variance for ANOVA. Means were compared by ANOVA followed by the Ryan–Einot–Gabriel–Welsh (REGW) multiple Q test (SPSS 16.0 for Windows) at α = 0.05.
Results
GC–MS analyses
GC–MS analyses showed that monoterpenes are the major volatile compounds from all the aeration samples of P. armandii logs with or without D. armandi attacks, accounted for >95 % of total volatile composition. α-Pinene, Δ3-carene, β-pinene, limonene and β-myrcene are the major monoterpenes (Fig. 1a; Table 2), while (+)-longifolene is the most dominant sesquiterpene (Fig. 1a, b), followed by trans-β-caryophyllene and α-copaene (Figs. S1, S2). Other minor sesquiterpenes included trans-β-farnesene, Δ-cadinene, γ-cadinene, germacrene, amorphene, gurjunene and longipinene (Fig. S2). Oxygenated monoterpenes, bornyl acetate and camphor, were also detected (Fig. S2). β-Myrcene and Δ3-carene co-eluted on the polar column (Fig. 1a and Fig. S1), but were easily separated on the non-polar column (Fig. S3).
a GC–MS traces of aeration extract samples of P. armandii logs with or without D. armandi attacks on a polar column. Upper trace total ion current (TIC) chromatogram of volatiles released from an un-attacked control log (#2); middle trace TIC chromatogram of volatiles released from a pine log with 30 D. armandi females (#3); lower trace TIC chromatogram of volatiles released from a pine log with 30 D. armandi males (#4). b Close-up view (with retention time ranged from 12 to 20 min) of the GC–MS traces presented in a, showing the minor volatile compounds
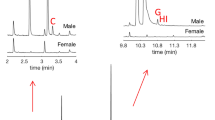
No significant differences in the plant volatile compositions and relative abundances among the pine logs with or without D. armandi attacks (natural or caged) were found. However, bark beetle-produced compounds, trans-verbenol (tV), exo-brevicomin (EBV), seudenol (SD; 3-methyl-2-cyclohexen-1-ol), 1-methyl-2-cyclohexen-1-ol (MCOL) and verbenone, were detected from the aeration (#3) extract of the P. armandii log with 30 D. armandi females caged for 3 days (Figs. 1b, 2, Fig. S2; Table 3), but not from the un-attacked control log (#2) or the log (#4) caged with 30 D. armandi males (Fig. 1b; Table 3). Some minor peaks from the non-attacked control log sample appeared to have similar retention times as some of beetle-produced compounds, but were either column junks or pine sesquiterpenes (Fig. 1b), not the beetle origin. In fact, most of the caged male D. armandi died during the 3-day caging period. trans-Verbenol is the most dominant component among the five bark beetle-produced compounds from the P. armandii log with 30 caged D. armandi females (Table 3; Figs. 1b, 2 and Fig. S2). Only three bark beetle-produced compounds, trans-verbenol, exo-brevicomin and verbenone, were identified from the naturally attacked P. armandii log (#1) (Table 3; Fig. S2), with verbenone being the most dominant, followed by the trans-verbenol. GC–MS analysis of the hind-gut extract (#1) of the caged D. armandi females (on the P. armandii log for 3 days) showed four bark beetle-produced compounds, trans-verbenol (major), exo-brevicomin (minor), seudenol (trace) and MCOL (trace) (Fig. 2; Table 3). No verbenone was detected from this female hindgut extract. The same four female-produced pheromone candidate compounds were also identified from another hindgut extract (#2) of D. armandi females (mostly mated) from naturally attacked P. arnandii logs taken in August 2013, but a significant amount of verbenone plus a trace amount of cis-verbenol were detected from this hindgut sample (Table 3). All the bark beetle-related compounds were identified and confirmed with synthetic standards (Fig. 2).
