Abstract
Anthocyanins are water-soluble naturally occurring flavonoids present in fruits, flowers, leaves, and roots of fruit plants and vegetables. One of the important anthocyanidin components of red wine and berries is delphinidin (DP). This review provides an update on the potential of DP in cancer therapy, with a further understanding of the mechanisms involved. Delphinidin has been shown to elicit inhibitory effects on catabolizing enzymes of human granulocytes and parasites, TNF-induced COX-2 expression in mouse epidermal cells, and reduce oxidative stress. It also inhibited anchorage-independent growth and caused cell death in breast cancer cell lines. Delphinidin increased Nrf2 expression, increased HO-1 production, and promoted mRNA expression of mitochondrial biogenesis-related factors. Further, DP has anti-proliferative and pro-apoptotic effects in various cancer cell lines such as lung, breast, and ovarian cancer cells. The mTOR-related pathway is the most important signaling pathway in the activation of autophagy, and DP has been shown to exert its cytotoxic effects on cancer cell lines via activating protein kinases. Among DP derivatives, delphinidin-3-O-glucoside has the best anticancer activity because it is easily absorbed. However, the metabolism of DP and its bioavailability in biological systems need to be explored to fully understand its benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In ancient times, medicines were primarily derived from natural sources due to their perceived safety, availability and affordability. Today, most drugs are synthetic, and are derived from scaffolds or plant-based molecules with known health benefits such as sterols, carotenoids, polyphenols, and anthocyanins [1]. The pharmacological activities of phytoconstituents have been extensively studied. In this context, flavonoids (flavones, flavanones, isoflavonoids, flavonols, flavans, and anthocyanins) feature as one of the well-known phytochemical groups which differs structurally at the heterocyclic oxygen ring. One of the most important flavonoids is the anthocyanin, delphinidin (Fig. 1).
Delphinidin (3,3,4,5,5,5,7-hexahydroxyflavylium) is abundant in colored fruits and vegetables, especially blueberries, pomegranates, grapes, beets and eggplants [2, 3]. Several derivatives of delphinidin (DP) have been reported in plants (Table 1; Fig. 2), and some have displayed interesting anti-mutagenic, anti-angiogenic, anti-oxidant, and anti-inflammatory activities [4]. Delphinidin is light-sensitive and remains stable only at pH 3. It is quickly degraded in physiological settings, poorly absorbed and thus its bioavailability is low [5]. Although DP and its derivatives have a vast array of therapeutic effects, the underlying molecular mechanisms are not yet clearly understood [6]. In this review, we delve into the anticancer potential of DP and its derivatives, giving the mechanistic insights into its mode of action.
Structure of delphinidin derivatives identified in various plants and herbal products. The numbers (1 to 20) refer to the molecules mentioned in Table 1
Structural characteristics of delphinidin and its derivatives, and their relationships with anticancer activities
Delphinidin is a polyphenolic compound with its basic structure based on the flavylium cation, which consists of a three-ring system (C6-C3-C6), otherwise called the anthocyanidin skeleton. This structure has two aromatic rings: A (resorcinol) and B (catechol), and C (3-O-subsituted-pyrylium), a heterocyclic ring [4] Delphinidin has hydroxyl groups (-OH) at the 3, 5, 7, 3′, 4′, and 5′ positions of the anthocyanidin skeleton. Oxygen in the first position is linked to the sugar moiety at the 3-O-β- position of the C ring. In the three rings, DP has 6 hydrogen bond donor and 6 hydrogen bond acceptor atoms. Because of the presence of numerous electron donor atoms, DP acts as a potent antioxidant by scavenging reactive oxygen species (ROS). The presence of a 3-hydroxyl group in ring B of DP distinguishes it from other anthocyanins. It also possesses two hydroxy groups in ring A. Because these -OH groups interact potently with a wide range of proteins, they are accountable for several important biological processes [7].
Although DP is more active in its aglycone form, its bioavailability depends on a sugar moiety being present in the third position of the C ring [8]. Due to the presence of several hydroxyl groups, DP is extremely polar in comparison to other anthocyanins and is hence readily soluble in methanol and water [9]. In the C-3 position, DP is connected to a range of sugar moieties, including glucoside and arabinoside. These glycosides (DP derivatives; Fig. 2) have improved solubility and stability compared to the aglycone form. The type, quantity, and location of sugars in the DP molecule, the degree of hydroxyl group methylation, and the number of aliphatic or aromatic acids attached to sugars in a DP derivative are the features that distinguish DP derivatives from one another [10]. Acylation (addition of acyl groups, for example, p-coumaric and caffeic acids) of the glycosyl moieties are typical modifications that can enhance the stability and bioavailability of DP derivatives. Such acylated DP derivatives tend to elicit enhanced anticancer activities owing to increased lipophilicity and improved interaction with cellular membranes [11]. Another important structural feature of DP derivatives is methylation at hydroxyl positions, which can lead to increased stability and altered bioactivity. For instance, the methylation of hydroxyl groups can affect the antioxidant properties and interaction with cellular targets as in malvidin, a DP derivative [12].
Regarding the relationship of the structural features of DP and its derivatives to their anticancer properties, several aspects can be highlighted. Firstly, the hydroxyl groups of DP and its derivatives are largely responsible for their scavenging free radicals and ROS, which are implicated in cancer development and progression. By reducing oxidative stress, DP and its derivatives can inhibit the initiation and promotion stages of carcinogenesis [13]. Secondly, pro-apoptotic effects (induction of apoptosis and cell cycle arrest) of DP are mediated through various pathways (discussed in later sections) in cancer cells, including the mitochondrial pathway and the activation of caspases. The presence of hydroxyl groups enhances its ability to interact with and modulate apoptotic proteins. Specifically, DP can cause cell cycle arrest, particularly in the G2/M phase, by modulating the expression of cell cycle regulatory proteins such as cyclins and cyclin-dependent kinases (CDKs) [14]. Thirdly, the anti-inflammatory properties of DP is achieved through inhibition of inflammatory pathways (key inflammatory mediators such as NF-κB and COX-2) which are often upregulated in cancer cells. This anti-inflammatory action contributes to its anticancer effects. Further, DP can exert an effect on signaling pathways, and by modulating signaling pathways involved in inflammation and cell proliferation, it can suppress tumor growth and metastasis [14]. Fourthly, DP can inhibit angiogenesis (the process of new blood vessel formation) which is essential for tumor growth and metastasis. This is partly achieved by downregulating pro-angiogenic factors like VEGF. The antioxidant and anti-inflammatory properties of DP contribute to its anti-angiogenic effects, thereby reducing the nutrient and oxygen supply to tumor cells [13] (Sharma et al. [13]). Lastly, the epigenetic modulatory effect of DP and its derivatives are reportedly crucial in inhibiting tumor progression. The molecules modulate the expression of genes involved in cancer progression through epigenetic mechanisms, such as histone modification and DNA methylation. Such epigenetic modulations can lead to the reactivation of tumor suppressor genes and the silencing of oncogenes, contributing to the anticancer effects of DP [15].
Taken together, the structural characteristics of DP and its derivatives, particularly the presence of hydroxyl groups and potential modifications like glycosylation, acylation, and methylation are crucial for their anticancer properties. These features enable DP to exert antioxidant, pro-apoptotic, anti-inflammatory, anti-angiogenic, and epigenetic effects, making it a promising candidate for cancer prevention and therapy. However, further research is evidently required to fully understand and optimize these properties for clinical applications.
Biosynthesis of delphinidin
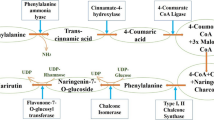
Naturally, DP is biosynthesised from coumaroyl-CoA and malonyl-CoA, with 3′,5′-hydroxylase serving as the primary enzyme (Fig. 3). The de novo assembly approach for anthocyanin biosynthesis involves the use of unigenes, the genes for chalcone synthase (CHS) and cinnamonate-4-hydroxylase (C4H) [16, 17]. The enzymes proanthocyanidins and flavonoid 3-O-glucosyltransferase (UFGT) compete to produce anthocyanins and reductase, respectively. Enzymatic reduction of DP and leucodelphinidin results in the formation of gallocatechin and epigallocatechin. Prodelphinidin is created by polymerization of both epigallocatechin and gallocatechin (catechin) [18].
Delphinidin biosynthetic pathway. F3′H flavonoid-3′5′-hydroxylase, CHI chalcone isomerase, CHS chalcone synthase, F3H flavanone 3-hydroxylase, UF3GT UDP-Glc flavonoid 3-O-glucosyl transferase, DFR dihydroflavonol-4-reductase, F3′5′H flavonoid-3′,5′-hydroxylase, ANS anthocyanidin synthase. Adapted from [4]
The flavonoid route, which follows a similar upstream pathway with pro-anthocyanidins until anthocyanins are formed by the catalysis of anthocyanidin synthase (leucoanthocyanidin dioxygenase) is responsible for the synthesis of free anthocyanins in grapes [19]. Examining the pre- and post-harvest mechanisms, blueberry anthocyanin production was found to be significantly boosted by pre-harvest UV-B, C, and post-harvest UV-A, B, and C irradiation [20]. Anthocyanin production was enhanced in purple-colored leaves, according to a variety of metabolites detected by HPLC-MS, with the highest concentrations of anthocyanidins, pro-anthocyanidins, and kaempferol glycoside [21].
