Abstract
In this study, 35 novel 2,4,6-trisubstituted quinazoline derivatives were designed and synthesized, and their anti-tumor activity in vitro were preliminarily explored. Among them, compound 19n exhibited the best anti-proliferative activity against MGC-803 cells with an IC50 of 4.61 μM. Further biological experiments showed that compound 19n could inhibit the cloning formation of MGC-803 cells. DAPI staining and apoptosis experiments showed that compound 19n could induce apoptosis of MGC-803 cells in a concentration-dependent manner, and the cell cycle experiment indicated that compound 19n could also arrest the MGC-803 cell cycle in G0/G1 phase. In addition, the ADME datas of compound 19n was predicted and the potential target of compound 19n was explained through molecular docking.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is caused by the loss of normal regulation and over-proliferation of body cells, which is an important cause of human death. According to literature reports, the incidence rate of cancer in China is on the rise. So far, humans have achieved significant results in cancer treatment, in addition to traditional surgical treatment, chemotherapy, and radiation therapy, various emerging treatment methods have also been continuously discovered in recent years, such as immunotherapy [1], DNA precision therapy [2], photothermal therapy [3], and stem cell therapy [4]. However, each treatment method has its limitations and cannot be effectively used for tumor treatment. Among them, chemical drug therapy is the most widely used in the field of cancer treatment, but chemotherapy drugs are prone to develop resistance, and it could cause serious adverse reactions, such as nausea, vomiting, hair loss, anemia, etc. Therefore, searching for safe, efficient, and low-toxicity anti-tumor drugs is still the hot spot of drug research and development [5,6,7,8].
Quinazoline is an important nitrogen-containing aromatic heterocyclic compound, with various pharmacological activities, such as anti-tumor [9, 10], antiviral [11, 12], antibacterial [13, 14], and anticonvulsant [15, 16], etc. Because of its diverse biological activities, it has aroused great interest of medical researchers and chemists [17,18,19], 4-aminoquinazoline can serve as the backbone structure for many kinase inhibitors, and many small molecule anti-tumor drugs based on the 4-aminoquinoline skeleton have been approved by FDA(Food and Drug Administration) for cancer treatment, such as gefitinib(1) [20], erlotinib(2) [21], afatinib(3) [22], etc. In addition, many quinazoline derivatives with anti-tumor activity have also been continuously reported in recent years, such as AURK inhibitor compound 4 [23] and compound LSD1 inhibitor compound 5 [24].
In addition, the amide group can act as the bioisosteres of many groups, such as 1,2,3-triazole, oxadiazole, imidazole, tetrazole, indole, pyridine, pyrazine, sulfonamide, etc, it play an important role in the design of anti-tumor drugs and the optimization or modification of lead compounds [25]. Benzylthiol is an important structural fragment for small drug molecules to exert pharmacological activities and improve pharmacokinetic characteristics, and previous research by our research group found that compounds 6 and 7 containing benzylthio groups have good anti-tumor activity [26, 27]. Furthermore, many compounds containing benzylthio groups were also reported and exhibited good anti-proliferative activity, such as compound 8 [28], it was speculated that the reason for its effectiveness may be that benzylthiol may be oxidized into sulfone in the body, but the true mechanism of action was still being further explored by our research group. In addition, through literature review, we found that there have been few reports on the simultaneous modification of positions 2, 4, and 6 of the quinazoline ring. Therefore, on the basis of extensive literature review and our research group’s previous work, further development and exploration were conducted on the quinazoline ring, we chose to design and modify these three sites of the quinazoline ring reasonably to obtain efficient new chemical entities with anti-tumor activity. The detailed design rationale was shown in Figure 1. Thus, we introduced the benzylthio and amide groups into 4-aminoquinazoline skeleton structure based on the combination principles to synthesize a series of 2,4,6-trisubstituted quinazoline derivatives and evaluated their anti-proliferative activities in vitro, and taking compound 19n as an example, the potential target of the target compounds was predicted through computer simulation and its ADME data was predicted, hope to obtain some quinazoline derivatives with good anti proliferative activity.
Results and discussion
Chemistry
The preparation of compounds 17a–17n and 19a–19u was shown in Scheme 1. There are two synthesis methods for compound 11. The first method: commercially available compound 9 reacted with thiourea at 140 °C for 8 h to obtain compound 11. The second method: at first, compound 9 reacted with SOCl2 to synthesize compound 10, then compound 10 reacted with NH4NCS for 30 min and cyclized to obtain compound 11. The compounds 12a–12n was obtained by a nucleophilic substitution stirred of compound 11 with different benzyl chloride. Next, compounds 12a–12n were chlorinated with SOCl2 to obtain compounds 13a–13n. The compounds 13a–13n were reacted with 4-amino-1-Boc-piperidine in DMF to yield the compounds 14a–14n, which were reduced by iron powder to obtain compounds 15a–15n. The compounds 16a–16n were prepared via condensation stirred of compounds 15a–15n with benzoic acid. Finally, the final compounds 17a–17n were prepared by removal of the N-Boc protection through CF3COOH treatment. Compounds 18a–18u were obtained by condensation stirred of compound 15c with different substituted benzoic acids under the action of condensation agent HATU. Then, the N‐Boc protecting group was removed to get compounds 19a–19u.
Reaction conditions and reagents: a) thiourea, polyethylene glycol 400, 140 °C, 8 h; b) SOCl2, 75 °C, 2 h; c) NH4NCS, acetone, room temperature, 30 min; d) different benzyl chlorides, NaOH, H2O, 80 °C, 1 h; e) SOCl2, N,N-Dimethylformamide, 65 °C, 2 h; f) 4-amino-1-Boc-piperidine, K2CO3, 80 °C, 1 h; g) iron, NH4Cl, ethanol, H2O, 85 °C, 4 h; h) benzoic acid, HATU, DIPEA, N,N-Dimethylformamide, room temperature, 6 h; i) CF3COOH, CH2Cl2, room temperature, 1 h; j) different benzoic acid, HATU, DIPEA, N,N-dimethylformamide, room temperature, 6 h
Biological activity
Anti-proliferative activity
Using 5-fluorouracil as the positive control, the anti-proliferative activities in vitro of compounds 17a–17n against A549(human non-small cell lung cancer cell line), PC-3(prostate cancer cell line), MGC-803(human gastric cancer cell line), and Eca-109(human esophageal cancer cell line) were determined by MTT method. The results were shown in Table 1.
As shown in Table 1, most target compounds displayed moderate anti-proliferative activities against the four cell lines. By comparing the activity data of compounds 17b–17d, 17e–17f, 17h–17i, and 17j–17k against A549 and PC-3, we can see that the anti-proliferative activities of the compounds that substituent in the meta position were superior to that of the ortho- or para- substitution. From the biological data of compounds 17b–17j, When R1 were halogen substituent, the anti-proliferative activities against MGC-803 were superior to the other three types of tumor cells. In addition, when R1 was a double substituent, it would not have a significant impact on the activities of the compounds, such as compounds 17m and 17n. The summary of structure-activity relationship of compounds 17a–17n were shown in Fig. 2. Compound 17c showed the best anti-proliferative activity against MGC-803 cells, in order to obtain compounds with better activities, further optimization was carried out on the basis of compound 17c, and different substituents were introduced into the benzamide at position 6 of the quinazoline ring, exploring the effects of electronic effect and the position of different substituents on the anti-proliferative activities of compounds. The results of anti-proliferative activities were shown in Table 2.
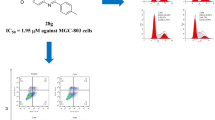
As shown in Table 2, when R2 was a strong electron-withdrawing group, such as -NO2 or -CN, it was found to have an adverse effect on the anti-proliferative activities of compounds. When R2 were -Cl, -Br, -CH3, -OCH3, and -NO2, we could conclude that compared to ortho and meta substitutions, compounds exhibit better anti-proliferative activity when the substituent was in the para position, such as compounds 19d–19f, 19g–19i, 19j–19l, 19m–19n, and 19r–19s. From the biological data of compounds 19t and 19u, we could see that when R2 was -CN, the activities of meta substitution were always better than that of para substitution. The summary of structure-activity relationship of compounds 19a–19u were shown in Fig. 2. Among them, compound 19n showed the best anti-proliferative activity against MGC-803 cells, with the IC50 value of 4.61 μM, which was better than the positive control 5-Fu. Therefore, Compound 19n was selected for further research.
Compound 19n inhibited the colony formation of MGC-803 cells
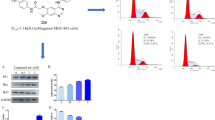
In order to further evaluate the anti-tumor ability in vitro of compound 19n, the colony formation experiment was conducted. The MGC-803 cells were treated with drug-containing medium containing different concentrations of compound 19n(0 μM, 1 μM, 2 μM, and 4 μM), as shown in Fig. 3, it could be seen that as the concentration of compound 19n gradually increases, the cell community gradually decreases, and the area of the cell community also decreases. This indicated that compound 19n could effectively inhibit the cloning formation of MGC-803 cells.
Compound 19n arrested MGC-803 cells at G0/G1 phase
Many anti-tumor drugs can effectively inhibit the development of tumor by arresting the cell cycle [29]. Therefore, the effects of compound 19n on the cell cycle of MGC-803 were evaluated using flow cytometry. The results were shown in Fig. 4, the cells in G0/G1 phase gradually increased from 47.52% of the blank control group to 59.44% after 24 h of culture in the drug-containing medium with different concentrations of compound 19n(0 μM, 2 μM, 4 μM, and 8 μM). The results suggested that compound 19n could arrest the cell cycle of MGC-803 in G0/G1 phase in a concentration-dependent manner.
Compound 19n induced MGC-803 cells apoptosis
Inducing cell apoptosis is an important pathway for many anti-tumor drugs to exert their function [30]. At first, the effects of compound 19n on the nuclear morphology of MGC-803 were observed through DAPI staining experiment. It could be seen from Fig. 5A that with the increase of compound 19n concentration(0 μM, 1 μM, 2 μM, and 4 μM), the number of cells with nuclear shrinkage and chromatin condensation gradually increases.
A Effects of compound 19n on nuclear morphology of MGC-803 cells. The arrows in the figure indicate the cells whose nuclei have shrunk or broken. B Effects of compound 19n on apoptosis of MGC-803 cells C Quantitative analysis of apoptotic cells; Compared with the control group, **P < 0.01, ***P < 0.001
In order to further evaluate the ability of compound 19n to induce cell apoptosis, propidium iodide (PI) and Annexin V-FITC double staining experiment was performed. The result was shown in Fig. 5B and C, the percentage of MGC-803 cells in G0/G1 phase increased from 9.21 to 38.64% after treatment of various concentrations compound 19n(0 μM, 2 μM, 4 μM, and 8 μM) for 48 h. The above results indicated that compound 19n could induce apoptosis of MGC-803 cells in a concentration-dependent manner.