GC–MS traces of aeration/hindgut extract or synthetic mixture samples on a polar column. Upper trace TIC chromatogram of a hindgut extract (#1) of D. armandi females fed on P. armandii log for 3 days; middle trace TIC chromatogram of volatiles released from a pine log with 30 D. armandi females (#3); lower trace TIC chromatogram of a synthetic mixture of common Dendroctonus aggregation pheromone components (100 ng/µl each). *Indicating the beetle-produced volatile compound
Field-trapping experiments
Experiment 1 carried out during both early and late summers of 2013 in Liuba, Shaanxi, showed that all the four female-produced individual pheromone candidates and their possible combinations (binary, ternary and quaternary) were not significantly attractive to D. armandi, since their trap catches were not different from the unbaited blank control traps (Fig. 3). In Experiment 2, two pheromone candidate blends [one ternary (tV/SD/EBV) and one quaternary (tV/SD/EBV/MCOL)], and a mixture of host terpene kairomone candidates were all inactive when tested alone. However, when combined with the host terpene mixture, the pheromone ternary or quaternary blend had trap catches that were significantly (4–10 times) higher than the blank control or their separate blends, indicating a strong synergistic effect (Fig. 4). The combination of the terpene-blend with the quaternary blend of pheromone candidates caught 59 % more D. armandi beetles than the combination of the terpene-blend with the pheromone ternary blend, but the difference was not statistically significant (Fig. 4), indicating a possible additive effect by MCOL. The sex ratio of captured beetles was estimated as close to 1:1, based on the pooled sample.
Mean captures (+SE) of D. armandi in cross-barrier type traps baited with four female-produced synthetic pheromone candidates, trans-verbenol (tV), (±)-seudenol (SD), (±)-exo-brevicomin (EBV) and (±)-1-methyl-2-cyclohexen-1-ol (MCOL) in a full factorial design, i.e., all individuals, binary, ternary and quaternary blends, plus an unbaited blank control, during March 20–April 18, 2013 ( ), and August 5 to September 10 (
), and August 5 to September 10 ( ), 2013, at Liuba county, Shaanxi, China. One-way ANOVA on the log (x + 1) transformed data showed no treatment effects (P > 0.05) for both trapping periods
), 2013, at Liuba county, Shaanxi, China. One-way ANOVA on the log (x + 1) transformed data showed no treatment effects (P > 0.05) for both trapping periods
Mean captures (+SE) of D. armandi in cross-barrier type traps baited with two blends of female-produced pheromone candidate compounds, a synthetic host terpene mixture, and their combinations, plus an unbaited blank control during July 6–28, 2014, Shengnongjia Nature Reserve, Hubei, China. Bars with the same letter were not statistically different (P > 0.05) by the Ryan–Einot–Gabriel-Welsh (REGW) multiple Q test after ANOVA on log (x +1) transformed data. tV trans-verbenol, SD (±)-seudenol, EBV (±)-exo-brevicomin, MCOL (±)-1-methyl-2-cyclohexen-1-ol; Terpene-Mix: α-pinene (64 %), β-pinene (8 %), β-myrcene (4 %), (+)-3-carene (14 %), d-limonene (6 %), and (+)-longifolene (2 %)
Discussion
This is the first detailed chemical and behavioral analysis/report on the aggregation pheromone system of the Chinese white pine beetle, D. armandi and its critical synergistic interaction with host tree terpene volatiles. It is commonly known that bark beetles, especially tree-killing Dendroctonus spp., use a combination of pheromones and host tree volatiles to colonize hosts and find mates (Wood 1982; Schlyter and Birgersson 1999; Byers and Zhang 2012). Significant synergism between the beetle-produced aggregation pheromones and host tree volatile kairomones has been reported for several tree-killing Dendroctonus species, such as D. ponderosae Hopkins, D. brevicomis LeConte, D. frontalis Zimmermann, D. rufipennis (Kirby), D. pseudotsugae Hopkins