Anticancer potential of delphinidin
Anthocyanins are widely promoted due to the vast array of their perceived health benefits. Both DP and its derivatives has anticancer efficacy against various molecular subtypes of well-established cancer cell lines (Table 2). DP inhibited anchorage-independent growth and caused apoptosis in HER2-overexpressing, ER-positive breast cancer cell lines. The upregulation of mitogenic kinases in breast cancer cells pretreated with DP was found to have been blocked [22]. DP was shown to reduce the risk of cancer by inducing apoptosis in endothelial cells [23, 24]. The mechanism of cytotoxicity involved DNA damage of the treated cells that were unable to produce tyrosyl-DNA-phosphodiesterase 1, suggesting that topoisomerase was not the primary cause of DNA strand breakage. In another study, DP prevented menadione-induced DNA damage in HT29 cells and exerted an antioxidative effect in the presence of catalase when hydrogen peroxide formation was reduced in cells [25].
Similarly, DP inhibited COX-2 expression and tumor progression in mouse skin epidermal cells by targeting mitogen protein kinase [26]. In human colon cancer cells, DP treatments upregulated caspases 3–8 and 9 while Bcl-2 protein decreased and the cellular division was arrested at the G2/M phase [27]. A mechanistic study found that DP exhibited anticancer effects in treating ovarian cancer with poor prognosis and resistance to treatment via inhibition of ERK1/2 MAPK and PI3K/AKT signal transduction cascades in ES2 cells from ovarian clear cell carcinoma [28]. This was in agreement with earlier studies which concluded that DP downregulated ERK-MAPK and PI3K/AKT signaling cascades in SKOV3 cells and inhibited cancer cell progression, suggesting that these signaling pathways are the main targets of DP for the prevention of epithelial ovarian cancer that is resistant to paclitaxel [29]. Brain-derived neurotrophic factor (BDNF) induced increased cell migration and invasion of SKOV3 ovarian cancer cells but DP treatments reinstated cell invasion and migration by downregulating the expression of MMP-2 and MMP-9, Akt and NF-κB [30]. In another investigation where HCT-116 and HT-29 human colorectal cancer cells were treated with pure phenolics such as delphinidin-3-O-glucoside (D3G), and cyanidin-3-O-glucoside (C3G) either alone or in combination (100–600 µg/mL), programmed death ligand 1 (PD-L1) fluorescence intensity was found to be 39% reduced by C3G. In peripheral blood mononuclear cells, anthocyanins reduced programmed death protein-1 (PD-1) expression by 41 and 55% in monoculture, 39 and 26% (for C3G), 50 and 51% (D3G) in co-culture with HCT-116 and HT-29 cells, respectively [31]. Huang et al. also inferred that DP (<100 μM) encumbered the progression of colorectal cell lines (DLD-1, SW480 and SW620) via inhibition of focal adhesion kinase (FAK)/Src/paxillin, integrin αV/β3, and interfered with cytoskeletal assembly as well as lowered the migratory capacity and invasiveness in the treated CRC cell lines [32].
Jang et al. performed proteomic analysis and western blotting and demonstrated that phosphoglycerate kinase 1 (PGK1) was increased by hydrogen peroxide in a dose-dependent manner and that its expression was inhibited by co-treatment with the recognized antioxidant DP [33]. According to Zhang et al. [34], DP treatments induced changes in mitochondrial membrane potential which triggered Bax, Caspase-3, 8, and 9, cytochrome C, and suppressed anti-apoptotic protein expression, phosphorylation of the activities of STAT-3 and MAPKinase signaling in colon cancer cells (HCT116). Yu and colleagues examined the antiproliferative and proapoptotic characteristics of DP in human colon cancer (HCT116) cells and unraveled that subjecting the cells to DP (30-240 µM; 48 h) led to: (i) a reduction in cell viability; (ii) apoptosis; (iii) PARP cleavage; (iv) upregulation of caspases-3, -8, and -9; (v) upregulation of Bax and downregulation of Bcl-2 protein; (vi) G2/M phase cell cycle arrest [27]. In A549 cells, DP decreased the activity of hypoxia response element (HRE) promoter triggered by cobalt chloride and epidermal growth factor (EGF) by inhibiting VEGF, ERK, PI3K/Akt/mTOR/p70S6K signaling pathways, reduced HIF-1 binding to the HRE promoter, and greatly prevented the development of new blood vessels caused by EGF in animal models [35].
According to another research group, DP upregulated the expression of autophagy-induced cell death-related protein and downregulated the phosphorylation of PI3K, AKT, and mTOR in non-small cell lung cancer (NSCLC) cells exposed to radiations [36]. Athymic nude mice treatment with DP caused (i) tumor growth inhibition, (ii) reduced proliferation of PCNA and Ki67 and (iii) increased programmed cell death [37]. Similarly, PC3 cells treated with DP elicited a dose-dependent reduction of (a) phosphorylation of IκB kinase γ (NEMO), (b) phosphorylation of the inhibitory protein IκBα of nuclear factor-κB (NF-κB), (c) phosphorylation of NF-κB/p65 at Ser536 and NF-κB/p50 at Ser529, (d) nuclear translocation of NF-κB/p65, and (e) NF-κB DNA binding activity [38]. PC3 cells were treated with DP and outcomes showed inhibition of the cell progression by intrinsic and extrinsic apoptotic pathways via death receptor 5 and cleavage of histone deacetylase 3 [39]. Jeong [40] also found that DP treatments of LNCaP cells—a human prostate cancer cell line with p53 wild-type—increased the activity of caspase-3, -7, and -8 and decreased histone deacetylase and HDAC3, one of the class I HDACs activities. In addition, DP (15–180 μM, 72 h) triggered the formation of Axin and glycogen synthase kinase 3β phosphorylation, blocked translocation of β-catenin and downregulated target β-catenin [41].
Based on the work of Afqaq and others, pretreating keratinocyte HaCaT cells with DP (1–20 µM for 24 h) shielded cells from UVB (15–30 mJ/cm2)-mediated (i) apoptosis induction; (ii) reduction in cell viability; (iii) lipid peroxidation, (iv) reduced 8-hydroxy-2’-deoxyguanosine (8-OHdG), (v) expression of the nuclear antigen; (vi)lowered poly(ADP-ribose) polymerase cleavage; (vii) caspase activation; (viii) downregulation of Bcl-2; (ix) upregulation of Bax; (x) increase in the expressions of Bid and Bak [42]. Actually, DP was found to directly target and inhibit Raf and mitogen-activated protein kinase (MEK), and COX2 to repress 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced transformation production in JB6 promotion-sensitive mouse skin epidermal (JB6 P + ) cells [26]. Other findings indicate that DP triggered Nrf2 promoter to modulate Nrf2-ARE pathway in JB6 P+ cells (skin cancer cells) and thereby could be a potent chemopreventive agent to treat skin cancer [15]. Evidently, UVB-exposed human keratinocyte cells (HaCaT) characterized by diminished metabolic activity, actin cytoskeleton rearrangement and elimination of 53BP1 cell repair marker had their normal activity restored by treatment with DP at 5 or (10 μM) [43]. Along with cyanidin and malvidin, DP exerted growth inhibitory effects against human hepatoma (HepG2) cells via activation of caspase-3 in a time-dependent manner and activated poly (ADP-ribose) polymerase (PARP) cleavage. In addition, DP upregulated the expression of JNK and c-Jun according to RT-PCR and Western blot studies [44].
Another study indicated that DP triggered LC3 lipidation, a hallmark of macroautophagy in hepatocellular carcinoma (HCC) cells as inhibited with 3-methyladenine treatments, and resulted in extensive necrosis. Consequently, anthocyanins may cause distinct forms of cell death in various malignancies. Moreover, a combination of anthocyanins and a macroautophagy inhibitor may be utilized to treat malignancies like HCC [45]. In human promyelocytic leukemia cells (HL-60), the effective induction of apoptosis by DP was recorded at a concentration of 100 µM in 6 h. In the study, DP promoted the activation of the JNK pathway, which included JNK phosphorylation, c-jun gene expression, and caspase-3 activation, concurrently with apoptosis in the HL-60 cells [46]. Tsai et al. was examined the impact of Hibiscus anthocyanins (HAs) that contain DP, in the human leukemia cell line HL-60 and the results of flow cytometry analysis were observed cell cycle arrest at the G2/M phase [47]. A previous study revealed that HAs in HL-60 cells, may induce apoptosis in cancer cells. In HL-60 cells, HAs administration (0–4 mg/ml) significantly and time- and dose-dependently promoted apoptosis and elevated phosphorylation in p38 and c-Jun, cytochrome c release, tBid, Fas, and FasL [48]. Takasawa et al. also probed into the inhibitory properties of anthocyanidins (including pelargonidin, cyanidin, and DP) but only DP caused dose- and time-dependent apoptosis in HL-60 cells [49]. According to molecular evidence, delphinidin 3-sambubioside (Dp3-Sam) treatment of HL-60 cells caused the release of cytochrome c from mitochondria into the cytosol, the truncation of Bid, and a decrease of mitochondrial membrane potential in a time- and dose-dependent manner [50]. In Jurkat cells treated with blackcurrant extract that were rich in cyanidin-3-O-rutinoside, delphinidin-3-O-rutinoside, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, apoptosis was induced along with G2/M phase cell cycle arrest, upregulation of p73 and caspase 3 but downregulation of Bad, Akt and Bcl-2 [51].