ADME properties of compound 19n
The process in vivo (absorption, distribution, metabolism, excretion) of a compound is an important index to evaluate whether it has druggability [31]. We predicted the ADME properties of compound 19n through pkCSM server (https://biosig.lab.uq.edu.au/pkcsm/). The predicted results were shown in Table 3. Absorption: The predicted results showed that compound 19n had moderate Caco-2(human colorectal adenocarcinoma cells) permeability (log Papp in 10−6 cm/s = 0.709), moderate water solubility (log mol/L = −4.356) and good intestinal absorption (%Absorbed >90%). P-glycoprotein (P-gp) is a transmembrane transport protein, it can reduce the concentration of drugs in cells and related to the formation of tumor drug resistance [32], and the results showed that compound 19n was an inhibitor of P-gp. Distribution: The logBB value and VDss value (log L/kg) were −1.225 and 0.523, respectively. The results meant that compound 19n was poorly distributed in the brain and had good distribution. Metabolism: CYP2D6 is involved in the metabolism of multiple drugs. And the metabolic prediction of compound 19n showed that compound 19n was neither substrate nor inhibitor of CYP2D6. Excretion: OCT2 (Renal organic cation transporter 2) plays a crucial role in the disposal of drugs and endogenous compounds, as well as in renal clearance, the prediction results showed that compound 19n was Renal OCT2 substrate.
Molecular docking
The quinazoline framework has been successfully used in the development of EGFR inhibitor. Therefore, in order to explore the potential target of the target compounds and discuss the pharmacophore of target compounds and the interaction leading to its antiproliferative activity, we conducted docking studies on compound 19n with EGFR kinase (PDB code: 3UG2) though MOE 2020. As shown in Fig. 6A and B, it can be seen that compound 19n could effectively bind to the catalytic pocket of EGFR and interacted with multiple amino acid residues. The N atom on the piperidine ring could form ionic and hydrogen bonding interactions with Asp855 and Asn842, respectively. The benzyl sulfide group: the S atom could interact with residues Cys797 and Asp800 through a hydrogen bond, respectively; the benzene ring can form an H-π conjugated interaction with residue Gly796. In addition, the oxygen atom on the amide bond could form a hydrogen bond interaction with Met793. The above results indicated that the target compound may exert anti proliferative activity by inhibiting EGFR activity, but the specific mechanism need to be further exploration in our future study.
Conclusions
In summary, we have designed, synthesized, and identified a novel series of 2,4,6-trisubstituted quinazoline derivatives. In vitro anti-proliferation experiments showed that most of the target compounds exhibited moderate to good anti-proliferation activity against the tested tumor cell lines, compound 19n exhibited the best activity against MGC-803 cells, with an IC50 value of 4.61 μM. Further biological experiments showed that compound 19n could inhibit the cloning of MGC-803 cells. DAPI staining and propidium iodide (PI) and Annexin V-FITC double staining experiments revealed that compound 19n could induce apoptosis of MGC-803 cells in a concentration-dependent manner. In addition, cell cycle experiments indicated that compound 19n could also arrest the cell cycle of MGC-803 cells in G0/G1 phase. The ADME prediction results showed that compound 19n had good druggability. Molecular docking experiment indicated that compound 19n may exert its antiproliferative activity by inhibiting EGFR kinase activity. Taken together, these results suggested that compound 19n might act as a promising lead compound to provide new ideas for the research and development of anti-tumor drugs.
Materials and methods
Materials
RPMI-1640 and MTT were purchased from Solarbio. Fetal Bovine Serum (FBS) was obtained from Biological Industries. Annexin V-FITC/PI Apoptosis Detection Kit and Cell Cycle Detection Kit were purchased from Keygen Biotech. All the other reagents used were of analytical grade.
MTT assay
The MTT experiment was conducted according to the methods in the literature [33]. After digestion and centrifugation, the tumor cells were inoculated into 96 well plate with 1500 to 3300 cells per well. After 24 h, the old culture medium was discarded, and 200 μL fresh medicinal culture media with different concentrations were added. After 72 h, under dark conditions, 5% MTT solution was added with 20 μL per well and incubated in constant temperature incubator at 37 °C with 5% CO2. After 4 h, remove the old culture medium and add 150 μL DMSO to each well. Finally, the absorbance value at 490 nm wavelength was tested through an enzyme-linked immunosorbent assay (ELISA), and the IC50 value was calculated using Graph Pad Prism 8.0.1. Three composite pores were set for each concentration.
Colony formation assay
Colony formation assay was conducted according to the methods in the literature [34]. The MGC-803 cells were seeded in a 6 well plate, after 24 h of cultivation, discard the old culture medium and add the medicated culture medium containing different concentrations of compound 19n. The drug-containing culture medium was discarded when the cells grows into a visible cell community. After carefully washing twice with PBS, add 1 mL of 4% paraformaldehyde to each well for 20 min, then discard the paraformaldehyde and add 1 mL of 0.1% crystalline violet dye to each well. After the plate drying, images were photographed. The experiment was repeated three times.
Cell morphology assay and DAPI staining
DAPI staining assay was conducted according to the methods in the literature [35]. MGC-803 cells were plated in a 6 well plate. After overnight, the cells were incubated with different concentrations (0, 0.5, 1, 2 μM) of compound 19n for 48 h. Afterward, the cells were fixed with ice-cold methanol at 4 °C for 10 min and treated with the DAPI dye solution in the dark. Then, the cells were photographed with fluorescence microscope, and the pictures were processed by Photoshop and Illustartor software. The experiment was repeated three times.
Cell cycle assay
Cycle experiments was conducted according to the instructions of the kit. MGC-803 cells were inoculated into a 6 well plate, and after 24 h of cultivation, replace the old medium with a drug-containing medium containing different concentrations of compound 19n. After 24 h, the cells were digested and collected, and washed twice with pre-cooled PBS. Then, 1 mL of DNA staining solution and 10 μL membrane breaker were added and incubate the cells in dark for 30 min. Finally, the cells were detected through the flow cytometer. The experiment was repeated three times.
Cell apoptosis assay
Cell apoptosis experiment was conducted according to the instructions of the kit. According to the manufacturer’s instructions of the apoptosis detection kit. MGC-803 cells were seeded in a 6 well culture plate and treated with compound 19n for 48 h. Then cells were harvested and resuspended in Annexin V-FITC solution and propidium iodide (PI) solution, then incubated for 15 min in a dark place. After that, samples were analyzed with flow cytometry. The experiment was repeated three times.
Molecular docking
The molecular docking experiment was conducted through MOE2020. The co-crystal structure of EGFR protein(PDB code: 3UG2) was downloaded from RCSB Protein Date Bank(https://www.rcsb.org/). At first, the protein was prepared through the ‘QuickPrep’ function. The 3D structure energy of compound 19n was minimized, and the molecular list was obtained by conformational search function. Next, the binding mode of compound 19n and with EGFR protein(PDB code: 3UG2) was obtained by docking the molecular list with the catalytic pocket position of protein.
Experimental section
General procedure
Reagents and solvents were purchased from commercial sources and were used without further purification. 1H NMR and 13C NMR spectra were recorded on a Bruker 400 MHz and 101 MHz spectrometer respectively. High resolution mass spectra (HRMS) were recorded on a Waters Micromass Q-T of Micromass spectrometer by electrospray ionization (ESI). Melting points were determined by the X-5 micromelting apparatus. The purity was determined by E2695 high-performance liquid chromatography, detection wavelength: 254 nm, mobile phase: compounds 17a–17n, 19a, 19c–19h and 19k–19u: MeOH: H2O = 70:30; compounds 19b, 19i, and 19j: MeOH: H2O = 90:10.
General procedure for the synthesis of compounds 11
Compound 11 was synthesized according to the method described in the literature [36]. Compound 9 (5.00 g, 27.45 mmol) was transferred to a 100 mL round-bottomed flask, and 40 mL thionyl chloride was added, and reacted at 75 °C for 2 h. Then, the excess thionyl chloride was removed by rotary evaporation to obtain compound 10. NH4NCS (2.09 g, 27.45 mmol) were dissolved in appropriate amounts of acetone, and then the acetone solution of compound 10 was slowly dropped into the NH4NCS acetone solution, stirred at room temperature for 30 min, filtered and dried to obtain compound 11, no need for purification, can directly proceed to the next step. The total yield of two-steps: 63.4%.
General procedure for the synthesis of compounds 12a–12n
Compound 12a–12n was synthesized according to the literature [37]. Compound 11 (5.00 g, 22.60 mmol) and sodium hydroxide (1.39 g, 24.86 mmol) were dissolved in water, and different substituted benzyl chlorides (24.86 mmol) were added, compounds 12a–12n were obtained by reacting at 80 °C for 1 h. After the reaction was completed, filtered, dried to obtain compounds 11a–11s, no need for purification, can directly proceed to the next step, yield: 80.1–89.2%.
General procedure for the synthesis of compounds 13a–13n
Compound 13a–13n was synthesized according to the method described in the literature [38]. Taking the synthesis of compound 13a as an example. Compound 12a (2.00 g, 6.39 mmol) was dissolved in 20 mL SOCl2, then adding 2 drops of N,N-dimethylformamide. Stirred on at 65 °C for 2 h., after the reaction was completed, the reaction solution was slowly dropped into ice water and filtered out the resulting precipitate to obtain compound 13a, no need for purification, can directly proceed to the next step. The synthesis method of compound 13b–13n was consistent with the preparation of compound 13a, yield: 85.2–90.7%.
General procedure for the synthesis of compounds 14a–14n
Compound 14a–14n was synthesized according to the method described in the literature [39]. Taking the synthesis of compound 14a as an example, compound 13a (1.00 g, 3.01 mmol), N-Boc-4-aminopiperidine (0.61 g, 3.32 mmol), and anhydrous potassium carbonate (0.46 g, 3.32 mmol) were transferred to a 25 mL round bottom flask. 10 mL of N, N-dimethylformamide was added as the solvent and reacted at 80 °C for 1 h. After the reaction was completed, the reaction system was extracted three times with ethyl acetate, concentrated, and purified by column chromatography to obtain yellow solid compound 14a. The synthesis method of compound 14b–14n was consistent with the preparation of compound 14a, yield: 78.2–87.5%.
General procedure for the synthesis of compounds 15a–15n
Compound 15a–15n was synthesized according to the method described in the literature [40]. Taking the synthesis of compound 15a as an example, compound 14a (1.00 g, 2.09 mmol), iron powder (0.82 g, 14.60 mmol), and ammonium chloride (0.33 g, 6.26 mmol) were weighed and transferred to a 50 mL circular bottom flask. 20 mL of a mixed solution of ethanol and water (VEtOH: VH2O = 4:1) was added, and the reaction was refluxed at 85 °C for 4 h. After the reaction was completed, the iron powder residue was removed by suction filtration, the crude product was extracted using ethyl acetate as the extraction solution, concentrated, and purified by column chromatography to obtain compound 15a, the preparation method of compound 15b–15n was consistent with that of compound 15a, with a yield of 75.3–86.2%.
General procedure for the synthesis of compounds 16a–16n
Compound 16a–16n was synthesized according to the method described in the literature [41]. At first, benzoic acid (0.16 g, 1.29 mmol), HATU (0.49 g, 1.29 mmol), compound 15a (0.50 g, 1.07 mmol) and diisopropylethylamine (281 μL, 1.61 mmol) were transferred to a 25 mL reaction flask and dissolved in 10 mL of N,N-dimethylformamide, stirred at room temperature for 6 h. Then, the reaction solution was extracted with ethyl acetate, and purified with column chromatography to obtain compound 16a. The preparation method of compound 16b–16n was consistent with that of compound 16a, with a yield of 75.3–88.2%.