and D. valens LeConte (Borden et al. 1987, 2008; Miller and Borden 2000; Pureswaran and Borden 2005; Hofstetter et al. 2008, 2012; Pureswaran et al. 2008a; Sullivan et al. 2011; Liu et al. 2013; Ryall et al. 2013), which might be a key characteristic trait necessary for successful mass attacks of these primary bark beetles to quickly overcome the defense system of the healthy trees. In the above-mentioned cases, either host kairomones (mostly monoterpene individuals or blends) or beetle aggregation pheromones, or both, were attractive (weakly to moderately) to both sexes of Dendroctonus adults in the field-trapping bioassays, but combining beetle-produced pheromones with monoterpene host volatiles resulted in highly significant synergistic increases in trap catches (Seybold et al. 2006). However, unlike these species, in our study, neither the beetle-produced pheromone blends (tV/SD/EBV and tV/SD/EBV/MCOL) nor the host volatile terpene mixture (5 monoterpenes plus one sesquiterpene) alone were active in attracting D. armandi at the dosages tested, but a strongly significant attraction to both sexes of D. armandi beetles was achieved only when combining host terpene mixture and pheromone blends in the same traps. Thus, the insect- and plant-derived signals are equally crucial for their successful mass attacks on healthy P. armandii host trees. This is the first report of non-attraction to either host kairomones or beetle aggregation pheromone components but a strong synergy between these two odor blends for bark beetles.
In nature, D. armandi females initiate attacks on healthy P. armandii tree trunks, excavate nuptial chambers/galleries in the phloem (Yin et al. 1984; Wang et al. 2014), and produce aggregation pheromones. At the same time, these attacks result in release of host volatile kairomones in high concentrations from the beetle-damaged host tissues or oleoresin flowing from the wound, from boring dusts that pass around the beetles during the excavation, from undigested tree tissues in fecal materials that pass through the alimentary canals of the beetles, or from potentially sequestered host monoterpenes that are re-released by the beetles. Releases of the bark beetle pheromone are always accompanied by release of host terpenes; thus, it makes ecological sense to have a critical synergistic interaction between these two types of olfactory communication signals that evidently are critical for finding and successfully mass attacking relatively healthy host trees.
Our GC–MS analysis on the headspace (aeration) samples of P. armandii logs showed similar monoterpene compositions as reported earlier (Chen et al. 2006, 2007; Li et al. 2006; He et al. 2009; Yang et al. 2010) with α-pinene, Δ3-carene, β-pinene, limonene and β-myrcene as the major monoterpenes; however, the most dominant sesquiterpene in our study was (+)-longifolene, not the reported trans-β-caryophyllene (Li et al. 2006, 2007; Chen et al. 2007). (+)-Longifolene was included in the host terpene mixture as part of the potential kairomone candidates in our field bioassay. trans-β-Caryophyllene was also identified from our P. armandii log aeration samples, but in a much lower amount than that of (+)-longifolene (Fig. S2). Such disparity in the abundance of the major sesquiterpenes might be due to geographical variations, different sampling techniques/timing, or other unknown reasons. Identification of trans-β-caryophyllene in these early reports was based on the GC–MS library search matches (not confirmed with synthetic standard); however, our identification of (+)-longifolene was confirmed by an authentic synthetic standard. Significant EAG responses by both sexes of D. armandi to major monoterpenes, including the ones identified from the P. armandii logs in our study, were recently reported (Zhang et al. 2010; Wang et al. 2011a, b), supporting their potential kairomone role in host selection and mass attacks.