Other than the foregoing, DP (10–60 μg/mL) showed a noteworthy cytotoxic impact on T24 cells with a considerable proportion of dead cells observed [52]. Further, Ouanouki et al. explored the effects of anthocyanidins on TGF-β-induced epithelial-mesenchymal transition (EMT) and their underlying mechanism(s), including cyanidin (Cy), DP, malvidin (Mv), pelargonidin (Pg), and petunidin (Pt). Anthocyanidins were added to human U-87 glioblastoma (U-87 MG) cells either before, concurrently with, or after the addition of TGF-β. The results showed that depending on the treatment circumstances, anthocyanidins had varying effects on TGF-β-induced EMT. Due to its ability to block both the TGF-β Smad and non-Smad signaling pathways, DP was shown to be the most powerful EMT inhibitor [53]. Previous research was elucidated that the combination of DP treatment and the transfection of miR-137 mimics resulted in the reduction of cell invasion and the growth factor receptor (EGFR), angiogenic factors (VEGF and b-FGF), invasive factors (MMP-9 and MMP-2), and survival factors (p-Akt and NF-κB) [54]. MCF-10A cell treatments with DP was consequently inhibited hepatocyte growth factor (HGF) induced the expression of Met receptors, phosphorylated downstream regulators such as Src and FAK, and blocked mediated tyrosyl-phosphorylation [42]. Every anthocyanin pigment under investigation suppressed the growth of the MCF-7 in a dose-dependent manner. MCF-7 cell treatments with Dp-3-gluc was increased the cytotoxicity as compared to the corresponding portosin. The data suggested that the moiety in the phenolic ring (ortho-trihydroxylated) is a crucial structural component to exert cytotoxic activity in breast cancer (MCF-7) cells than dihydroxylated compounds [55]. DP treatments resulted in a partial reduction of MAPK signaling in ER-negative chemically altered MCF10A cells and triple-negative cells (Table 2).
In HER2-overexpressing cells, DP significantly increased the rate of apoptosis along with HER2 and MAPK signaling reductions [22]. In MCF-7 (human breast) cancer cells, DP dramatically reduced the expression of the MMP-9 protein generated by phorbol 12-myristate 13-acetate (PMA). It blocked the activation of NFkappaB (NF-κB) through MAPK signaling pathways, hence inhibiting the transcriptional activity of the MMP-9 gene. Furthermore, DP inhibited PMA-induced cancer cell invasion. According to these findings, DP may be an effective antimetastatic molecule that inhibits PMA-induced cancer cell invasion by selectively blocking the expression of the MMP-9 gene, which is dependent on NF-κB [56]. It is important to mention that among the DP derivatives, delphinidin-3-O-glucoside has the best anticancer activity because it is easily absorbed and appears in the blood plasma within 15 min of oral administration. Catechol-O-methyl transferase metabolizes delphinidin-3-O-glucoside by methylating the 4′ OH group in the B-ring and the metabolite shows a better distribution profile [57].
Insights into the mechanisms of anticancer activity of DP and its derivatives
Anticancer activity by differentiation induction
The phenomenon known as differentiation induction occurs as a result of differentiation inducers, malignant cells differentiate into mature, normal cells. Many cancerous cells go through cell division and generate less differentiated cells [58, 59]. Anthocyanins can impede tumorigenesis and cause cancer cells to differentiate terminally. Cyanidin-3-O-βglucopyranoside (Cy-g), for example, triggered PI3K and PKC in a dose-dependent manner to induce the differentiation of the human acute promyelocytic leukemia cell line (HL-60). This was understood by identifying indicators and kinase inhibitors during cell differentiation. Moreover, Cy-g (200 mg/mL) administration caused HL-60 cells to prevent the oncogene (c-Myc) and exhibit differentiation features such as improved adhesion and greater esterase activity. However, after being treated with PI3K and PKC inhibitors, marked decrement in the differentiation activity of Cy-g against HL-60 was observed [60]. Lastly, Cy-g upregulated the expression of cAMP, tyrosinase, and MART-1 which induced the differentiation in the melanoma cell line (TVM-A12) [61]. Thus, DP and its derivatives can hinder cancer at early stages by inducing differentiation, which in turn affects the final size of the tumor and malignancy. Such extents of differentiation influence the malignancy to some degree.
Anticancer effect via inhibition of cellular transformations
One of the processes which favor tumorigenesis are cellular changes. Certain carcinogens, including 12-O-tetradecanoylphorbol-13-acetate (TPA) and EGF, trigger transcription factors AP-1 and NF-κB in different cancer cell lines via PI3K-Akt and MEK-ERK pathways [62]. Interestingly, DP has been shown to suppress TPA-induced AP-1, NF-κB, COX-2, PGE2 expression in JB6P+ cells [26]. DP also mitigated TPA-cellular transformation brought via inhibiting the phosphorylation of ERK, MEK, ribosomal protein S6 kinase, and mitogen stress activator protein kinase in JB6P mouse epidermal cells [63]. Cyanidin can directly interact with PI3K in an ATP-competitive manner to block AP-1 and NF-κB production via the PI3K/Akt/p70S6 pathway. It can also prevent JB6P+ cells from undergoing cellular transformation when treated with EGF [64]. These studies indicate that DP and its derivatives (in part) elicit their anticancer effect through prevention of cellular transformation.
Modulation of anti-oncogene and relative protein expression
Ha et al. elucidated that anthocyanins was triggered the transcription of p21 and p27 in colon and prostate cancer cells as well as upregulated p53 to activate the DNA repair system. Cancer cells underwent cell cycle arrest on the coupling of p21 and CDKs [65]. Anwar et al. discovered that Caco-2 cell growth was suppressed by berry anthocyanin-rich extract. This was achieved by upregulating the expression of p21Waf/Cif1, arresting the cell cycle, and further causing death by activating caspase-3 [66]. According to Chen et al. [67], anthocyanins (peonidin 3-Glucoside and cyanidin 3-Glucoside) was induced cell cycle arrest at G0/G1 and G2/M, downregulated the expressions of CDK-1 and CDK-2, cyclin-E, cyclin-B, cyclin-A in breast cancer cells. Consequently, anthocyanins was inhibited the progression of breast cancer cells by arresting the cell cycle at various stages of division by up-regulating the expression of anti-oncogenes and down-regulating the expression of oncogenes, along with the expression of various cyclins and their partners, CDKs and/or CDKIs [67].
Chemoprotective effect via modulation of the Nrf2 pathway
The epidermal growth factor receptor (EGFR) inhibitor is the potential inhibitor of tumor development and metastasis. In one of the earlier attempts, DP suppressed vascular endothelial growth factor receptor 2 (VEGFR2), receptor tyrosine kinase 2 (ErbB2), VEGF receptor-3 (VEGFR3), and insulin-like growth factor 1 receptor (IGF1R), indicating that the molecule has a wide range of receptor tyrosine kinases inhibitory activity [68]. In another study, DP reduced VEGF-induced angiogenesis and enhanced antioxidative activities [69]. It was also shown to considerably improve tetradecanoylphorbol acetate -induced in mouse epidermal cells, increase the activity of ARE-driven luciferase, and upregulated Nrf2-related genes. Demethylation at CpG positions of the Nrf2 promoter was associated with activation of the Nrf2-ARE pathway in the mouse. Other researchers identified that DNA methyltransferase and histone deacetylase protein expression decreased in tandem after reduction of CpG methylation in the Nrf2 promoter area [15]. The findings of Xu et al. indicated that hydrogen peroxide reduced liver cancer cell line viability via ROS generation, but DP pretreatment promoted cell survival. DP pretreatment increased Nrf2 expression by facilitating the uncoupling of Nrf2 and Keap1 and Nrf2 was modulated its expression via an ARE-like element located in the proximal region of NFE2L2 gene promoter and DP-assisted to inhibit NF-Kb pathway that results in inhibition of translation of genes related to pro-informatory cytokines [70] (Fig. 4).