General procedure for the synthesis of compounds 17a
Compound 17a was synthesized according to the method described in the literature [42]. Compound 16a (0.50 g, 0.90 mmol) was dissolved in 5 mL dichloromethane, and then trifluoroacetic acid (1 mL) was slowly added and reacted at room temperature for 1 h. After the reaction was completed, the reaction solution was extracted with ethyl acetate, after adjusting the pH, the crude product was purified using column chromatography to give the 17a. White solid, yield: 90.2%, mp: 131.4–132.0 °C, purity: 95.05%. 1H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H, -NH-C = O), 8.64–8.44 (m, 1H, Ar-NH-), 8.10 (d, J = 7.6 Hz, 1H, Ar-H), 8.04 (d, J = 7.4 Hz, 2H, Ar-H), 7.86 (dd, J = 8.9, 2.2 Hz, 1H, Ar-H), 7.65–7.60 (m, 1H, Ar-H), 7.57 (d, J = 7.9 Hz, 3H, Ar-H), 7.47 (d, J = 7.5 Hz, 2H, Ar-H), 7.31 (t, J = 7.4 Hz, 2H, Ar-H), 7.23 (t, J = 7.3 Hz, 1H, Ar-H), 4.43 (s, 2H, -S-CH2-), 4.24 (m, 1H, Piperidine-H), 3.06 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.63 (t, J = 12.1 Hz, 2H, Piperidine-H), 1.86 (d, J = 11.9 Hz, 2H, Piperidine-H), 1.68–1.53 (m, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.34(-NH-C = O), 164.89(Ar-C), 157.78, 146.94, 138.84, 134.74, 134.35, 131.73, 128.70, 128.47, 128.25, 127.54, 126.72, 126.32, 114.97, 112.77, 47.62(-S-CH2-), 44.36(Piperidine-C), 33.99(Piperidine-C), 30.76(Piperidine-C). HRMS (ESI) calcd for C27H27N5OS [M + H]+ : 470.2015, found: 470.2020.
N-(2-((2-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17b)
White solid, yield: 89.3%, mp: 132.3–132.9 °C, purity: 95.12%. 1H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H, -NH-C = O), 8.57 (s, 1H, Ar-H), 8.14 (d, J = 7.6 Hz, 1H, Ar-H), 8.05 (d, J = 7.4 Hz, 2H, Ar-H), 7.90 (d, J = 8.8 Hz, 1H, Ar-H), 7.65–7.53 (m, 4H, Ar-H), 7.26 (d, J = 12.5 Hz, 2H, Ar-H), 7.20 (d, J = 7.3 Hz, 1H, Ar-H), 7.04 (d, J = 7.6 Hz, 1H, Ar-H), 4.43 (d, J = 19.2 Hz, 2H, -S-CH2-), 4.28 (m, 1H, Piperidine-H), 3.19 (s, 1H, Piperidine-H), 3.12 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.71 (t, J = 12.5 Hz, 2H, Piperidine-H), 1.92 (d, J = 12.9 Hz, 2H, Piperidine-H), 1.67 (t, J = 12.2 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.27(-NH-C = O), 164.60(Ar-C), 163.18(d, J = 244.1 Hz), 157.75, 146.94, 142.22(d, J = 7.5 Hz), 140.97, 134.78, 134.40, 131.68, 130.11(d, J = 8.6 Hz), 128.44, 127.55, 126.26, 124.74, 115.23(d, J = 20.6 Hz), 115.08, 113.55(d, J = 21.2 Hz), 112.85, 48.67(-S-CH2-), 45.36(Piperidine-C), 33.41(Piperidine-C), 32.38(Piperidine-C). HRMS (ESI) calcd for C27H26FN5OS [M + H]+: 488.1920, found: 488.1907.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17c)
White solid, yield: 85.3%, mp: 147.6–148.3 °C, purity: 94.10%. 1H NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, -NH-C = O), 8.57 (s, 1H, Ar-H), 8.15 (d, J = 7.6 Hz, 1H, Ar-H), 8.05 (d, J = 7.5 Hz, 2H, Ar-H), 7.89 (dd, J = 8.8, 2.2 Hz, 1H, Ar-H), 7.62 (d, J = 7.5 Hz, 2H, Ar-H), 7.58 (d, J = 7.1 Hz, 3H, Ar-H), 7.30 (q, J = 7.0, 6.6 Hz, 1H, Ar-H), 7.20 (t, J = 9.2 Hz, 1H, Ar-H), 7.14 (t, J = 7.5 Hz, 1H, Ar-H), 4.46 (s, 2H, -S-CH2-), 4.23 (m, 1H, Piperidine-H), 3.04 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.59 (t, J = 12.1 Hz, 2H, Piperidine-H), 1.88–1.79 (m, 2H, Piperidine-H), 1.57 (qd, J = 12.1, 4.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.26(-NH-C = O), 164.40(Ar-C), 161.60(d, J = 246.0 Hz), 157.84, 146.89, 134.87, 134.36, 131.71, 131.02(d, J = 3.9 Hz), 128.97(d, J = 8.4 Hz), 128.50, 128.45, 127.57, 126.32, 125.71(d, J = 21.2 Hz), 124.24, 115.23(d, J = 21.7 Hz), 115.05, 112.83, 47.48(-S-CH2-), 44.12(Piperidine-C), 30.52(Piperidine-C), 27.43(Piperidine-C). HRMS (ESI) calcd for C27H26FN5OS [M + H]+: 488.1920, found: 488.1906.
N-(2-((4-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17d)
White solid, yield: 83.2%, mp: 144.6–145.4 °C, purity: 95.10%. 1H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H, -NH-C = O), 8.58 (d, J = 8.4 Hz, 1H, Ar-H), 8.23–8.11 (m, 1H, Ar-H), 8.05 (d, J = 7.4 Hz, 2H, Ar-H), 7.90 (d, J = 8.7 Hz, 1H, Ar-H), 7.61 (d, J = 8.0 Hz, 2H, Ar-H), 7.53 (dd, J = 28.0, 7.2 Hz, 4H, Ar-H), 7.12 (t, J = 8.6 Hz, 2H, Ar-H), 4.43 (s, 2H, -S-CH2-), 4.24 (m, 1H, Piperidine-H), 3.09 (d, J = 12.2 Hz, 2H, Piperidine-H), 1.89 (d, J = 12.6 Hz, 2H, Piperidine-H), 1.62 (q, J = 12.2 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.30(-NH-C = O), 164.74(Ar-C), 162.26 (d, J = 243.5 Hz), 157.80, 146.90, 135.22 (d, J = 3.03 Hz), 135.19, 134.84, 134.37, 131.70, 130.61 (d, J = 8.08 Hz), 128.44, 127.55, 126.31, 115.03 (d, J = 21.2 Hz), 114.96, 112.83, 47.80(-S-CH2-), 44.53(Piperidine-C), 33.14(Piperidine-C), 31.08(Piperidine-C). HRMS (ESI) calcd for C27H26FN5OS [M + H]+: 488.1920, found: 488.1920.
N-(2-((2-chlorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17e)
White solid, yield: 87.3%, mp: 166.1–167.0 °C, purity: 95.19%. 1H NMR (400 MHz, DMSO-d6) δ 10.52 (s, 1H, -NH-C = O), 8.57 (s, 1H, Ar-H), 8.15 (d, J = 7.6 Hz, 1H, Ar-H), 8.05 (d, J = 7.5 Hz, 2H, Ar-H), 7.92 (d, J = 9.0 Hz, 1H, Ar-H), 7.68 (dd, J = 6.4, 3.3 Hz, 1H, Ar-H), 7.60 (d, J = 8.1 Hz, 2H, Ar-H), 7.54 (t, J = 7.5 Hz, 2H, Ar-H), 7.47 (dd, J = 6.2, 3.3 Hz, 1H, Ar-H), 7.29 (dt, J = 6.0, 3.0 Hz, 2H, Ar-H), 4.52 (s, 2H, -S-CH2-), 4.23–4.07 (m, 1H, Piperidine-H), 3.00 (d, J = 12.1 Hz, 2H, Piperidine-H), 2.56 (d, J = 12.1 Hz, 2H, Piperidine-H), 1.80 (d, J = 12.1 Hz, 2H, Piperidine-H), 1.54 (q, J = 12.1 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.25(-NH-C = O), 164.41(Ar-C), 157.77, 146.85, 136.13, 134.88, 134.37, 133.16, 131.67, 130.90, 129.23, 128.72, 128.42, 128.31, 127.57, 127.11, 126.25, 115.06, 112.87, 48.38(-S-CH2-), 45.05(Piperidine-C), 31.91(Piperidine-C), 22.55(Piperidine-C). HRMS (ESI) calcd for C27H26ClN5OS [M + H]+: 504.1625, found: 504.1611.
N-(2-((3-chlorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17f)
White solid, yield: 86.1%, mp: 117.2–117.9 °C, purity: 95.15%. 1H NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, -NH-C = O), 8.56 (d, J = 2.2 Hz, 1H, Ar-H), 8.15 (d, J = 7.7 Hz, 1H, Ar-H), 8.08–7.98 (m, 2H, Ar-H), 7.88 (dd, J = 8.9, 2.2 Hz, 1H, Ar-H), 7.65–7.60 (m, 1H, Ar-H), 7.60–7.54 (m, 4H, Ar-H), 7.45 (d, J = 7.4 Hz, 1H, Ar-H), 7.32 (dt, J = 16.2, 8.2 Hz, 2H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.27–4.15 (m, 1H, Piperidine-H), 3.02 (d, J = 11.9 Hz, 2H, Piperidine-H), 2.61–2.52 (m, 2H, Piperidine-H), 1.86–1.79 (m, 2H, Piperidine-H), 1.55 (qd, J = 12.1, 4.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.26(-NH-C = O), 164.49(Ar-C), 157.82, 146.90, 141.89, 134.86, 134.36, 132.72, 131.70, 130.04, 128.48, 128.44, 127.57, 127.39, 126.62, 126.26, 115.05, 112.83, 47.86(-S-CH2-), 44.55(Piperidine-C), 33.29(Piperidine-C), 31.16(Piperidine-C). HRMS (ESI) calcd for C27H26ClN5OS [M + H]+: 504.1625, found: 504.1623.
N-(2-((3-bromobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17g)
White solid, yield: 82.0%, mp: 129.1–129.5 °C, purity: 96.62%. 1H NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, -NH-C = O), 8.56 (d, J = 2.2 Hz, 1H, Ar-H), 8.15 (d, J = 7.7 Hz, 1H, Ar-H), 8.05 (d, J = 7.4 Hz, 2H, Ar-H), 7.89 (dd, J = 9.0, 2.1 Hz, 1H, Ar-H), 7.70 (s, 1H, Ar-H), 7.65–7.60 (m, 1H, Ar-H), 7.58 (d, J = 8.1 Hz, 3H, Ar-H), 7.49 (d, J = 7.7 Hz, 1H, Ar-H), 7.45–7.39 (m, 1H, Ar-H), 7.27 (t, J = 7.8 Hz, 1H, Ar-H), 4.43 (s, 2H, -S-CH2-), 4.20 (ddp, J = 11.6, 7.9, 3.9 Hz, 1H, Piperidine-H), 3.00 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.60–2.51 (m, 2H, Piperidine-H), 1.89–1.79 (m, 2H, Piperidine-H), 1.54 (qd, J = 12.1, 4.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.26(-NH-C = O), 164.49(Ar-C), 157.79, 146.90, 142.21, 134.85, 134.37, 131.70, 131.38, 130.33, 129.50, 128.44, 127.77, 127.57, 126.24, 121.36, 115.06, 112.85, 48.25(-S-CH2-), 44.93(Piperidine-C), 33.25(Piperidine-C), 31.70(Piperidine-C). HRMS (ESI) calcd for C27H26BrN5OS [M + H]+: 548.1120, found: 548.1108.