At least five bark beetle-related compounds were identified from both hindgut extracts of female D. armandi and headspace samples of P. armandii logs infested with D. armandi females in our study. The major component, trans-verbenol, was reported as an aggregation pheromone component of several tree-killing Dendroctonus spp. in North America, such as D. ponderosae (Borden et al. 1987), D. pseudotsugae (Rudinsky et al. 1972) and D. frontalis (Payne et al. 1978; Pureswaran et al. 2008b). It was also found as the most attractive pheromonal volatile in another Hylesini bark beetle, Tomicus minor Hart, but not active in the sympatric T. piniperda (L.) (Lanne et al. 1987). The other three minor components, exo-brevicomin, seudenol and 1-methyl-2-cyclohexen-1-ol (MCOL), are also common aggregation pheromone components of various Dendroctonus species (Byers et al. 1984; Borden et al. 1987; Lindgren et al. 1992, 2012; Hofstetter et al. 2008; Pureswaran et al. 2008a; Ryall et al. 2013). cis-Verbenol, a common pheromone compound from Ips spp., was also detected in one of our hindgut extracts of females as a trace component, but it has never been reported as an aggregation pheromone component of any Dendroctonus species. Verbenone, a well-known anti-aggregation pheromone of conifer bark beetles (Byers 1989), was also detected from several aeration and hindgut samples, indicating the post-mating status (later attack phases) of the beetles from which these samples were prepared.
Since 2005, efforts to identify D. armandi aggregation pheromone have been made by several research groups in China. Two papers related to chemical identification of hindgut extracts of females were recently published by the same group (Xie and Ding 2010; Xie and Lv 2012). In Xie and Ding (2010), 23 and 25 volatile compounds, mainly composed of fatty acids, monoterpenes and sequiterpenes, were identified from hindgut extracts of females and males, respectively. No bark beetle-related compounds were detected from any of their hindgut extracts. This result indicates that their hindgut sampling procedure was unreliable for chemical identifications. This could occur because the D. armandi hindguts in their study were taken together with large amounts of fat body and other beetle tissues, or breakdown of compounds occurred before samples were analyzed chemically. In their second paper (Xie and Lv 2012), eight volatile compounds, including three bark beetle-related compounds: cis-verbenol (3.3 %), trans-verbenol (5 %) and verbenone (2.2 %); five monoterpenes: α-pinene (8.7 %), (+)-3-carene (39 %), myrcene (2.8 %) and limonene (4.4 %); plus a sesquiterpene, β-caryophyllene (34.6 %), were identified by GC–MS and quantified by GC-FID using polar columns from the hindgut extracts of un-mated D. armandi females (see Table 1; Fig. 3 in their paper). Surprisingly, the retention times and elution order of these eight volatiles reported in their Table 1 and Fig. 3 are obviously incorrect. The two bark beetle-related compounds, cis-verbenol and trans-verbenol, reportedly eluted before 10.00 min (and >4 min apart), and both eluted >20 min before the first eluted monoterpene hydrocarbon, α-pinene; and the last monoterpene, limonene, eluted at 42.28 min which is not physically possible [see Table 1; Fig. 3 in Xie and Lv (2012)]. Improbably, the GC-FID trace in Fig. 3 of Xie and Lv (2012) was exactly the same as the GC-TIC trace (with DB-17MS column) in the Fig. 3 of Xie and Ding (2010), which had fatty acids as major constitutes without any bark beetle-related compounds. Furthermore, the GC-FID trace reported in Fig. 2 of Xie and Ding (2010) on the volatiles from the hindgut extract of D. armandi females appears identical to that in Fig. 2 of another paper by the same group (Xie and Lv 2013) on volatiles from hindgut extract of Ips typographus (L.) males. These serious technical shortcomings and scientific inaccuracies make the publications of Xie and Ding (2010), Xie and Lv (2012, 2013) unreliable.
In their field bioassays, two “female-produced pheromone candidate compounds”, cis-verbenol and trans-verbenol, were included in some treatments with monoterpenes, but no additive or synergistic effects were found (Xie and Lv 2012). Surprisingly and inexplicably, α-pinene alone or in combinations with other monoterpenes/sesquiterpenes supposedly showed significant attraction to both sexes of D. armandi adults in their study, but these combinations were inactive in our field bioassay. Another paper on GC–MS analysis of SPME samples of fresh frass (boring dusts and feces) of D. armandi adults showed verbenone (2.3 % of total volatiles) as the only bark beetle-related compound (Wu et al. 2012), likely due to inadequate sampling timing and procedure.