Inhibition of cancer via modulation of ERK1/2 and PI3K/AKT signaling pathways
The signaling pathway of protein kinases is inhibited due to autophagy [50]. More research is required to understand how it regulates redox equilibrium. For example, through antioxidative activity, and the possible mechanisms. An investigation involving endothelial cells revealed the antiproliferative and antiangiogenic effects of DP, which could have been mediated through the deactivation of PDE2 in VEGF-induced upregulation of extracellular kinases MEK/ERK, PI3K, and activating transcription factors (CREB/ATF1). Hypoxia-inducible factor-1 (HIF-1), VEGF, and epidermal growth factor (EGF) expression was reduced after the exposure of DP in cobalt chloride-induced lung cancer due to inhibition of PI3K/Akt/mTOR and ERK signaling [71]. Human lung cancer cell (A549) treatments with DP reduced cell proliferation by inducing overexpression of PI3K/Akt and EGFR/VEGFR2 signaling [35]. Another report indicated that DP exerted antiproliferative activity against colon and rat breast cancer cells, where endothelial cells after exposure to DP mitigated mitochondrial respiration by activating Akt [72].
Usually, the tumor growth factor (TGF)-β triggers the EMT which facilitates tumor cell invasion and advancement. It has since been shown that DP is an effective EMT inhibitor in human glioblastoma cell line by downregulating the TGF/ERK, TGF/Smad2, and EMT-related proteins i.e., snail and fibronectin [40]. In agreement with the foregoing results, EMT induction was prevented in hepatocellular carcinoma cells after treatments with DP. This was achieved via modulation of the expressions of matrix metalloproteinase-2 (MMP2), EGFR, AKT, and ERK. In ovarian cancer cell lines, DP inhibited cell proliferation and promoted apoptosis via modulating PI3K/Akt and ERK1/2/JN phosphorylation [73].
This is further supported by the anti-proliferative and pro-apoptotic effects of DP in ovarian cancer cells that are resistant to paclitaxel via downregulating the expressions of PI3K/AKT and ERK1/2 signaling [74]. It is expected that autophagy-mediated vacuolization and growth inhibition is another possible mechanism of DP anticancer activity in HCC cell lines. One such probe found that DP reduced cell proliferation, promoted apoptosis, and induced protective autophagy against breast cancer cells by downregulating AKT/mTOR and upregulating AMPK/FOXO3a [75]. Ozbay and Nahta were elucidated that DP has a significant degree of cytotoxicity for breast cancer cells and has strong interaction with the Her2 receptor and in vitro experiment was detected downregulation of ERK1/2 signaling pathway by treatments of DP (12.5 to 100 μg/mL.) in DHER2 and ER-positive breast cancer cells in a dose-dependent manner [22].
Inhibition of cancer progression via regulation of mTOR-related pathway
The mTOR-related pathway is involved in autophagy and is the most important in the activation of autophagy. In breast cancer cell types bearing the MCF-7 phenotype, several phytochemical substances influence autophagy via the mTOR pathway [76]. The link between DP, autophagy, and the mTOR pathway was investigated. The eukaryotic translation initiation factor 4E (eIF4e) and 70 kDa ribosomal S6 kinase (p70s6k) were shown to be adversely affected following DP treatment, which showed that delphinidin had an influence on mTOR activity. Treatment with delphinidin particularly inhibits the Akt branch upstream of mTOR [77, 78]. According to Steelman et al. breast cancer is typically caused by a malfunction in the mTOR pathway in mammary tissue rather than uterine tissue [78]. Delphinidin reduced the proliferation of positive breast cancer cells (HER-2) through the mTOR pathway, which suggests that DP reduces proliferation and autophagy produced via modulating the mTOR pathway. Under situations of oxidative stress and energy shortage in eukaryotic cells, 5′ AMP-activated protein kinase (AMPK), an energy sensor in cells, activates autophagy. According to Aryal et al., autophagy was triggered in pancreatic cells by AMPK and directly activates the downstream Unc-51 Like Autophagy Activating Kinase 1 (ULK1) via inhibiting mTOR phosphorylation [79]. The study found that AMPK phosphorylated ULK1 at ser317 and reduced the activation of mTOR, which showed a link between ULK1 and mTOR in delphinidin-induced autophagy. So, it was concluded that delphinidin was inhibited the proliferation of breast cancer cell lines (BT474 and MDA-MB-453) via activating liver kinase B1 (LKB1) and AMPK [80] Forkhead box O (FoxO) transcription factor FOXO3a was shown to promote the expression of genes involved in autophagy. When AMPK was activated, FOXO3a was increased, which led to the activation of autophagy. It was indicated that FOXO3a triggers autophagy-related genes. Autophagy-related genes are regulated by the AMPK-FOXO3a axis in a variety of cell types, according to several studies [81]. The PI3K/Akt/mTOR pathway is a desirable target for anticancer drugs and DP targets these pathways to exert its anticancer activity. The mTOR, protein kinase B (Akt), and phospho-inositol-3 kinase (PI3K) signal transduction pathways are crucially involved in the control of numerous vital physiological processes, including growth, angiogenesis, apoptosis, survival, and metabolism [82, 83]. These signaling pathways are typically downregulated in a variety of malignancies and other inflammatory diseases like psoriasis [84]. DP-regulated PI3K/Akt/mTOR pathway by feedback loops, partly through the upregulation of mTOR. Two functionally different protein complexes contain mTOR: mTORC1 and mTORC2. Protein translation results from phosphorylated mTORC1 of p70S6 kinase (p70S6K), which phosphorylates 4E-BP1 and the S6 ribosomal protein [85]. As part of the feedback loop, mTORC2 phosphorylates serine 473 to activate Akt, which then phosphorylates TSC2 and PRAS40 to activate mTORC1 and promote keratinocyte hyperproliferation while suppressing differentiation [86, 87] (Fig. 5).
Delphinidin modulates PI3K/Akt/mTOR signaling by inhibiting both upstream and downstream signals in the system, PI3K/Akt and mTOR can be targeted at the same time to reduce cell and tissue development, promote angiogenesis, and restore normal tissue architecture. Delphinidin inhibits cell survival and proliferation by blocking the PI3K/Akt pathway as well as the mTOR pathway
Clinical evidence on the anticancer potential of delphinidin and its derivatives
While preclinical studies provide strong evidence for the anticancer potential of DP and its derivatives, clinical evidence supporting the same is still limited and requires further validation through rigorous trials. Most of the evidence supporting DP’s anticancer effects comes from in vitro studies and animal models. Some observational studies have shown that diets rich in anthocyanins, including delphinidin, are associated with a reduced risk of certain cancers [88, 89]. For example, Zhang et al. [90] found that dietary intake of total anthocyanidins and of all six subclasses including cyanidin, DP and its derivatives (malvidin and petunidin), peonidin, and pelargonidin was related to a reduced risk of lung cancer in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening cohort.
In general, there is still limited clinical trial data to validate the efficacy and safety of DP and its derivatives in humans. Such studies when conducted in the future could also consider employing combination therapies by investigating the potential synergistic effects of DP with other anticancer agents which could provide new therapeutic strategies and improve the stability of DP to exploit its anticancer benefits.
Limitations of delphinidin and its derivatives as sources of anticancer drugs
Delphinidin has shown promise as an anticancer agent due to its antioxidant, anti-inflammatory, and antiproliferative properties. However, several limitations must be addressed before it can be considered a viable anticancer drug. These include:
-
(a)
Poor solubility of DP in water poses challenges for formulation and delivery. Such low solubilities lead to poor absorption in the gastrointestinal tract, which can limit its bioavailability and efficacy when administered orally. Moreover, DP undergoes rapid metabolism and degradation in the body, which further reduces its effective concentration at the target site.
-
(b)
The pharmacokinetics of DP is another striking medical limitation of its use in anticancer therapy. For example, DP is a molecule with a short biological half-life, emphasizing that it can be rapidly eliminated from the body. This would necessitate frequent dosing to maintain its therapeutic concentrations at any given time. It is to date not very clear how the distribution of DP to cancerous tissues occurs, and its ability to reach and penetrate tumors in effective concentrations is under debate.
-
(c)
There are stability issues around DP and its derivatives. DP is in principle a chemically unstable molecule, especially under physiological conditions, which can lead to rapid degradation and loss of activity. Thus, specific storage conditions and facilities are inevitably required to keep it stable, and this ultimately complicates its use in clinical settings.
-
(d)
As with most phytochemicals, there are probable toxicity and side effects associated with DP. The molecule is generally considered to be safe but its cytotoxicity on normal (healthy non-cancerous) cells at higher concentrations raise concerns about potential side effects. Thus, long-term safety data and potential toxicological effects of DP in humans will need to be explored further in future.
-
(e)
The exact mechanisms through which DP exerts its anticancer effects are yet to be fully elucidated. This complexity can make it difficult to predict its behavior in different cancer types and stages. It should also be expected that the effectiveness of DP and its derivatives will vary significantly depending on the type of cancer, the stage of the disease, and individual patient factors.
-
(f)
Delivering of DP is another challenge in its utilization for cancer therapy, and at the moment, effective delivery systems that can specifically target cancer cells while sparing normal cells are still under development.
Overall, DP and its derivatives hold a great promise as source of anticancer moleucles. However, the aforementioned limitations have to be addressed through advanced research and development efforts, including improved delivery systems, comprehensive clinical trials, and detailed studies on its mechanisms of action and long-term safety.