N-(2-((2-methylbenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17h)
White solid, yield: 81.9%, mp: 150.2–150.8 °C, purity: 95.21%. 1H NMR (400 MHz, DMSO-d6) δ 10.66 (s, 1H, -NH-C = O), 8.65 (s, 1H, Ar-H), 8.23 (d, J = 7.6 Hz, 1H, Ar-H), 8.08 (d, J = 7.5 Hz, 2H, Ar-H), 8.00–7.93 (m, 1H, Ar-H), 7.65–7.59 (m, 1H, Ar-H), 7.55 (t, J = 8.0 Hz, 3H, Ar-H), 7.49–7.41 (m, 1H, Ar-H), 7.19 (d, J = 7.2 Hz, 1H, Ar-H), 7.16–7.09 (m, 2H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.25–4.13 (m, 1H, Piperidine-H), 2.98 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.52 (d, J = 10.7 Hz, 2H, Piperidine-H), 2.40 (s, 3H, Piperidine-H), 1.86–1.76 (m, 2H, Piperidine-H), 1.55 (td, J = 11.9, 4.1 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.24(-NH-C = O), 164.97(Ar-C), 157.72, 146.88, 136.31, 136.12, 134.88, 134.41, 131.67, 130.07, 129.46, 128.41, 128.14, 127.59, 127.02, 126.18, 125.82, 115.07, 112.91, 48.78(-S-CH2-), 45.44(Piperidine-C), 32.45(Piperidine-C), 32.28(Piperidine-C), 18.94(Piperidine-C). HRMS (ESI) calcd for C28H29N5OS [M + H]+: 484.2171, found: 484.2173.
N-(2-((3-methylbenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17i)
White solid, yield: 84.6%, mp: 125.2–125.8 °C, purity: 95.60%. 1H NMR (400 MHz, DMSO-d6) δ 10.50 (s, 1H, -NH-C = O), 8.60–8.52 (m, 1H, Ar-H), 8.14 (d, J = 7.7 Hz, 1H, Ar-H), 8.04 (d, J = 7.5 Hz, 2H, Ar-H), 7.88 (dd, J = 8.8, 2.1 Hz, 1H, Ar-H), 7.66–7.60 (m, 1H, Ar-H), 7.56 (dd, J = 8.6, 5.5 Hz, 3H, Ar-H), 7.26 (d, J = 13.4 Hz, 2H, Ar-H), 7.18 (t, J = 7.5 Hz, 1H, Ar-H), 7.04 (d, J = 7.5 Hz, 1H, Ar-H), 4.39 (s, 2H, -S-CH2-), 4.30–4.17 (m, 1H, Piperidine-H), 3.01 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.56 (t, J = 12.0 Hz, 2H, Piperidine-H), 2.27 (s, 3H, Piperidine-H), 1.90–1.79 (m, 2H, Piperidine-H), 1.57 (td, J = 11.9, 4.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.25(-NH-C = O), 164.95(Ar-C), 157.73, 146.97, 138.72, 137.30, 134.75, 134.39, 131.68, 129.33, 128.44, 128.34, 128.13, 127.56, 127.36, 126.24, 125.82, 115.05, 112.83, 48.47(-S-CH2-), 45.14(Piperidine-C), 33.98(Piperidine-C), 32.00(Piperidine-C), 20.95(Piperidine-C). HRMS (ESI) calcd for C28H29N5OS [M + H]+: 484.2171, found: 484.2156.
N-(4-(piperidin-4-ylamino)-2-((2-(trifluoromethyl)benzyl)thio)quinazolin-6-yl)benz-amide(17j)
White solid, yield: 85.2%, mp: 181.9–182.5 °C, purity: 96.17%. 1H NMR (400 MHz, DMSO-d6) δ 10.55 (s, 1H, -NH-C = O), 8.59 (d, J = 2.2 Hz, 1H, Ar-H), 8.22 (d, J = 7.6 Hz, 1H, Ar-H), 8.05 (d, J = 7.6 Hz, 2H, Ar-H), 7.91 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.83 (d, J = 7.8 Hz, 1H, Ar-H), 7.75 (d, J = 7.8 Hz, 1H, Ar-H), 7.63 (dd, J = 10.0, 6.9 Hz, 2H, Ar-H), 7.60–7.53 (m, 3H, Ar-H), 7.48 (t, J = 7.7 Hz, 1H, Ar-H), 4.64 (s, 2H, -S-CH2-), 4.17 (m, 1H, Piperidine-H), 3.00 (d, J = 12.1 Hz, 2H, Piperidine-H), 1.86–1.76 (m, 2H, Piperidine-H), 1.55 (qd, J = 12.1, 4.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.25(-NH-C = O), 164.28(Ar-C), 157.84, 146.84, 136.96, 134.96, 134.37, 132.70, 131.69, 131.32, 128.43, 128.37, 127.51, 127.08(d, J = 29.6 Hz,), 126.27, 125.88(d, J = 281.3 Hz), 125.83, 115.01, 112.90, 48.33(-S-CH2-), 44.93(Piperidine-C), 31.75(Piperidine-C), 30.56(Piperidine-C). HRMS (ESI) calcd for C28H26F3N5OS [M + H]+: 538.1888, found: 538.1883.
N-(4-(piperidin-4-ylamino)-2-((4-(trifluoromethyl)benzyl)thio)quinazolin-6-yl)benz-amide(17k)
White solid, yield: 85.3%, mp: 112.8–113.4 °C, purity: 96.18%. 1H NMR (400 MHz, DMSO-d6) δ 10.47 (s, 1H, -NH-C = O), 8.56 (s, 1H, Ar-H), 8.12 (d, J = 7.8 Hz, 1H, Ar-H), 8.05 (d, J = 7.4 Hz, 2H, Ar-H), 7.89 (d, J = 9.3 Hz, 2H, Ar-H), 7.80 (d, J = 7.5 Hz, 1H, Ar-H), 7.59 (dt, J = 13.9, 9.1 Hz, 6H, Ar-H), 4.53 (s, 2H, -S-CH2-), 4.27–4.10 (m, 1H, Piperidine-H), 3.20 (s, 2H, Piperidine-H), 2.98 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.51 (d, J = 10.3 Hz, 2H, Piperidine-H), 1.81 (d, J = 12.0 Hz, 2H, Piperidine-H), 1.53 (qd, J = 12.1, 4.1 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.35(-NH-C = O), 164.47(Ar-C), 157.77, 146.88, 140.95, 134.87, 134.38, 132.87, 131.73, 129.20, 129.04(d, J = 31.6 Hz), 128.44, 128.40, 127.54, 126.20, 125.52(d, J = 273.3 Hz), 125.24(q, J = 3.74 Hz), 123.33, 114.99, 112.85, 48.68(-S-CH2-), 45.28(Piperidine-C), 33.33(Piperidine-C), 32.27(Piperidine-C). HRMS (ESI) calcd for C28H26F3N5OS [M + H]+: 538.1888, found: 538.1876.
N-(2-((2-cyanobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzamide(17l)
White solid, yield: 82.5%, mp: 208.6–209.1 °C, purity: 96.05%. 1H NMR (400 MHz, DMSO-d6) δ 10.58 (s, 1H, -NH-C = O), 8.61 (d, J = 2.2 Hz, 1H, Ar-H), 8.18 (d, J = 7.7 Hz, 1H, Ar-H), 8.07 (d, J = 7.4 Hz, 2H, Ar-H), 7.95 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.81 (dd, J = 16.0, 7.8 Hz, 2H, Ar-H), 7.67–7.59 (m, 3H, Ar-H), 7.56 (t, J = 7.3 Hz, 2H, Ar-H), 7.42 (t, J = 7.6 Hz, 1H, Ar-H), 4.59 (s, 2H, -S-CH2-), 4.16 (m, 1H, Piperidine-H), 2.97 (dt, J = 12.5, 3.3 Hz, 2H, Piperidine-H), 2.59–2.50 (m, 2H, Piperidine-H), 1.80–1.74 (m, 2H, Piperidine-H), 1.52 (qd, J = 12.1, 4.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.24(-NH-C = O), 163.95(Ar-C), 157.79, 146.77, 142.67, 135.04, 134.38, 133.08, 132.77, 131.67, 129.96, 128.42, 128.24, 127.65, 127.59, 126.23, 117.70, 115.05, 112.97, 111.81, 48.67(-S-CH2-), 45.39(Piperidine-C), 32.57(Piperidine-C), 32.43(Piperidine-C). HRMS (ESI) calcd for C28H27N6OS [M + H]+: 495.1967, found: 495.1964.
N-(2-((2,4-dichlorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(17m)
White solid, yield: 83.6%, mp: 167.1–167.5 °C, purity: 95.19%. 1H NMR (400 MHz, DMSO-d6) δ 10.44 (s, 1H, -NH-C = O), 8.52 (d, J = 2.2 Hz, 1H, Ar-H), 8.08 (d, J = 7.7 Hz, 1H, Ar-H), 8.05–8.01 (m, 2H, Ar-H), 7.86 (dd, J = 8.9, 2.2 Hz, 1H, Ar-H), 7.69 (d, J = 8.4 Hz, 1H, Ar-H), 7.63 (d, J = 2.3 Hz, 1H, Ar-H), 7.61 (d, J = 7.9 Hz, 2H, Ar-H), 7.58–7.54 (m, 2H, Ar-H), 7.37 (dd, J = 8.3, 2.2 Hz, 1H, Ar-H), 4.48 (s, 2H, -S-CH2-), 4.20–4.05 (m, 1H, Piperidine-H), 2.95 (dd, J = 9.6, 6.4 Hz, 2H, Piperidine-H), 2.50–2.44 (m, 2H, Piperidine-H), 1.75 (dd, J = 12.4, 3.7 Hz, 2H, Piperidine-H), 1.49 (qd, J = 12.0, 4.1 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.28(-NH-C = O), 164.18(Ar-C), 157.76, 146.86, 135.45, 134.84, 134.37, 134.05, 132.24, 132.07, 131.71, 128.63, 128.45, 128.42, 127.54, 127.20, 126.30, 115.03, 112.86, 48.67(-S-CH2-), 45.32(Piperidine-C), 32.32(Piperidine-C), 31.47(Piperidine-C). HRMS (ESI) calcd for C27H25Cl2N5OS [M + H]+: 538.1235, found: 538.1224.