Our GC–MS analyses demonstrate that D. armandi females produce and release trans-verbenol, exo-brevicomin, seudenol and MCOL after feeding under the bark of P. armandii tree trunks, as their potential aggregation pheromone components and, at the same time, large amounts of monoterpenes plus minor amounts of sesquiterpenes are released from the wounded bark tissues and bark beetle attack sites, as a host kairomone significantly synergistic with the bark beetle pheromone components. Both D. armandi aggregation pheromone and the P. armandii host terpene kairomone are equally, and critically important for successful D. armandi mass attacks on the healthy P. armandii trees. All D. armandi female-produced pheromone components are chiral; however, our current study did not determine enantiomeric compositions. Nevertheless, (−)-trans-verbenol is expected be the dominant natural enantiomer, since its antipode does not commonly occur in nature. More work is surely needed to determine their enantiomeric compositions, preferably from hindgut samples of un-mated females that just finished the nuptial chambers. Furthermore, field-trapping experiments on optimal attractant compositions (for both the pheromone blend and the terpene mixture), ratios, release rates, and dispenser technology are under way. These synergistically attractive semiochemicals (in an optimized/simplified lure formulation) should have great potential as a monitoring and mass-trapping tool in integrated pest management programs against this serious forest pest of Chinese white pine forests in the western China (Schlyter et al. 2001).
References
Borden JH, Ryker LC, Chong LJ, Pierce HD Jr, Johnston BD, Oehlschlager C (1987) Response of the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae), to five semiochemicals in British Columbia lodgepole pine forests. Can J For Res 17:118–128
Borden JH, Pureswaran DS, Lafontaine JP (2008) Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae). J Econ Entomol 101:1266–1275
Byers JA (1989) Behavioral mechanisms involved in reducing competition in bark beetles. Holarctic Ecol 12:466–476
Byers JA (1993) Randomization algorithms in BASIC for experimental design. Comput Biol Med 23:167–176
Byers JA, Zhang Q-H (2012) Chemical ecology of bark beetles in regard to search and selection of host trees. In: Liu T-X, Le K (eds) Recent advances in entomological research. Springer, New York, pp 150–190
Byers JA, Wood DL, Craig J, Hendry LB (1984) Attractive and inhibitory pheromones produced in the bark beetle, Dendroctonus brevicomis, during host colonization: regulation of inter-and intraspecific competition. J Chem Ecol 10:861–877
Chen H, Tang M (2007) Spatial and temporal dynamics of bark beetles in Chinese white pine in Qinling Mountains of Shaanxi Province, China. Environ Entomol 36:1124–1130
Chen H, Tang M, Gao J, Chen X, Li Z (2006) Changes in the composition of volatile monoterpenes and sesquiterpenes of Pinus armandii, P. tabulaeformis, and P. bungeana in Northwest China. Chem Nat Comp 42:534–538
Chen M, Li Y-H, Wang X-J (2007) Analysis of volatile constituents in Pinus armandii bark with GC/MS. For Res 20:428–432
Fettig CJ, Dabney CP, McKelvey SR, Borys RR (2006) An assessment of re-randomization methods in bark beetle (Scolytidae) trapping bioassays. Agri For Entomol 8:267–271
He M-J, Liao C-L, Guo H-J, He Y-S, Liao L-J, Wang H, Zhang Y, Zhang G-H (2009) Analysis of constituents of the volatile materials in the bark of Pinus armandii from Enshi. J Hubei Univ Natl 27:254–257