Conclusions
Delphinidin and its derivatives have been shown to exert anticancer properties against various cell lines, including breast cancer, endothelial cells, and human colon cancer cells. DP and derivatives promote apoptosis in cancer cells and have potential health benefits. The anticancer mechanism of action involved targeting cyclooxygenase-2-Prostaglandin E2 pathway, mitogen-activated protein kinases/extracellular signal-regulated kinase signaling pathway, induction of epithelial-to-mesenchymal transition, intrinsic apoptotic pathway, and inhibition of Poly ADP-ribose polymerase, PI3K/AKT pathways, and modulate mTOR, and Nrf2 signaling. The review highlighted important signaling pathways that showed DP and its derivatives influence a broad range of signaling mediators. The review has narrated preclinical trials to report the anticancer effect of DP and mentioned the occurrence of DP derivatives in different plants that can be isolated and investigated for their anticancer effects and possible development into anticancer drugs.
Abbreviations
- ATG-12:
-
Autophagy-related gene 12
- ATG-5:
-
Autophagy-related gene 5
- b-FGF:
-
Basic fibroblast growth factor
- CDK1/2:
-
Cyclin-dependent kinase 1/2,
- CD31:
-
Cluster of differentiation 31
- CPD:
-
Cyclobutane pyrimidine dimer
- DHT:
-
Dihydrotestosterone
- DNMT:
-
DNA methyltransferase
- EGFR:
-
Epidermal growth factor receptor
- DR5:
-
Death receptor 5
- HDACs:
-
Histone deacetylases
- HDAC3:
-
Histone deacetylase 3
- HIF-α:
-
Hypoxia-inducible factor alpha, Hmox1: heme oxygenase 1
- LC3-II:
-
Microtubule-associated protein light chain 3-II
- MCL-1:
-
Myeloid cell leukemia 1
- MMP-2:
-
Matrix metalloproteinases 2
- MMP-9:
-
Matrix metalloproteinase-9
- mTOR:
-
Mammalian target of rapamycin
- NQO1:
-
Nad(p)h/quinone oxidoreductase 1
- PARP:
-
Poly(adp-ribose) polymerase
- p-Akt:
-
Phosphorylated akt
- p-BAD:
-
Phosphorylated bcl-2-associated death promoter
- p-ERK:
-
Phosphorylated extracellular signal-regulated protein kinase
- p-FAK:
-
Phosphorylated focal adhesion kinase
- PGE2:
-
Prostaglandin E2
- PGK1:
-
Phosphoglycerate kinase 1
- p-GSK3β:
-
Phosphorylated glycogen synthase kinase-3β
- PI3K:
-
Phosphatidylinositol-3-kinase
- p-MSK:
-
Phosphorylated mitogen and stress-activated protein kinase,
- p-p90RSK:
-
Phosphorylated p90 ribosomal S6 kinase,
- PSA:
-
Prostate-specific antigen
- p-SHP-2:
-
Phosphorylated SH2 domain-containing protein tyrosine phosphatase-2
- pS6:
-
Phosphorylated S6,
- PUMA:
-
p53 upregulated modulator of apoptosis
- p70S6K:
-
Phosphorylated 70S6 kinase,
- SRD5A1:
-
Steroid 5 α-reductase type I
- TGF β:
-
Transforming growth factor β
- UHRF1:
-
Ubiquitin-like PHD ring finger 1
- XIAP:
-
X-linked inhibitor of apoptosis protein
- 8-OHdG:
-
8-hydroxy-20-deoxyguanosine.
References
Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2018;24:551–60. https://doi.org/10.1007/s11655-014-1960-x.
do Carmo Brito BN, da Silva Pena R, Santos Lopes A, Campos Chisté R. Anthocyanins of Jambolão (Syzygium cumini): extraction and pH-dependent color changes. J Food Sci. 2017;82:2286–90. https://doi.org/10.1111/1750-3841.13847.
Zhang J, Celli GB, Brooks MS. “Natural sources of anthocyanins”. Lancet Respiratory Med. 2019;10:1–33. https://doi.org/10.1016/S2213-2600(21)00559-2.
Husain A, Chanana H, Khan SA, Dhanalekshmi UM, Ali M, Alghamdi AA, et al. Chemistry and pharmacological actions of delphinidin, a dietary purple pigment in anthocyanidin and anthocyanin forms. Front Nutr. 2022;9:746881 https://doi.org/10.3389/fnut.2022.746881.
Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly) phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–92.
Han B, Peng X, Cheng D, Zhu Y, Du J, Li J, et al. Delphinidin suppresses breast carcinogenesis through the HOTAIR/microRNA-34a axis. Cancer Sci. 2019;110:3089–97. https://doi.org/10.1111/cas.14133.
Chamcheu JC, Adhami VM, Esnault S, Sechi M, Siddiqui IA, Satyshur KA, et al. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant, delphinidin, ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like disease in mice. Antioxid redox Signal. 2017;26:49–69.
Sogo T, Kumamoto T, Ishida H, Hisanaga A, Sakao K, Terahara N, et al. Comparison of the inhibitory effects of delphinidin and its glycosides on cell transformation. Planta Med. 2015;81:26–31.
Kumoro AC, Retnowati DS, Budiyati CS. Solubility of delphinidin in water and various organic solvents between (298.15 and 343.15) K. J Chem Eng Data. 2010;55:2603–6.
Dudek A, Spiegel M, Strugała-Danak P, Gabrielska J. (2022) Analytical and theoretical studies of antioxidant properties of chosen anthocyanins; a structure-dependent relationships. Int J Mol Sci. 2022;23:5432. https://doi.org/10.3390/ijms23105432.
Chen K, Kortesniemi MK, Linderborg KM, Yang B. Anthocyanins as promising molecules affecting energy homeostasis, inflammation, and gut microbiota in type 2 diabetes with special reference to impact of acylation. J Agric Food Chem. 2023;71:1002–17. https://doi.org/10.1021/acs.jafc.2c05879.
Merecz-Sadowska A, Sitarek P, Kowalczyk T, Zajdel K, Jęcek M, Nowak P, et al. Food anthocyanins: malvidin and its glycosides as promising antioxidant and anti-inflammatory agents with potential health benefits. Nutrients. 2023;15:3016. https://doi.org/10.3390/nu15133016.
Sharma A, Choi HK, Kim YK, Lee HJ. Delphinidin and its glycosides’ war on cancer: preclinical perspectives. Int J Mol Sci. 2021;22:11500. https://doi.org/10.3390/ijms222111500.
Wu A, Zhu Y, Han B, Peng J, Deng X, Chen W, et al. Delphinidin induces cell cycle arrest and apoptosis in HER-2 positive breast cancer cell lines by regulating the NF-kappaB and MAPK signaling pathways. Oncol Lett. 2021;22:832 https://doi.org/10.3892/ol.2021.13093.
Kuo HD, Wu R, Li S, Yang AY, Kong AN. Anthocyanin delphinidin prevents neoplastic transformation of mouse skin JB6 P+ cells: epigenetic re-activation of Nrf2-ARE pathway. AAPS J. 2019;21:83 https://doi.org/10.1208/s12248-019-0355-5.
Bu C, Zhang Q, Zeng J, Cao X, Hao Z, Qiao D, et al. Identification of a novel anthocyanin synthesis pathway in the fungus Aspergillus sydowii H-1. BMC Genomics. 2020;21:29 https://doi.org/10.1186/s12864-019-6442-2.
Liu C, Yao X, Li G, Huang L, Xie Z. Transcriptomic profiling of purple broccoli reveals light-induced anthocyanin biosynthetic signaling and structural genes. PeerJ. 2020;8:e8870 https://doi.org/10.7717/peerj.8870.
James AM, Ma D, Mellway R, Gesell A, Yoshida K, Walker V, et al. Poplar MYB115 and MYB134 transcription factors regulate proanthocyanidin synthesis and structure. Plant Physiol. 2017;174:154–71. https://doi.org/10.1104/pp.16.01962.
He F, Mu L, Yan GL, Liang NN, Pan QH, Wang J, et al. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules. 2010;15:9057–91. https://doi.org/10.3390/molecules15129057.
Yang J, Shi W, Li B, Bai Y, Hou Z. Preharvest and postharvest UV radiation affected flavonoid metabolism and antioxidant capacity differently in developing blueberries (Vaccinium corymbosum L.). Food Chem. 2019;301:125248 https://doi.org/10.1016/j.foodchem.2019.125248.
Zhang Q, Hu J, Liu M, Shi Y, De Vos R, Ruan J. Stimulated biosynthesis of delphinidin-related anthocyanins in tea shoots reducing the quality of green tea in summer. J Sci Food Agric. 2020;100:1505–14. https://doi.org/10.1002/jsfa.10158.
Ozbay T, Nahta R. Delphinidin inhibits HER2 and Erk1/2 signaling and suppresses growth of HER2-overexpressing and triple negative breast cancer cell lines. Breast Cancer (Auckl). 2011;5:143–54. https://doi.org/10.4137/BCBCR.S7156.