N-(2-((3,4-dichlorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benzami-de(17n)
White solid, yield: 79.8%, mp: 125.6–126.3 °C, purity: 95.51%. 1H NMR (400 MHz, DMSO-d6) δ 10.48 (s, 1H, -NH-C = O), 8.55 (s, 1H, Ar-H), 8.14 (d, J = 8.0 Hz, 1H, Ar-H), 8.04 (d, J = 7.7 Hz, 2H, Ar-H), 7.87 (d, J = 9.2 Hz, 1H, Ar-H), 7.75 (s, 1H, Ar-H), 7.68–7.61 (m, 2H, Ar-H), 7.57 (dd, J = 8.3, 5.6 Hz, 3H, Ar-H), 7.48 (d, J = 8.6 Hz, 1H, Ar-H), 4.43 (d, J = 10.2 Hz, 2H, -S-CH2-), 4.24–4.05 (m, 1H, Piperidine-H), 2.99 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.55 (s, 2H, Piperidine-H), 1.79 (d, J = 12.3 Hz, 2H, Piperidine-H), 1.54 (td, J = 12.1, 4.4 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.26(-NH-C = O), 164.33(Ar-C), 157.78, 146.87, 140.75, 134.85, 134.37, 131.69, 130.60, 130.52, 130.28, 129.17, 129.01, 128.44, 127.55, 126.24, 115.05, 112.86, 48.58(-S-CH2-), 45.26(Piperidine-C), 32.72(Piperidine-C), 32.23(Piperidine-C). HRMS (ESI) calcd for C27H25Cl2N5OS [M + H]+: 538.1235, found: 538.1237.
General procedure for the synthesis of compounds 18a–18u
The synthesis methods of compounds 18a–18u were consistent with the preparation of compounds 16a–16n.
General procedure for the synthesis of compounds 19a–19u
The synthesis methods of compounds 19a–19u were consistent with the preparation of compounds 17a–17n.
2-fluoro-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19a)
White solid, yield: 90.1%, mp: 182.3–182.9 °C, purity: 95.56%. 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H, -NH-C = O), 8.54 (d, J = 2.2 Hz, 1H, Ar-H), 8.14 (d, J = 7.7 Hz, 1H, Ar-H), 7.81 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.73 (td, J = 7.5, 1.7 Hz, 1H, Ar-H), 7.59 (dd, J = 16.5, 8.2 Hz, 2H, Ar-H), 7.42–7.36 (m, 2H, Ar-H), 7.35–7.27 (m, 3H, Ar-H), 7.11–7.00 (m, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.26–4.12 (m, 1H, Piperidine-H), 3.02–2.94 (m, 2H, Piperidine-H), 2.54 (d, J = 2.2 Hz, 2H, Piperidine-H), 1.79 (d, J = 10.0 Hz, 2H, Piperidine-H), 1.52 (qd, J = 12.0, 2.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.63(-NH-C = O), 163.17(d, J = 244.1 Hz, Ar-C), 162.64(d, J = 244.3 Hz), 160.22, 157.74, 146.97, 142.17(d, J = 7.5 Hz), 134.46, 132.71(d, J = 8.2 Hz), 130.12(d, J = 8.7 Hz), 129.90, 127.57, 126.47, 124.74, 124.59, 124.44, 116.32(d, J = 22.5 Hz), 115.42(d, J = 22.1 Hz), 114.15, 113.56(d, J = 20.2 Hz), 112.88, 48.66(-S-CH2-), 45.38(Piperidine-C), 33.38(Piperidine-C), 32.36(Piperidine-C). HRMS (ESI) calcd for C27H25F2N5OS [M + H]+: 506.1826, found: 506.1814.
3-fluoro-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19b)
White solid, yield: 90.2%, mp: 201.3–201.9 °C, purity: 98.05%. 1H NMR (400 MHz, DMSO-d6) δ 10.55 (s, 1H, -NH-C = O), 8.62 – 8.50 (m, 1H, Ar-H), 8.13 (d, J = 7.7 Hz, 1H, Ar-H), 7.98–7.81 (m, 3H, Ar-H), 7.69–7.55 (m, 2H, Ar-H), 7.48 (td, J = 8.5, 2.7 Hz, 1H, Ar-H), 7.32 (td, J = 10.9, 9.2, 5.4 Hz, 3H, Ar-H), 7.12–7.00 (m, 1H, Ar-H), 4.45 (s, 2H, -S-CH2-), 4.27–4.14 (m, 1H, Piperidine-H), 3.00 (d, J = 12.1 Hz, 2H, Piperidine-H), 2.56 (d, J = 11.3 Hz, 2H, Piperidine-H), 1.87–1.76 (m, 2H, Piperidine-H), 1.55 (qd, J = 11.2, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.73(-NH-C = O), 163.87(d, J = 244.4 Hz, Ar-C), 163.85(d, J = 244.6 Hz), 163.17, 157.74, 147.04, 142.19(d, J = 7.5 Hz), 136.73(d, J = 6.8 Hz), 134.46, 130.71(d, J = 8.4 Hz), 130.12(d, J = 8.0 Hz), 128.42, 126.33, 124.75, 123.78, 123.76, 118.73(d, J = 23.0 Hz), 115.44, 115.23, 114.47(d, J = 23.1 Hz), 113.56(d, J = 21.1 Hz), 48.55(-S-CH2-), 45.23(Piperidine-C), 33.38(Piperidine-C), 32.18(Piperidine-C). HRMS (ESI) calcd for C27H25F2N5OS [M + H]+ : 506.1826, found: 506.1817.
4-fluoro-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benz-amide(19c)
White solid, yield: 91.2%, mp: 116.0–116.5 °C, purity: 95.89%. 1H NMR (400 MHz, DMSO-d6) δ 10.54 (s, 1H, -NH-C = O), 8.55 (d, J = 2.2 Hz, 1H, Ar-H), 8.13 (dd, J = 8.5, 5.7 Hz, 3H, Ar-H), 7.88 (dd, J = 8.9, 2.2 Hz, 1H, Ar-H), 7.57 (d, J = 8.9 Hz, 1H, Ar-H), 7.40 (t, J = 8.7 Hz, 2H, Ar-H), 7.32 (td, J = 11.2, 9.5, 4.2 Hz, 3H, Ar-H), 7.05 (td, J = 8.0, 7.0, 2.7 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.24–4.12 (m, 1H, Piperidine-H), 2.97 (dd, J = 12.6, 3.3 Hz, 2H, Piperidine-H), 2.53 (s, 2H, Piperidine-H), 1.84–1.73 (m, 2H, Piperidine-H), 1.51 (qd, J = 11.9, 2.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.37(d, J = 250.5 Hz), 164.63(-NH-C = O), 164.15(Ar-C), 163.17(d, J = 244.2 Hz), 157.74, 146.94, 142.21(d, J = 7.47 Hz), 134.69, 130.82(d, J = 2.7 Hz), 130.34(d, J = 9.1 Hz), 130.11(d, J = 7.9 Hz), 128.42, 126.26, 124.75, 115.52(d, J = 22.0 Hz), 115.44(d, J = 21.9 Hz), 115.18, 113.55(d, J = 21.5 Hz), 112.85, 48.62(-S-CH2-), 45.36(Piperidine-C), 33.38(Piperidine-C), 32.36(Piperidine-C). HRMS (ESI) calcd for C27H25F2N5OS [M + H]+ : 506.1826, found: 506.1815.
2-chloro-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19d)
White solid, yield: 89.2 %, mp: 131.1–131.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.69 (s, 1H, -NH-C = O), 8.58 (d, J = 2.3 Hz, 1H, Ar-H), 8.19 (d, J = 7.7 Hz, 1H, Ar-H), 7.78 (dd, J = 8.8, 2.2 Hz, 1H, Ar-H), 7.59 (q, J = 9.8, 8.3 Hz, 3H, Ar-H), 7.51 (dt, J = 20.5, 6.8 Hz, 2H, Ar-H), 7.10–6.99 (m, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.18–4.28 (m, 1H, Piperidine-H), 3.06 (d, J = 12.1 Hz, 2H, Piperidine-H), 2.62 (t, J = 12.1 Hz, 2H, Piperidine-H), 1.91–1.78 (m, 2H, Piperidine-H), 1.60 (qd, J = 12.3, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.83(-NH-C = O), 164.53(Ar-C), 163.17(d, J = 244.1 Hz), 157.81, 146.93, 142.12(d, J = 7.47 Hz), 136.70, 134.65, 131.17, 130.14(d, J = 8.69 Hz), 129.95, 129.71, 128.90, 127.30, 127.26, 126.57, 124.76, 115.45(d, J = 20.5 Hz), 113.57(d, J = 20.9 Hz), 113.55, 112.92, 48.06(-S-CH2-), 44.73(Piperidine-C), 33.40(Piperidine-C), 31.35(Piperidine-C). HRMS (ESI) calcd for C27H25ClFN5OS [M + H]+: 522.1531, found: 522.1511.
3-chloro-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19e)
White solid, yield: 89.7%, mp: 191.2–191.8 °C, purity: 97.68%. 1H NMR (400 MHz, DMSO-d6) δ 10.60 (s, 1H, -NH-C = O), 8.54 (d, J = 2.3 Hz, 1H, Ar-H), 8.19–8.06 (m, 2H, Ar-H), 8.00 (d, J = 7.7 Hz, 1H, Ar-H), 7.89 (dd, J = 8.9, 2.2 Hz, 1H, Ar-H), 7.75–7.67 (m, 1H, Ar-H), 7.60 (dd, J = 14.3, 8.3 Hz, 2H, Ar-H), 7.33 (ddt, J = 14.7, 9.4, 5.2 Hz, 3H, Ar-H), 7.06 (td, J = 7.8, 6.9, 2.6 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.20 (s, 1H, Piperidine-H), 3.00 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.62–2.52 (m, 2H, Piperidine-H), 1.86–1.77 (m, 2H, Piperidine-H), 1.55 (qd, J = 12.0, 3.0 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.72(-NH-C = O), 163.78(Ar-C), 163.17(d, J = 244.1 Hz), 157.77, 147.01, 142.19(d, J = 7.6 Hz), 136.35, 134.54, 133.29, 131.53, 130.47, 130.11(d, J = 8.4 Hz), 128.29, 127.33, 126.38, 126.31, 124.75, 115.43(d, J = 19.6 Hz), 115.13, 113.55(d, J = 21.1 Hz), 112.85, 48.35(-S-CH2-), 45.09(Piperidine-C), 33.40(Piperidine-C), 31.97(Piperidine-C). HRMS (ESI) calcd for C27H25ClFN5OS [M + H]+: 522.1531, found: 522.1514.
4-chloro-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19f)
White solid, yield: 87.2%, mp: 216.2–217.0 °C, purity: 97.86%. 1H NMR (400 MHz, DMSO-d6) δ 10.55 (s, 1H, -NH-C = O), 8.53 (d, J = 2.2 Hz, 1H, Ar-H), 8.09 (dd, J = 22.5, 7.9 Hz, 3H, Ar-H), 7.86 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.65 (d, J = 8.2 Hz, 2H, Ar-H), 7.58 (d, J = 8.9 Hz, 1H, Ar-H), 7.32 (td, J = 11.1, 9.1, 4.4 Hz, 3H, Ar-H), 7.05 (t, J = 8.3 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.27–4.10 (m, 1H, Piperidine-H), 3.02 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.63–2.52 (m, 2H, Piperidine-H), 1.89–1.75 (m, 2H, Piperidine-H), 1.55 (qd, J = 12.1, 2.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.68(-NH-C = O), 164.17(Ar-C), 163.17(d, J = 244.1 Hz), 157.76, 147.00, 142.18(d, J = 7.5 Hz), 136.57, 134.57, 133.07, 130.11(d, J = 8.08 Hz), 129.51, 128.54, 128.46, 126.30, 124.74, 115.45, 115.23, 113.56(d, J = 20.9 Hz), 112.83, 48.28(-S-CH2-), 44.98(Piperidine-C), 33.39(Piperidine-C), 31.81(Piperidine-C). HRMS (ESI) calcd for C27H25ClFN5OS [M + H]+: 522.1531, found: 522.1515.