Hofstetter R, Chen Z, Gaylord M, McMillin J, Wagner M (2008) Synergistic effects of α-pinene and exo-brevicomin on pine bark beetles and associated insects in Arizona. J Appl Entomol 132:387–397
Hofstetter RW, Gaylord ML, Martinson S, Wagner MR (2012) Attraction to monoterpenes and beetle-produced compounds by syntopic Ips and Dendroctonus bark beetles and their predators. Agri For Entomol 14:207–215
Lanne BS, Schlyter F, Byers JA, Löfqvist J, Leufvén A, Bergström G, Van Der Pers JNC (1987) Differences in attraction to semiochemicals present in sympatric pine shoot beetles, Tomicus minor and T. piniperda. J Chem Ecol 13:1045–1067
Li Z-B, Chen H, Chen X (2006) Analysis of chemical constituents of volatile oil from resin of Pinus armandii. J Northwest For Univ 21:138–141
Li Y, Chen M, Ye H (2007) Relationship between volatile matters from Armand pine and endangerments of Armand pine bark-weevil. J Northeast For Univ 35:7–10
Lindgren B, Gries G, Pierce H Jr, Mori K (1992) Dendroctonus pseudotsugae Hopkins (Coleoptera: Scolytidae): production of and response to enantiomers of 1-methylcyclohex-2-en-1-ol. J Chem Ecol 18:1201–1208
Lindgren BS, Miller DR, Lafontaine J-P (2012) MCOL, frontalin and ethanol: a potential operational trap lure for Douglas-fir beetle in British Columbia. J Entomol Soc Brit Col 109:72–74
Liu Z, Xu B, Miao Z, Sun J (2013) The pheromone frontalin and its dual function in the invasive bark beetle Dendroctonus valens. Chem Senses 38:485–495
Miller DR, Borden JH (2000) Dose-dependent and species-specific responses of pine bark beetles (Coleoptera: Scolytidae) to monoterpenes in association with pheromones. Can Entomol 132:183–195
Payne T, Coster J, Richerson J, Edson L, Hart E (1978) Field response of the southern pine beetle to behavioral chemicals. Environ Entomol 7:578–582
Pu X-J, Chen H (2007) Relations between attacking of Dendroctonus armandi and nutrition and resistance material of host trees (Pinus armandii). J Northwest A F Univ 35:106–110
Pureswaran DS, Borden JH (2005) Primary attraction and kairomonal host discrimination in three species of Dendroctonus (Coleoptera: Scolytidae). Agric For Entomol 7:219–230
Pureswaran DS, Hofstetter RW, Sullivan BT (2008a) Attraction of the southern pine beetle, Dendroctonus frontalis, to pheromone components of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae: Scolytinae), in an allopatric zone. Environ Entomol 37:70–78
Pureswaran DS, Sullivan BT, Ayres MP (2008b) High individual variation in pheromone production by tree-killing bark beetles (Coleoptera: Curculionidae: Scolytinae). Naturwissenschaften 95:33–44
Rudinsky J, Kinzer G, Fentiman A, Foltz R (1972) trans-Verbenol isolated from Douglas-fir beetle: laboratory and field bioassays in Oregon. Environ Entomol 1:485–488
Ryall K, Silk P, Thurston G, Scarr T, de Groot P (2013) Elucidating pheromone and host volatile components attractive to the spruce beetle, Dendroctonus rufipennis (Coleoptera: Curculionidae), in eastern Canada. Can Entomol 145:406–415
Schlyter F, Birgersson G (1999) Forest beetles. In: Hardie J, Minks AK (eds) Pheromones in non-lepidopteran insects associated with agricultural plants. CAB International, Oxford, pp 113–148
Schlyter F, Zhang Q-H, Liu G-T, Ji L-Z (2001) A successful case of pheromone mass trapping of the bark beetle Ips duplicatus in a forest island, analysed by 20-year time-series data. Integr Pest Manag Rev 6:185–196