Clere N, Faure S, Martinez MC, Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem. 2011;9:62–77. https://doi.org/10.2174/187152511796196498.
Paixao J, Dinis TC, Almeida LM. Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: an in vitro approach. Apoptosis. 2011;16:976–89. https://doi.org/10.1007/s10495-011-0632-y.
Fritz J, Roth M, Holbach P, Esselen M, Marko D. Impact of delphinidin on the maintenance of DNA integrity in human colon carcinoma cells. J Agric Food Chem. 2008;56:8891–6. https://doi.org/10.1021/jf801522x.
Kang NJ, Lee KW, Kwon JY, Hwang MK, Rogozin EA, Heo YS, et al. Delphinidin attenuates neoplastic transformation in JB6 Cl41 mouse epidermal cells by blocking Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling. Cancer Prev Res (Philos). 2008;1:522–31. https://doi.org/10.1158/1940-6207.CAPR-08-0071.
Yun JM, Afaq F, Khan N, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol Carcinog. 2009;48:260–70. https://doi.org/10.1002/mc.20477.
Lim W, Jeong W, Song G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol Cell Endocrinol. 2016;422:172–81. https://doi.org/10.1016/j.mce.2015.12.013.
Lim W, Song G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via PI3K/AKT and ERK 1/2 MAPK signal transduction. Oncol Lett. 2017;14:810–8. https://doi.org/10.3892/ol.2017.6232.
Lim WC, Kim H, Kim YJ, Park SH, Song JH, Lee KH, et al. Delphinidin inhibits BDNF-induced migration and invasion in SKOV3 ovarian cancer cells. Bioorg Medicinal Chem Lett. 2017;27:5337–43. https://doi.org/10.1016/j.bmcl.2017.09.024.
Mazewski C, Kim MS, Gonzalez de Mejia E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci Rep. 2019;9:11560 https://doi.org/10.1038/s41598-019-47903-0.
Huang CC, Hung CH, Hung TW, Lin YC, Wang CJ, Kao SH. Dietary delphinidin inhibits human colorectal cancer metastasis associating with upregulation of miR-204-3p and suppression of the integrin/FAK axis. Sci Rep. 2019;9:18954 https://doi.org/10.1038/s41598-019-55505-z.
Jang CH, Lee IA, Ha YR, Lim J, Sung MK, Lee SJ, et al. PGK1 induction by a hydrogen peroxide treatment is suppressed by antioxidants in human colon carcinoma cells. Biosci Biotechnol Biochem. 2008;72:1799–808. https://doi.org/10.1271/bbb.80079.
Zhang Z, Pan Y, Zhao Y, Ren M, Li Y, Lu G, et al. Delphinidin modulates JAK/STAT3 and MAPKinase signaling to induce apoptosis in HCT116 cells. Environ Toxicol. 2021;36:1557–66. https://doi.org/10.1002/tox.23152.
Kim MH, Jeong YJ, Cho HJ, Hoe HS, Park KK, Park YY, et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1alpha and VEGF expression in A549 lung cancer cells. Oncol Rep. 2017;37:777–84. https://doi.org/10.3892/or.2016.5296.
Kang SH, Bak DH, Chung BY, Bai HW, Kang BS. Delphinidin enhances radio-therapeutic effects via autophagy induction and JNK/MAPK pathway activation in non-small cell lung cancer. Korean J Physiol Pharm. 2020;24:413–22. https://doi.org/10.4196/kjpp.2020.24.5.413.
Pal HC, Sharma S, Strickland LR, Agarwal J, Athar M, Elmets CA, et al. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One. 2013;8:e77270 https://doi.org/10.1371/journal.pone.0077270.
Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–72. https://doi.org/10.1158/0008-5472.CAN-08-2232.
Ko H, Jeong MH, Jeon H, Sung GJ, So Y, Kim I, et al. Delphinidin sensitizes prostate cancer cells to TRAIL-induced apoptosis, by inducing DR5 and causing caspase-mediated HDAC3 cleavage. Oncotarget. 2015;6:9970–84. https://doi.org/10.18632/oncotarget.3667.
Jeong MH, Ko H, Jeon H, Sung GJ, Park SY, Jun WJ, et al. Delphinidin induces apoptosis via cleaved HDAC3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget. 2016;7:56767–80. https://doi.org/10.18632/oncotarget.10790.
Lee W, Yun JM. Suppression of beta-catenin signaling pathway in human prostate cancer PC3 cells by delphinidin. J Cancer Prev. 2016;21:110–4. https://doi.org/10.15430/JCP.2016.21.2.110.
Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127:222–32. https://doi.org/10.1038/sj.jid.5700510.
Sobiepanek A, Milner-Krawczyk M, Bobecka-Wesołowska K, Kobiela T. The effect of delphinidin on the mechanical properties of keratinocytes exposed to UVB radiation. J Photochemistry Photobiol B: Biol 2016;164:264–70. https://doi.org/10.1016/j.jphotobiol.2016.09.038.
Yeh CT, Yen GC. Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J Agric Food Chem. 2005;53:1740–9. https://doi.org/10.1021/jf048955e.
Feng R, Wang SY, Shi YH, Fan J, Yin XM. Delphinidin induces necrosis in hepatocellular carcinoma cells in the presence of 3-methyladenine, an autophagy inhibitor. J Agric Food Chem. 2010;58:3957–64. https://doi.org/10.1021/jf9025458.
Hou DX, Ose T, Lin S, Harazoro K, Imamura I, Kubo M, et al. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: structure-activity relationship and mechanisms involved. Int J Oncol. 2003;23:705–12.
Tsai MC, Chen CC, Tseng TH, Chang YC, Lin YJ, Tsai IN, et al. Hibiscus anthocyanins extracts induce apoptosis by activating AMP-activated protein kinase in human colorectal cancer cells. Nutrients. 2023;15:3972. https://doi.org/10.3390/nu15183972.
Chang YC, Huang HP, Hsu JD, Yang SF, Wang CJ. Hibiscus anthocyanins rich extract-induced apoptotic cell death in human promyelocytic leukemia cells. Toxicol Appl Pharmacol. 2005;205:201–12. https://doi.org/10.1016/j.taap.2004.10.014.
Takasawa R, Saeki K, Tao A, Yoshimori A, Uchiro H, Fujiwara M, et al. Delphinidin, a dietary anthocyanidin in berry fruits, inhibits human glyoxalase I. Bioorg Medicinal Chem. 2010;18:7029–33. https://doi.org/10.1016/j.bmc.2010.08.012.
Hou DX, Tong X, Terahara N, Luo D, Fujii M. Delphinidin 3-sambubioside, a Hibiscus anthocyanin, induces apoptosis in human leukemia cells through reactive oxygen species-mediated mitochondrial pathway. Arch Biochem Biophys. 2005;440:101–9. https://doi.org/10.1016/j.abb.2005.06.002.
León-González AJ, Sharif T, Kayali A, Abbas M, Dandache I, Etienne-Selloum N, et al. Delphinidin-3-O-glucoside and delphinidin-3-O-rutinoside mediate the redox-sensitive caspase 3-related pro-apoptotic effect of blackcurrant juice on leukaemia Jurkat cells. J Funct Foods. 2015;17:847–56. https://doi.org/10.1016/j.jff.2015.06.043.
Kang Y, Li J, Jing L, Zhang Y, Wang X. Antiproliferative and apoptosis inducing effect of delphinidin against human bladder cancer cell line. Pharmacogn Mag. 2021;17:101.
Ouanouki A, Lamy S, Annabi B. Anthocyanidins inhibit epithelial-mesenchymal transition through a TGFbeta/Smad2 signaling pathway in glioblastoma cells. Mol Carcinog. 2017;56:1088–99. https://doi.org/10.1002/mc.22575.
Chakrabarti M, Ray SK. Direct transfection of miR-137 mimics is more effective than DNA demethylation of miR-137 promoter to augment anti-tumor mechanisms of delphinidin in human glioblastoma U87MG and LN18 cells. Gene. 2015;573:141–52. https://doi.org/10.1016/j.gene.2015.07.034.
Fernandes I, Faria A, Azevedo J, Soares S, Calhau C, De Freitas V, et al. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. J Agric Food Chem. 2010;58:3785–92. https://doi.org/10.1021/jf903714z.
Im NK, Jang WJ, Jeong CH, Jeong GS. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-kappaB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J Med Food. 2014;17:855–61. https://doi.org/10.1089/jmf.2013.3077.
Ichiyanagi T, Rahman MM, Kashiwada Y, Ikeshiro Y, Shida Y, Hatano Y, et al. Absorption and metabolism of delphinidin 3-O-beta-D-glucopyranoside in rats. Free Radic Biol Med. 2004;36:930–7. https://doi.org/10.1016/j.freeradbiomed.2004.01.005.
Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, Agarwal R, Vanamala JK. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J Nutr Biochem. 2015;26:1641–9. https://doi.org/10.1016/j.jnutbio.2015.08.005.