2-bromo-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19g)
White solid, yield: 92.1%, mp: 151.7–152.4 °C, purity: 95.18%. 1H NMR (400 MHz, DMSO-d6) δ 10.68 (s, 1H, -NH-C = O), 8.59 (s, 1H, Ar-H), 8.26 (d, J = 7.5 Hz, 1H, Ar-H), 7.77 (dd, J = 17.0, 8.5 Hz, 2H, Ar-H), 7.61–7.56 (m, 2H, Ar-H), 7.53 (d, J = 7.4 Hz, 1H, Ar-H), 7.45 (t, J = 7.7 Hz, 1H, Ar-H), 7.37–7.29 (m, 3H, Ar-H), 7.06 (s, 1H, Ar-H), 4.45 (s, 2H, -S-CH2-), 4.28–4.10 (m, 1H, Piperidine-H), 3.18 (d, J = 12.3 Hz, 2H, Piperidine-H), 2.80 (t, J = 12.3 Hz, 2H, Piperidine-H), 1.92 (d, J = 12.8 Hz, 2H, Piperidine-H), 1.78–1.69 (m, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.74(-NH-C = O), 164.48(Ar-C), 163.16(d, J = 244.02 Hz), 157.91, 146.92, 142.06(d, J = 7.47 Hz), 138.84, 134.76, 132.80, 131.28, 130.16(d, J = 8.9 Hz), 128.83, 127.73, 127.34, 126.62, 124.78, 118.96, 115.49(d, J = 23.3 Hz), 113.60(d, J = 19.8 Hz), 113.42, 112.90, 46.93(-S-CH2-), 43.54(Piperidine-C), 33.40(Piperidine-C), 29.54(Piperidine-C). HRMS (ESI) calcd for C27H25BrFN5OS [M + H]+: 566.1025, found: 566.1011.
3-bromo-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19h)
White solid, yield: 90.4%, mp: 134.2–134.9 °C, purity: 95.27%. 1H NMR (400 MHz, DMSO-d6) δ 10.57 (s, 1H, -NH-C = O), 8.51 (d, J = 2.2 Hz, 1H, Ar-H), 8.23 (s, 1H, Ar-H), 8.11 (d, J = 7.7 Hz, 1H, Ar-H), 8.03 (d, J = 7.8 Hz, 1H, Ar-H), 7.91–7.80 (m, 2H, Ar-H), 7.59–7.51 (m, 2H, Ar-H), 7.31 (td, J = 11.4, 9.6, 4.4 Hz, 3H, Ar-H), 7.05 (td, J = 7.9, 6.9, 2.4 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.22–4.12 (m, 1H, Piperidine-H), 3.03–2.95 (m, 2H, Piperidine-H), 2.55 (s, 2H, Piperidine-H), 1.84–1.76 (m, 2H, Piperidine-H), 1.53 (qd, J = 11.9, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.74(-NH-C = O), 163.71(Ar-C), 163.17(d, J = 244.32 Hz), 157.74, 147.04, 142.19(d, J = 7.58 Hz), 136.54, 134.46, 134.42, 130.72, 130.16, 130.03(d, J = 8.38 Hz), 128.35, 126.75, 126.31, 124.74, 121.74, 115.44(d, J = 22.6 Hz), 115.16, 113.55(d, J = 20.6 Hz), 112.84, 48.57(-S-CH2-), 45.30(Piperidine-C), 33.40(Piperidine-C), 32.29(Piperidine-C). HRMS (ESI) calcd for C27H25BrFN5OS [M + H]+: 566.1025, found: 566.1012.
4-bromo-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19i)
White solid, yield: 86.4%, mp: 211.2–212.1 °C, purity: 99.61%. 1H NMR (400 MHz, DMSO-d6) δ 10.53 (s, 1H, -NH-C = O), 8.51 (d, J = 2.2 Hz, 1H, Ar-H), 8.10 (d, J = 7.7 Hz, 1H, Ar-H), 7.98 (d, J = 8.2 Hz, 2H, Ar-H), 7.85 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.79 (d, J = 8.2 Hz, 2H, Ar-H), 7.57 (d, J = 8.8 Hz, 1H, Ar-H), 7.32 (ddt, J = 14.9, 9.5, 5.1 Hz, 3H, Ar-H), 7.10–7.01 (m, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.23–4.13 (m, 1H, Piperidine-H), 3.02–2.93 (m, 2H, Piperidine-H), 2.55–2.51 (m, 2H, Piperidine-H), 1.79 (d, J = 12.3 Hz, 2H, Piperidine-H), 1.53 (qd, J = 12.0, 2.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.70(-NH-C = O), 164.29(Ar-C), 163.17(d, J = 244.22 Hz), 157.73, 147.02, 142.20(d, J = 7.47 Hz), 134.52, 133.45, 131.49, 130.11(d, J = 8.0 Hz), 129.67, 128.43, 126.31, 125.53, 124.73, 115.43, 115.23, 113.55(d, J = 20.6 Hz), 112.84, 48.67(-S-CH2-), 45.35(Piperidine-C), 33.40(Piperidine-C), 32.35(Piperidine-C). HRMS (ESI) calcd for C27H25BrFN5OS [M + H]+: 566.1025, found: 566.1011.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-2-methylbenza-mide(19j)
White solid, yield: 88.6%, mp: 139.4–140.2 °C, purity: 98.11%. 1H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H, -NH-C = O), 8.62 (s, 2H, Ar-H), 8.24 (d, J = 7.3 Hz, 1H, Ar-H), 7.78 (d, J = 8.9 Hz, 1H, Ar-H), 7.59 (d, J = 8.9 Hz, 1H, Ar-H), 7.50 (d, J = 7.4 Hz, 1H, Ar-H), 7.42 (t, J = 7.5 Hz, 1H, Ar-H), 7.33 (q, J = 8.0, 7.2 Hz, 4H, Ar-H), 7.06 (t, J = 8.6 Hz, 1H, Ar-H), 4.46 (s, 2H, -S-CH2-), 4.42–4.35 (m, 1H, Piperidine-H), 3.17 (d, J = 3.4 Hz, 2H, Piperidine-H), 3.04 (t, J = 12.5 Hz, 2H, Piperidine-H), 2.43 (s, 3H, Piperidine-H), 2.04 (d, J = 13.3 Hz, 2H, Piperidine-H), 1.83 (q, J = 12.2 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 167.73(-NH-C = O), 164.30, 163.15(d, J = 244.2 Hz, Ar-C), 157.97, 146.75, 142.04(d, J = 7.4 Hz), 136.79, 135.36, 135.13, 130.60, 130.16(d, J = 8.1 Hz), 129.76, 127.67, 127.20, 126.50, 125.65, 124.81, 115.53(d, J = 21.0 Hz), 113.60(d, J = 21.0 Hz), 113.40, 112.85, 46.13(-S-CH2-), 42.87(Piperidine-C), 33.40(Piperidine-C), 28.53(Piperidine-C), 19.36(Piperidine-C). HRMS (ESI) calcd for C28H28FN5OS [M + H]+: 502.2077, found: 502.2064.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-3-methylbenza-mide(19k)
White solid, yield: 88.6%, mp: 208.7–209.6 °C, purity: 95.01%. 1H NMR (400 MHz, DMSO-d6) δ 10.42 (s, 1H, -NH-C = O), 8.52 (d, J = 2.2 Hz, 1H, Ar-H), 8.10 (d, J = 7.7 Hz, 1H, Ar-H), 7.92–7.78 (m, 3H, Ar-H), 7.57 (d, J = 8.9 Hz, 1H, Ar-H), 7.44 (d, J = 6.2 Hz, 2H, Ar-H), 7.33 (ddt, J = 14.4, 9.2, 5.1 Hz, 3H, Ar-H), 7.11–7.00 (m, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.30–4.15 (m, 1H, Piperidine-H), 2.99 (d, J = 12.1 Hz, 2H, Piperidine-H), 2.54 (d, J = 12.1 Hz, 2H, Piperidine-H), 2.42 (s, 3H, Piperidine-H), 1.80 (d, J = 12.2 Hz, 2H, Piperidine-H), 1.54 (qd, J = 11.6, 2.8, Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.35(-NH-C = O), 164.54(Ar-C), 163.17(d, J = 244.2 Hz), 157.75, 146.88, 142.21(d, J = 7.5 Hz), 137.72, 134.86, 134.40, 132.26, 130.12(d, J = 8.8 Hz), 128.38, 128.35, 128.03, 126.24, 124.74, 115.43(d, J = 21.5 Hz), 114.99, 113.55(d, J = 21.3 Hz), 112.85, 48.48(-S-CH2-), 45.20(Piperidine-C), 33.40(Piperidine-C), 32.13(Piperidine-C), 20.98(Piperidine-C). HRMS (ESI) calcd for C28H28FN5OS [M + H]+: 502.2077, found: 502.2064.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-4-methylbenza-mide(19l)
White solid, yield: 89.3%, mp: 193.5–194.2 °C, purity: 95.52%. 1H NMR (400 MHz, DMSO-d6) δ 10.37 (s, 1H, -NH-C = O), 8.52 (d, J = 2.3 Hz, 1H, Ar-H), 8.09 (d, J = 7.7 Hz, 1H, Ar-H), 7.95 (d, J = 7.9 Hz, 2H, Ar-H), 7.86 (dd, J = 8.9, 2.2 Hz, 1H, Ar-H), 7.56 (d, J = 8.9 Hz, 1H, Ar-H), 7.36 (d, J = 7.9 Hz, 2H, Ar-H), 7.31 (q, J = 9.4, 8.3 Hz, 3H, Ar-H), 7.09–7.01 (m, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.23–4.15 (m, 1H, Piperidine-H), 2.99 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.58–2.51 (m, 2H, Piperidine-H), 2.40 (s, 3H, -CH3), 1.85–1.75 (m, 2H, Piperidine-H), 1.52 (qd, J = 11.9, 2.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.06(-NH-C = O), 164.51(Ar-C), 163.17(d, J = 244.2 Hz), 157.75, 146.85, 142.14(d, J = 7.5 Hz), 141.72, 134.88, 131.50, 130.04(d, J = 8.2 Hz), 128.97, 128.45, 127.59, 126.21, 124.75, 115.44(d, J = 20.8 Hz), 115.03, 113.55(d, J = 20.5 Hz), 112.84, 48.46(-S-CH2-), 45.19(Piperidine-C), 33.38(Piperidine-C), 32.11(Piperidine-C), 20.96(Piperidine-C). HRMS (ESI) calcd for C28H28FN5OS [M + H]+ : 502.2077, found: 502.2061.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-3-methoxybenz-amide(19m)