Seybold SJ, Huber DP, Lee JC, Graves AD, Bohlmann J (2006) Pine monoterpenes and pine bark beetles: a marriage of convenience for defense and chemical communication. Phytochem Rev 5:143–178
Sullivan BT, Dalusky MJ, Mori K, Brownie C (2011) Variable responses by southern pine beetle, Dendroctonus frontalis Zimmermann, to the pheromone component endo-Brevicomin: influence of enantiomeric composition, release rate, and proximity to infestations. J Chem Ecol 37:403–411
Wang K-Q, Wang X-B (2010) Dendroctonus armandi Control in Taibai Mountains. Shaanxi For Sci Tech 6:58–60
Wang X, Chen H, Ma C, Li Z (2010) Chinese white pine beetle, Dendroctonus armandi (Coleoptera: Scolytinae), population density and dispersal estimated by mark-release-recapture in Qinling Mountains, Shaanxi, China. Appl Entomol Zool 45:557–567
Wang R-L, Yang W, Yang Z-Z, Chen X-P, Yang C-P, Li Q, Li F, Chen C-M (2011a) Electroantennographic and behavioral responses of Dendroctonus armandi (Coleoptera: Ipidae) to host plant volatiles. Chin J Ecol 30:724–729
Wang R-L, Yang W, Yang Z-Z, Chen X, Wang XW, Yang C-P (2011b) Electroantennographic and behavioral responses of Dendroctonus armandi (Tsai et Li) to 9 plant volatiles. For Pest Dis 30:23–26
Wang X-W, Zhang Z-J, Yang W, Yang C-P, Li F (2014) A preliminary study of the biological character of Dendroctonus armandi. J Sichuan For Sci Tech 35:56–58
Wood DL (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Ann Rev Entomol 27:411–446
Wu SP, Chen H, Wu Q (2012) Volatile compounds in the frass of adult Chinese white pine beetle. J Northwest A F Univ 27:111–116
Xie S-A, Ding Y (2010) Semiochemicals of Dendroctonus armandi. J Northeast For Univ 38:91–94
Xie S-A, Lv S-J (2012) An improved lure for trapping the bark beetle Dendroctonus armandi (Coleoptera: Scolytinae). Euro J Entomol 109:569–577
Xie S-A, Lv S-J (2013) Effect of different semiochemicals blends on spruce bark beetle, Ips typographus (Coleoptera: Scolytidae). Entomol Sci 16:179–190
Yang X, Zhao H, Wang J, Meng Q, Zhang H, Yao L, Zhang Y, Dong A, Ma Y, Wang Z (2010) Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the Southwest region of China. J Med Plant Res 4:1668–1672
Yin H-F, Huang F-S, Li Z-L (1984) Dendroctonus armandi Tsai & Li. Economic insect fauna of China (Coleoptera: Scolytidae), vol 29. Science Press, Beijing, pp 26–35
Zhang L, Chen H, Ma C, Tian Z (2010) Electrophysiological responses of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) to volatiles of Chinese white pine as well as to pure enantiomers and racemates of some monoterpenes. Chemoecol 20:265–275
Zhang L, Chen H, Chen X (2011) Effect of Dendroctonus armandi infection on the volatile constituents of Pinus armandii in Qinling Mountains. J Northwest For Univ 26:114–118
Acknowledgments
We thank Dr. Jeff Aldrich (Jeffrey R. Aldrich consulting, LLC, USA), Dr. John A. Byers (USDA-ARS) and Prof. Fredrik Schlyter (SLU, Sweden) for valuable comments and suggestions on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Günther Raspotnig.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, G., Song, Y., Wang, P. et al. Semiochemistry of Dendroctonus armandi Tsai and Li (Coleoptera: Curculionidae: Scolytinae): both female-produced aggregation pheromone and host tree kairomone are critically important. Chemoecology 25, 135–145 (2015). https://doi.org/10.1007/s00049-014-0182-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0182-1