Thwe A, Valan Arasu M, Li X, Park CH, Kim SJ, Al-Dhabi NA, et al. Effect of different agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (fagopyrum tataricum gaertn). Front Microbiol. 2016;7:318 https://doi.org/10.3389/fmicb.2016.00318.
Fimognari C, Berti F, Nüsse M, Cantelli-Forti G, Hrelia P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanidin-3-O-beta-glucopyranoside. Biochem Pharm. 2004;67:2047–56. https://doi.org/10.1016/j.bcp.2004.02.021.
Serafino A, Sinibaldi-Vallebona P, Lazzarino G, Tavazzi B, Rasi G, Pierimarchi P, et al. Differentiation of human melanoma cells induced by cyanidin-3-O-beta-glucopyranoside. FASEB J. 2004;18:1940–2. https://doi.org/10.1096/fj.04-1925fje.
Burton LJ, Smith BA, Smith BN, Loyd Q, Nagappan P, McKeithen D, et al. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis. 2015;36:1019–27. https://doi.org/10.1093/carcin/bgv084.
Hou DX, Kai K, Li JJ, Lin S, Terahara N, Wakamatsu M, et al. Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis. 2004;25:29–36. https://doi.org/10.1093/carcin/bgg184.
Song NR, Yang H, Park J, Kwon JY, Kang NJ, Heo YS, et al. Cyanidin suppresses neoplastic cell transformation by directly targeting phosphatidylinositol 3-kinase. Food Chem. 2012;133:658–64. https://doi.org/10.1016/j.foodchem.2012.01.045.
Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J cancer Prev. 2015;16:2129–44.
Anwar S, Fratantonio D, Ferrari D, Saija A, Cimino F, Speciale A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol Med Rep. 2016;14:1397–403. https://doi.org/10.3892/mmr.2016.5397.
Chen PN, Chu SC, Chiou HL, Chiang CL, Yang SF, Hsieh YS. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr Cancer. 2005;53:232–43. https://doi.org/10.1207/s15327914nc5302_12.
Chen J, Zhu Y, Zhang W, Peng X, Zhou J, Li F, et al. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer. 2018;18:342 https://doi.org/10.1186/s12885-018-4231-y.
Matsunaga N, Tsuruma K, Shimazawa M, Yokota S, Hara H. Inhibitory actions of bilberry anthocyanidins on angiogenesis. Phytother Res. 2010;24:S42–47. https://doi.org/10.1002/ptr.2895.
Xu J, Zhang Y, Ren G, Yang R, Chen J, Xiang X, et al. Inhibitory effect of delphinidin on oxidative stress induced by H2O2 in HepG2 cells. Oxid Med Cell Longev. 2020;2020:4694760 https://doi.org/10.1155/2020/4694760.
Keravis T, Favot L, Abusnina AA, Anton A, Justiniano H, Soleti R, et al. Delphinidin inhibits tumor growth by acting on VEGF signalling in endothelial cells. PLoS One. 2015;10:e0145291 https://doi.org/10.1371/journal.pone.0145291.
Thiele W, Rothley M, Teller N, Jung N, Bulat B, Plaumann D, et al. Delphinidin is a novel inhibitor of lymphangiogenesis but promotes mammary tumor growth and metastasis formation in syngeneic experimental rats. Carcinogenesis. 2013;34:2804–13. https://doi.org/10.1093/carcin/bgt291.
Lamy S, Lafleur R, Bédard V, Moghrabi A, Barrette S, Gingras D, et al. Anthocyanidins inhibit migration of glioblastoma cells: structure-activity relationship and involvement of the plasminolytic system. J Cell Biochem. 2007;100:100–11. https://doi.org/10.1002/jcb.21023.
Filipiak K, Hidalgo M, Silvan JM, Fabre B, Carbajo RJ, Pineda-Lucena A, et al. Dietary gallic acid and anthocyanin cytotoxicity on human fibrosarcoma HT1080 cells. A study on the mode of action. Food Funct. 2014;5:381–9. https://doi.org/10.1039/c3fo60465a.
Dreiseitel A, Korte G, Schreier P, Oehme A, Locher S, Hajak G, et al. sPhospholipase A(2) is inhibited by anthocyanidins. J Neural Transm (Vienna). 2009;116:1071–7. https://doi.org/10.1007/s00702-009-0268-z.
Zhou Y, Liang X, Chang H, Shu F, Wu Y, Zhang T, et al. Ampelopsin-induced autophagy protects breast cancer cells from apoptosis through Akt-mTOR pathway via endoplasmic reticulum stress. Cancer Sci. 2014;105:1279–87. https://doi.org/10.1111/cas.12494.
Li YC, He SM, He ZX, Li M, Yang Y, Pang JX, et al. Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells. Cancer Lett. 2014;344:239–59. https://doi.org/10.1016/j.canlet.2013.11.001.
Steelman LS, Martelli AM, Cocco L, Libra M, Nicoletti F, Abrams SL, et al. The therapeutic potential of mTOR inhibitors in breast cancer. Br J Clin Pharm. 2016;82:1189–212. https://doi.org/10.1111/bcp.12958.
Aryal P, Kim K, Park PH, Ham S, Cho J, Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014;281:4644–58. https://doi.org/10.1111/febs.12969.
Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–23. https://doi.org/10.1007/s00018-010-0454-z.
Chi Y, Shi C, Zhao Y, Guo C (2016) Forkhead box O (FOXO) 3 modulates hypoxia-induced autophagy through AMPK signalling pathway in cardiomyocytes. Biosci Rep. 36 https://doi.org/10.1042/BSR20160091.
Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway–beyond rapalogs. Oncotarget. 2010;1:530–43.
Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–46.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. cell 2012;149:274–93.
Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. science 2011;332:1317–22.
Huang T, Lin X, Meng X, Lin M. Phosphoinositide-3 kinase/protein kinase-B/mammalian target of rapamycin pathway in psoriasis pathogenesis. A potential therapeutic target? Acta Derm-venereologica. 2014;94:371–9.
Chamcheu JC, Chaves-Rodriquez M-I, Adhami VM, Siddiqui IA, Wood GS, Longley BJ, et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPARβ/δ in human psoriasis and imiquimod-induced murine psoriasiform dermatitis model. Acta Derm-venereologica. 2016;96:854–6.
Xu X, Zhu Y, Li S, Xia D. Dietary intake of anthocyanidins and renal cancer risk: a prospective study. Cancers (Basel). 2023;15:1406. https://doi.org/10.3390/cancers15051406.
Xiang L, Wu D, Xu Z, Tang Y, He H, Wang Y, et al. Association between dietary anthocyanidins and biliary cancer risk in 98,458 participants: results from a prospective study. Cancer Epidemiol, Biomark Prev. 2024;33:151–7. https://doi.org/10.1158/1055-9965.EPI-23-0759.
Zhang Y, Zhu M, Wan H, Chen L, Luo F. Association between dietary anthocyanidins and risk of lung cancer. Nutrients. 2022;14:2643. https://doi.org/10.3390/nu14132643.
Mazza G, Miniati E. Effects of selected lactobacilli on the functional properties and stability of gluten-free sourdough bread, Anthocyanins in Fruits, Vegetables, and Grains. Boca Raton: CRC Press; 1993. https://doi.org/10.1007/s00217-017-3020-1.
Chen Y, Du F, Wang W, Li Q, Zheng D, Zhang W, et al. Large-scale isolation of high-purity anthocyanin monomers from mulberry fruits by combined chromatographic techniques. J Sep Sci. 2017;40:3506–12. https://doi.org/10.1002/jssc.201700471.
Khalifa I, Zhu W, Li K, Li C. Polyphenols of mulberry fruits as multifaceted compounds: compositions, metabolism, health benefits, and stability—A structural review. J Funct Foods. 2018;40:28–43. https://doi.org/10.1016/j.jff.2017.10.041.
Chen X, Parker J, Krueger CG, Shanmuganayagam D, Reed JD. Validation of HPLC assay for the identification and quantification of anthocyanins in black currants. Anal Methods. 2014;6:8141–7. https://doi.org/10.1039/C4AY01500B.
Pati S, Liberatore MT, Gambacorta G, Antonacci D, La Notte E. Rapid screening for anthocyanins and anthocyanin dimers in crude grape extracts by high performance liquid chromatography coupled with diode array detection and tandem mass spectrometry. J Chromatogr A. 2009;1216:3864–8. https://doi.org/10.1016/j.chroma.2009.02.068.
Dugo P, Mondello L, Morabito D, Dugo G. Characterization of the anthocyanin fraction of sicilian blood orange juice by micro-HPLC-ESI/MS. J Agric Food Chem. 2003;51:1173–6. https://doi.org/10.1021/jf026078b.
Alighourchi H, Barzegar M, Abbasi S. Anthocyanins characterization of 15 Iranian pomegranate (Punica granatum L.) varieties and their variation after cold storage and pasteurization. Eur Food Res Technol. 2008;227:881–7. https://doi.org/10.1007/s00217-007-0799-1.