White solid, yield: 86.2%, mp: 144.6–145.3 °C, purity: 95.14%. 1H NMR (400 MHz, DMSO-d6) δ 10.45 (s, 1H, -NH-C = O), 8.53 (d, J = 2.2 Hz, 1H, Ar-H), 8.11 (d, J = 7.4 Hz, 1H, Ar-H), 7.87 (dd, J = 8.8, 2.2 Hz, 1H, Ar-H), 7.64–7.55 (m, 3H, Ar-H), 7.48 (t, J = 7.9 Hz, 1H, Ar-H), 7.36–7.27 (m, 3H, Ar-H), 7.18 (dd, J = 8.2, 2.6 Hz, 1H, Ar-H), 7.05 (td, J = 9.4, 2.5 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.24–4.13 (m, 1H, Piperidine-H), 3.86 (s, 3H, -CH3), 2.97 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.53 (s, 2H, Piperidine-H), 1.84–1.72 (m, 2H, Piperidine-H), 1.52 (qd, J = 11.9, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.93(-NH-C = O), 164.62(Ar-C), 163.17(d, J = 244.0 Hz), 159.21, 157.73, 146.93, 142.14(d, J = 7.47 Hz), 135.75, 134.71, 130.12(d, J = 19.7 Hz), 129.61, 128.48, 126.22, 124.75, 119.79, 117.47, 115.44, 115.19, 113.55(d, J = 20.7 Hz), 112.84, 112.75, 55.35, 48.69(-S-CH2-), 45.38(Piperidine-C), 33.41(Piperidine-C), 32.39(Piperidine-C). HRMS (ESI) calcd for C28H28FN5O2S [M + H]+: 518.2026, found: 518.2014.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-4-methoxybenz-amide(19n)
White solid, yield: 87.3%, mp: 216.0–216.7 °C, purity: 96.01%. 1H NMR (400 MHz, DMSO-d6) δ 10.31 (s, 1H, -NH-C = O), 8.51 (d, J = 2.2 Hz, 1H, Ar-H), 8.10 (d, J = 7.7 Hz, 1H, Ar-H), 8.04 (d, J = 8.4 Hz, 2H, Ar-H), 7.90–7.79 (m, 1H, Ar-H), 7.56 (d, J = 8.8 Hz, 1H, Ar-H), 7.39–7.27 (m, 3H, Ar-H), 7.14–7.02 (m, 3H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.27–4.16 (m, 1H, Piperidine-H), 3.85 (s, 3H, -CH3), 3.03 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.59 (t, J = 12.0 Hz, 2H, Piperidine-H), 1.82 (dd, J = 12.7, 3.7 Hz, 2H, Piperidine-H), 1.56 (qd, J = 12.1, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.64(-NH-C = O), 164.43(Ar-C), 163.17(d, J = 244.1 Hz), 161.97, 157.77, 146.77, 142.20(d, J = 7.5 Hz), 135.03, 130.12(d, J = 8.7 Hz), 129.51, 128.50, 126.38, 126.20, 124.76, 115.44(d, J = 21.0 Hz), 114.96, 113.67, 113.36(d, J = 20.6 Hz), 112.83, 55.37(Piperidine-C), 48.13(-S-CH2-), 44.87(Piperidine-C), 33.39(Piperidine-C), 31.63(Piperidine-C). HRMS (ESI) calcd for C28H28FN5O2S [M + H]+: 518.2026, found: 518.2017.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin)-2-(trifluoromethyl)-benzamide(19o)
White solid, yield: 86.3%, mp: 167.9–168.7 °C, purity: 95.13%. 1H NMR (400 MHz, DMSO-d6) δ 10.75 (s, 1H, -NH-C = O), 8.51 (d, J = 2.2 Hz, 1H, Ar-H), 8.18 (d, J = 7.6 Hz, 1H, Ar-H), 7.88 (d, J = 7.8 Hz, 1H, Ar-H), 7.85–7.77 (m, 2H, Ar-H), 7.74 (t, J = 7.8 Hz, 2H, Ar-H), 7.58 (d, J = 8.9 Hz, 1H, Ar-H), 7.39–7.27 (m, 3H, Ar-H), 7.06 (dt, J = 9.6, 4.6 Hz, 1H, Ar-H), 4.45 (s, 2H, -S-CH2-), 4.26–4.16 (m, 1H, Piperidine-H), 3.10–2.96 (m, 2H, Piperidine-H), 2.64–2.54 (m, 2H, Piperidine-H), 1.90–1.77 (m, 2H, Piperidine-H), 1.58 (qd, J = 12.1, 3.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 165.51(-NH-C = O), 164.58(Ar-C), 163.18(d, J = 244.3 Hz), 157.78, 147.00, 142.14(d, J = 7.6 Hz), 136.00(d, J = 2.1 Hz), 134.67, 132.59, 130.13, 130.04, 128.54, 127.28, 126.61, 126.41(q, J = 4.7 Hz), 126.03(d, J = 31.5 Hz), 125.11(d, J = 274.7 Hz), 124.75, 115.44(d, J = 22.0 Hz), 113.57(d, J = 21.1 Hz), 113.52, 112.93, 48.55(-S-CH2-), 48.33(Piperidine-C), 44.99(Piperidine-C), 33.40(Piperidine-C), 31.72(Piperidine-C). HRMS (ESI) calcd for C28H25F4N5OS [M + H]+: 556.1794, found: 556.1780.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin)-3-(trifluoromethyl)-benzamide(19p)
White solid, yield: 88.2%, mp: 125.6–125.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.71 (s, 1H, -NH-C = O), 8.51 (d, J = 2.2 Hz, 1H, Ar-H), 8.40 (s, 1H, Ar-H), 8.34 (d, J = 7.9 Hz, 1H, Ar-H), 8.13 (d, J = 7.7 Hz, 1H, Ar-H), 8.01 (d, J = 7.8 Hz, 1H, Ar-H), 7.91 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.83 (t, J = 7.8 Hz, 1H, Ar-H), 7.59 (d, J = 8.9 Hz, 1H, Ar-H), 7.32 (td, J = 11.2, 9.6, 4.2 Hz, 3H, Ar-H), 7.05 (tt, J = 7.0, 2.2 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.25–4.15 (m, 1H, Piperidine-H), 2.98 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.55 (s, 2H, Piperidine-H), 1.84–1.76 (m, 2H, Piperidine-H), 1.52 (qd, J = 12.1, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.82(-NH-C = O), 163.72(Ar-C), 163.17(d, J = 243.8 Hz), 157.73, 147.11, 142.12(d, J = 7.6 Hz), 135.21, 134.37, 131.75, 130.03(d, J = 8.4 Hz), 129.83, 129.40, 128.48, 128.26(d, J = 2.42 Hz), 126.34, 125.29, 124.74, 124.12(q, J = 3.74 Hz), 115.39, 115.22(d, J = 20.8 Hz), 113.55(d, J = 20.9 Hz), 112.86, 48.55(-S-CH2-), 45.32(Piperidine-C), 33.38(Piperidine-C), 32.32(Piperidine-C). HRMS (ESI) calcd for C28H25F4N5OS [M + H]+: 556.1794, found: 556.1782.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin)-4-(trifluoromethyl)-benzamide(19q)
White solid, yield: 87.1%, mp: 175.9–176.4 °C, purity: 93.43%. 1H NMR (400 MHz, DMSO-d6) δ 10.71 (s, 1H, -NH-C = O), 8.56 (d, J = 2.2 Hz, 1H, Ar-H), 8.23 (d, J = 8.1 Hz, 2H, Ar-H), 8.14 (d, J = 7.7 Hz, 1H, Ar-H), 7.96 (d, J = 8.1 Hz, 2H, Ar-H), 7.88 (dd, J = 8.9, 2.1 Hz, 1H, Ar-H), 7.59 (d, J = 8.9 Hz, 1H, Ar-H), 7.33 (ddt, J = 14.6, 9.4, 5.3 Hz, 3H, Ar-H), 7.06 (dd, J = 9.5, 6.8 Hz, 1H, Ar-H), 4.45 (s, 2H, -S-CH2-), 4.25–4.12 (m, 1H, Piperidine-H), 3.00 (d, J = 12.1 Hz, 2H, Piperidine-H), 2.56 (d, J = 12.1 Hz, 2H, Piperidine-H), 1.88–1.76 (m, 2H, Piperidine-H), 1.54 (qd, J = 12.1, 2.9 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.79(-NH-C = O), 164.10(Ar-C), 163.17(d, J = 243.61 Hz), 157.76, 147.09, 142.18(d, J = 7.6 Hz), 138.19, 134.40, 131.64(d, J = 31.9 Hz), 130.12(d, J = 8.1 Hz), 128.49, 128.37, 126.37, 125.49(q, J = 3.7 Hz), 125.22, 124.74, 115.45, 115.27, 113.56(d, J = 21.3 Hz), 112.84, 48.43(-S-CH2-), 45.13(Piperidine-C), 33.39(Piperidine-C), 32.03(Piperidine-C). HRMS (ESI) calcd for C28H25F4N5OS [M + H]+: 556.1794, found: 556.1783.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-2-nitrobenz-amide(19r)
White solid, yield: 89.3%, mp: 162.5–162.9 °C, purity: 96.94%. 1H NMR (400 MHz, DMSO-d6) δ 10.74 (s, 1H, -NH-C = O), 8.58 (s, 1H, Ar-H), 8.49 (s, 1H, Ar-H), 8.33 (d, J = 8.0 Hz, 1H, Ar-H), 8.18 (d, J = 7.8 Hz, 1H, Ar-H), 8.10 (d, J = 7.7 Hz, 1H, Ar-H), 7.91 (d, J = 9.1 Hz, 1H, Ar-H), 7.79 (t, J = 7.8 Hz, 1H, Ar-H), 7.58 (d, J = 8.9 Hz, 1H, Ar-H), 7.33 (ddt, J = 15.7, 10.0, 5.1 Hz, 3H, Ar-H), 7.05 (dd, J = 9.6, 7.0 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.24–4.09 (m, 1H, Piperidine-H), 2.98 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.65–2.56(m, 2H, Piperidine-H), 1.86–1.76 (m, 2H, Piperidine-H), 1.53 (qd, J = 11.6, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.78(-NH-C = O), 163.36(Ar-C), 163.17(d, J = 244.12 Hz), 157.75, 147.04, 142.19(d, J = 7.6 Hz), 135.41, 135.10, 134.43, 132.40, 131.20, 130.13(d, J = 8.3 Hz), 129.93, 128.10, 126.36, 124.76, 118.26, 115.44(d, J = 23.0 Hz), 113.56(d, J = 20.7 Hz), 112.87, 111.60, 48.62(-S-CH2-), 45.28(Piperidine-C), 33.37(Piperidine-C), 32.26(Piperidine-C). HRMS (ESI) calcd for C27H25FN6O3S [M + H]+: 533.1771, found: 533.1761.