Hasnaoui N, Jbir R, Mars M, Trifi M, Kamal-Eldin A, Melgarejo P, et al. Organic acids, sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. Int J Food Prop. 2011;14:741–57. https://doi.org/10.1080/10942910903383438.
Hosseinian FS, Beta T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba Berries. J Agric Food Chem. 2007;55:10832–8. https://doi.org/10.1021/jf072529m.
Wu X, Prior RL. Identification and characterization of anthocyanins by high-performance liquid chromatography−electrospray ionization−tandem mass spectrometry in common foods in the United States: Vegetables, Nuts, and Grains. J Agric Food Chem. 2005;53:3101–13. https://doi.org/10.1021/jf0478861.
Lin L-Z, Harnly JM, Pastor-Corrales MS, Luthria DL. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008;107:399–410. https://doi.org/10.1016/j.foodchem.2007.08.038.
Li H, Deng Z, Zhu H, Hu C, Liu R, Young JC, et al. Highly pigmented vegetables: anthocyanin compositions and their role in antioxidant activities. Food Res Int. 2012;46:250–9. https://doi.org/10.1016/j.foodres.2011.12.014.
Mennella G, Lo Scalzo R, Fibiani M, D'Alessandro A, Francese G, Toppino L, et al. Chemical and bioactive quality traits during fruit ripening in Eggplant (S. melongena L.) and Allied Species. J Agric Food Chem. 2012;60:11821–31. https://doi.org/10.1021/jf3037424.
Takeoka GR, Dao LT, Tamura H, Harden LA. Delphinidin 3-O-(2-O-β-d-Glucopyranosyl-α-l-arabinopyranoside): a novel anthocyanin identified in Beluga Black Lentils. J Agric Food Chem. 2005;53:4932–7. https://doi.org/10.1021/jf040493h.
Giusti F, Caprioli G, Ricciutelli M, Vittori S, Sagratini G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017;221:689–97. https://doi.org/10.1016/j.foodchem.2016.11.118.
Terahara N, Honda T, Hayashi M, Ishimaru K. New anthocyanins from purple pods of pea (Pisum spp.). Biosci, Biotechnol, Biochem. 2000;64:2569–74. https://doi.org/10.1271/bbb.64.2569.
Terahara N, Honda T, Hayashi M, Ishimaru K. New anthocyanins from purple pods of pea (Pisum spp.). J Biosci. 2006;61:527–35. https://doi.org/10.1271/bbb.64.2569.
Takeoka GR, Dao L, Harden L, et al. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (Rheum spp.) varieties. Int J Food Sci Technol. 2013;48:172–8. https://doi.org/10.1111/j.1365-2621.2012.03174.x.
Choung M-G, Baek I-Y, Kang S-T, Han WY, Shin DC, Moon HP, et al. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr. J Agric Food Chem. 2001;49:5848–51. https://doi.org/10.1021/jf010550w.
Koh K, Youn JE, Kim H-S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr.) varieties. J Food Sci Technol. 2014;51:377–81. https://doi.org/10.1007/s13197-011-0493-y.
Kim M-J, Hyun J-N, Kim J-A, Park JC, Kim MY, Kim JG, et al. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J Agric Food Chem. 2007;55:4802–9. https://doi.org/10.1021/jf0701943.
Bellido GG, Beta T. Anthocyanin composition and oxygen radical scavenging capacity (ORAC) of milled and pearled purple, black, and common Barley. J Agric Food Chem. 2009;57:1022–8. https://doi.org/10.1021/jf802846x.
Diczházi I, Kursinszki L. Anthocyanin content and composition in winter blue barley cultivars and lines. Cereal Chem. 2014;91:195–200. https://doi.org/10.1094/CCHEM-05-13-0091-R.
Asem ID, Imotomba RK, Mazumder PB, Laishram JM. Anthocyanin content in the black scented rice (Chakhao): its impact on human health and plant defense. Symbiosis. 2015;66:47–54. https://doi.org/10.1007/s13199-015-0329-z.
Hao J, Zhu H, Zhang Z, Yang S, Li H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J Cereal Sci. 2015;64:92–99. https://doi.org/10.1016/j.jcs.2015.05.003.
Pihlava J-M, Hellström J, Kurtelius T, Mattila P. Flavonoids, anthocyanins, phenolamides, benzoxazinoids, lignans and alkylresorcinols in rye (Secale cereale) and some rye products. J Cereal Sci. 2018;79:183–92. https://doi.org/10.1016/j.jcs.2017.09.009.
Abdel-Aal E-SM, Young JC, Rabalski I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J Agric Food Chem. 2006;54:4696–704. https://doi.org/10.1021/jf0606609.
Abdel-Aal e, Abou-Arab AA, Gamel TH, Hucl P, Young JC, Rabalski I. Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. J Agric Food Chem. 2008;56:11171–7. https://doi.org/10.1021/jf802168c.
Shams Najafabadi N, Sahari MA, Barzegar M, Hamidi Esfahani Z. Effects of concentration method and storage time on some bioactive compounds and color of jujube (Ziziphus jujuba var vulgaris) concentrate. J Food Sci Technol. 2017;54:2947–55. https://doi.org/10.1007/s13197-017-2733-2.
Carazzone C, Mascherpa D, Gazzani G, Papetti A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013;138:1062–71. https://doi.org/10.1016/j.foodchem.2012.11.060.
Zhang J, Wang L-S, Gao J-M, Xu YJ, Li LF, Li CH. Rapid Separation and Identification of Anthocyanins from Flowers of Viola yedoensis and V. prionantha by High-performance Liquid Chromatography–Photodiode Array Detection–Electrospray Ionisation Mass Spectrometry. Phytochem Anal. 2012;23:16–22. https://doi.org/10.1002/pca.1320.
Chen S, Xiang Y, Deng J, Liu Y, Li S. Simultaneous analysis of anthocyanin and non-anthocyanin flavonoid in various tissues of different lotus (nelumbo) cultivars by HPLC-DAD-ESI-MSn. PLOS ONE. 2013;8:e62291 https://doi.org/10.1371/journal.pone.0062291.
Deng J, Chen S, Yin X, Wang K, Liu Y, Li S, et al. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013;139:307–12. https://doi.org/10.1016/j.foodchem.2013.02.010.
de Rosso VV, Mercadante AZ. HPLC–PDA–MS/MS of Anthocyanins and Carotenoids from Dovyalis and Tamarillo Fruits. J Agric Food Chem. 2007;55:9135–41. https://doi.org/10.1021/jf071316u.
Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, et al. Bioactive Depsides and Anthocyanins from Jaboticaba (Myrciaria cauliflora). J Nat Products. 2006;69:1228–30. https://doi.org/10.1021/np0600999.
Escribano-Bailón MT, Alcalde-Eon C, Muñoz O, Rivas-Gonzalo JC, Santos-Buelga C. Anthocyanins in berries of Maqui [Aristotelia chilensis (Mol.) Stuntz. Phytochem Anal. 2006;17:8–14. https://doi.org/10.1002/pca.872.
Gironés-Vilaplana A, Mena P, García-Viguera C, Moreno DA. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT. 2012;47:279–86. https://doi.org/10.1016/j.lwt.2012.01.020.
Rojo LE, Ribnicky D, Logendra S, Poulev A, Rojas-Silva P, Kuhn P, et al. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012;131:387–96. https://doi.org/10.1016/j.foodchem.2011.08.066.
Fredes C, Yousef GG, Robert P, Grace MH, Lila MA, Gómez M, et al. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J Sci Food Agric. 2014;94:2639–48. https://doi.org/10.1002/jsfa.6602.
Du CT, Francis FJ. Anthocyanins of roselle (Hibiscus sabdariffa, L.). J Food Sci. 1973;38:810–2. https://doi.org/10.1111/j.1365-2621.1973.tb02081.x.
Lee D-Y, Park Y-J, Hwang S-C, Kim KD, Moon DK, Kim DH. Cytotoxic effects of delphinidin in human osteosarcoma cells. Acta Orthopaedica et Traumatologica Turc. 2018;52:58–64.
Kang HM, Park BS, Kang HK, Park HR, Yu SB, Kim IR. Delphinidin induces apoptosis and inhibits epithelial‐to‐mesenchymal transition via the ERK/p38 MAPK‐signaling pathway in human osteosarcoma cell lines. Environ Toxicol. 2018;33:640–9.
Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V, et al. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol. 2008;231:52–60.
Jang H-Y, Lee S-H, An I-J, Lee HN, Kim HR, Park YS, et al. Effects of delphinidin in anthocyanin on MDA-MB-231 breast cancer cells. J Korean Soc Food Sci Nutr. 2014;43:231–7.
Author contributions:
SI conceptualized and performed literature search. SI and TO drafted the initial version of the manuscript. Visualization of collected data were done by IK and UMK. All the authors read and approved the manuscript.
Funding
Open access funding provided by University of the Free State.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iqbal, S., Omara, T., Kahwa, I. et al. Anticancer potential of delphinidin and its derivatives: therapeutic and mechanistic insights. Med Chem Res (2024). https://doi.org/10.1007/s00044-024-03296-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00044-024-03296-y