N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)-3-nitrobenz-amide(19 s)
White solid, yield: 86.2%, mp: 132.1–132.7 °C, purity: 94.97%. 1H NMR (400 MHz, DMSO-d6) δ 10.88 (s, 1H, -NH-C = O), 8.48 (d, J = 2.2 Hz, 1H, Ar-H), 8.18 (d, J = 7.9 Hz, 2H, Ar-H), 7.90 (t, J = 7.5 Hz, 1H, Ar-H), 7.84–7.76 (m, 3H, Ar-H), 7.59 (d, J = 8.9 Hz, 1H, Ar-H), 7.33 (ddt, J = 14.4, 9.0, 5.1 Hz, 3H, Ar-H), 7.06 (dt, J = 9.7, 4.6 Hz, 1H, Ar-H), 4.45 (s, 2H, -S-CH2-), 4.26–4.16 (m, 1H, Piperidine-H), 3.03 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.65–2.54 (m, 2H, Piperidine-H), 1.89–1.78 (m, 2H, Piperidine-H), 1.57 (qd, J = 12.2, 2.8 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.64(-NH-C = O), 164.05(Ar-C), 163.17(d, J = 244.1 Hz), 157.77, 147.01, 146.51, 142.13(d, J = 7.6 Hz), 134.60, 134.05, 132.43, 131.06, 130.12(d, J = 8.1 Hz), 129.22, 127.23, 126.66, 124.76, 124.28, 115.45(d, J = 21.8 Hz), 113.53, 113.36, 112.95, 48.29(-S-CH2-), 44.94(Piperidine-C), 33.41(Piperidine-C), 31.66(Piperidine-C). HRMS (ESI) calcd for C27H25FN6O3S [M + H]+: 533.1771, found: 533.1761.
3-cyano-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benza-mide(19t)
White solid, yield: 90.6%, mp: 139.6–140.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.88 (s, 1H, -NH-C = O), 8.89 (d, J = 2.1 Hz, 1H, Ar-H), 8.61–8.44 (m, 3H, Ar-H), 8.22 (d, J = 7.5 Hz, 1H, Ar-H), 7.99–7.84 (m, 2H, Ar-H), 7.61 (d, J = 8.9 Hz, 1H, Ar-H), 7.44–7.25 (m, 3H, Ar-H), 7.13–6.93 (m, 1H, Ar-H), 4.45 (s, 2H, -S-CH2-), 4.32–4.22 (m, 1H, Piperidine-H), 3.14 (d, J = 12.2 Hz, 2H, Piperidine-H), 2.75 (t, J = 12.2 Hz, 2H, Piperidine-H), 1.90 (d, J = 12.7 Hz, 2H, Piperidine-H), 1.73–1.57 (m, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.83(-NH-C = O), 163.14(Ar-C), 160.75(d, J = 244.0 Hz), 157.84, 147.81, 147.13, 142.13(d, J = 7.6 Hz), 135.76, 134.35, 134.05, 130.30, 130.14(d, J = 6.7 Hz), 128.48, 126.41, 126.31, 124.77, 122.30, 115.44, 115.37, 115.23, 113.58(d, J = 19.8 Hz), 112.84, 47.53(-S-CH2-), 44.21(Piperidine-C), 33.42(Piperidine-C), 30.68(Piperidine-C). HRMS (ESI) calcd for C28H25FN6OS [M + H]+: 513.1873, found: 513.1863.
4-cyano-N-(2-((3-fluorobenzyl)thio)-4-(piperidin-4-ylamino)quinazolin-6-yl)benz-amide(19u)
White solid, yield: 89.1%, mp: 142.6–143.4 °C, purity: 95.36%. 1H NMR (400 MHz, DMSO-d6) δ 11.04 (s, 1H, -NH-C = O), 8.66 (s, 1H, Ar-H), 8.39 (d, J = 8.5 Hz, 2H, Ar-H), 8.29 (d, J = 8.5 Hz, 3H, Ar-H), 7.97 (d, J = 9.0 Hz, 1H, Ar-H), 7.58 (d, J = 8.9 Hz, 1H, Ar-H), 7.35–7.28 (m, 3H, Ar-H), 7.05 (dd, J = 9.5, 6.9 Hz, 1H, Ar-H), 4.44 (s, 2H, -S-CH2-), 4.22–4.12 (m, 1H, Piperidine-H), 2.98 (d, J = 12.0 Hz, 2H, Piperidine-H), 2.65–2.54(m, 2H, Piperidine-H), 1.83–1.74 (m, 2H, Piperidine-H), 1.53 (qd, J = 12.0, 3.2 Hz, 2H, Piperidine-H). 13C NMR (101 MHz, DMSO-d6) δ 164.82(-NH-C = O), 163.56(Ar-C), 163.17(d, J = 244.2 Hz), 157.79, 149.18, 147.02, 142.19(d, J = 7.5 Hz), 140.06, 134.53, 130.11(d, J = 8.3 Hz), 129.15, 129.10, 127.97, 126.26, 124.73, 123.54, 115.43(d, J = 22.7 Hz), 115.27, 113.54(d, J = 21.3 Hz), 112.92, 48.68(-S-CH2-), 45.33(Piperidine-C), 33.40(Piperidine-C), 32.33(Piperidine-C). HRMS (ESI) calcd for C28H25FN6OS [M + H]+: 513.1873, found: 513.1862.
References
Vivekanandhan S, Bahr D, Kothari A, Ashary MA, Baksh M, Gabriel E. Immunotherapies in rare cancers. Mol Cancer. 2023;22:23 https://doi.org/10.1186/s12943-023-01720-2
O’Donohue T, Farouk Sait S, Glade Bender J. Progress in precision therapy in pediatric oncology. Curr Opin Pediatr. 2023;35:41–7. https://doi.org/10.1097/mop.0000000000001198
He X, Zhang S, Tian Y, Cheng W, Jing H. Research progress of nanomedicine-based mild photothermal therapy in tumor. Int J Nanomed. 2023;18:1433–68. https://doi.org/10.2147/ijn.S405020
Gogola S, Rejzer M, Bahmad HF, Alloush F, Omarzai Y, Poppiti R. Anti-cancer stem-cell-targeted therapies in prostate cancer. Cancers (Basel). 2023;15:1621 https://doi.org/10.3390/cancers15051621
Abu El-Kass S, Ragheb MM, Hamed SM, Turkman AM, Zaki AT. Needs and self-care efficacy for cancer patients suffering from side effects of chemotherapy. J Oncol. 2021;2021:8880366 https://doi.org/10.1155/2021/8880366
Chen W, Sun Z, Lu L. Targeted engineering of medicinal chemistry for cancer therapy: recent advances and perspectives. Angew Chem Int Ed. 2021;60:5626–43. https://doi.org/10.1002/anie.201914511
Liang Y, Huang W, Zeng D, Huang X, Chan L, Mei C, et al. Cancer-targeted design of bioresponsive prodrug with enhanced cellular uptake to achieve precise cancer therapy. Drug Deliv. 2018;25:1350–61. https://doi.org/10.1080/10717544.2018.1477862
O’Reilly M, Mellotte G, Ryan B, O’Connor A. Gastrointestinal side effects of cancer treatments. Ther Adv Chronic Dis. 2020;11:1432 https://doi.org/10.1177/2040622320970354
Long S, Resende D, Kijjoa A, Silva AMS, Fernandes R, Xavier CPR, et al. Synthesis of new proteomimetic quinazolinone alkaloids and evaluation of their neuroprotective and antitumor effects. Molecules. 2019;24:534 https://doi.org/10.3390/molecules24030534
Niu Z, Ma S, Zhang L, Liu Q, Zhang S. Discovery of novel quinazoline derivatives as potent antitumor agents. Molecules. 2022;27:3906 https://doi.org/10.3390/molecules27123906
Abuelizz HA, Bakheit AH, Marzouk M, El-Senousy WM, Abdellatif MM, Mostafa GAE, et al. Antiviral activity of some benzo[g]quinazolines against coxsackievirus B4: biological screening and docking study. Pharmacol Rep. 2023. https://doi.org/10.1007/s43440-023-00495-z
Zaytsev VP, Lovtsevich LV, Pokazeev KM, Sorokina EA, Dorovatovskii PV, Khrustalev VN, et al. The IMDAV approach towards thieno- and furoisoindolo[2,1-a]quinazolines-11(13)-carboxylic acids possessing antimicrobial and antiviral activities. Tetrahedron. 2023;131:133205 https://doi.org/10.1016/j.tet.2022.133205
Li PJ, Yan Y, Wu N, Yang YH, An L, Tian GM, et al. Design, synthesis, crystal structure, and antimicrobial activities of new quinazoline derivatives containing both the sulfonate ester and piperidinylamide moieties. Pest Manag Sci. 2023. https://doi.org/10.1002/ps.7459
Patel AB. Investigation of the antibacterial activity of new quinazoline derivatives against methicillin and quinolone resistant staphylococcus aureus. J Chem Res. 2020;44:315–21. https://doi.org/10.1177/1747519819895887
Abuelizz HA, Al-Salahi R. An overview of triazoloquinazolines: pharmacological significance and recent developments. Bioorg Chem. 2021;115:105263 https://doi.org/10.1016/j.bioorg.2021.105263
Kubacka M, Mogilski S, Zadrozna M, Nowak B, Szafarz M, Pomierny B, et al. MH-76, a novel non-quinazoline alpha(1)-Adrenoceptor Antagonist, but not prazosin reduces inflammation and improves insulin signaling in adipose tissue of fructose-fed rats. Pharmaceuticals (Basel). 2021;14:477 https://doi.org/10.3390/ph14050477
Auti PS, George G, Paul AT. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020;10:41353–92. https://doi.org/10.1039/d0ra06642g
Bansal R, Malhotra A. Therapeutic progression of quinazolines as targeted chemotherapeutic agents. Eur J Med Chem. 2021;211:113016 https://doi.org/10.1016/j.ejmech.2020.113016
Faisal M, Saeed A. Chemical insights into the synthetic chemistry of quinazolines: recent advances. Front Chem. 2020;8:594717 https://doi.org/10.3389/fchem.2020.594717
Gridelli C, De Marinis F, Di Maio M, Cortinovis D, Cappuzzo F, Mok T. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutation: review of the evidence. Lung Cancer. 2011;71:249–257. https://doi.org/10.1016/j.lungcan.2010.12.008
How J, Mann J, Laczniak AN, Baggstrom MQ. Pulsatile Erlotinib in EGFR-positive non-small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin Lung Cancer. 2017;18:354–63. https://doi.org/10.1016/j.cllc.2017.01.013
Masood A, Kancha RK, Subramanian J. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: focus on afatinib. Semin Oncol. 2019;46:271–283. https://doi.org/10.1053/j.seminoncol.2019.08.004
Long L, Wang YH, Zhuo JX, Tu ZC, Wu R, Yan M, et al. Structure-based drug design: synthesis and biological evaluation of quinazolin-4-amine derivatives as selective Aurora A kinase inhibitors. Eur J Med Chem. 2018;157:1361–1375. https://doi.org/10.1016/j.ejmech.2018.08.053
Menna M, Fiorentino F, Marrocco B, Lucidi A, Tomassi S, Cilli D, et al. Novel non-covalent LSD1 inhibitors endowed with anticancer effects in leukemia and solid tumor cellular models. Eur J Med Chem. 2022;237:114410 https://doi.org/10.1016/j.ejmech.2022.114410
Kumari S, Carmona AV, Tiwari AK, Trippier PC. Amide bond bioisosteres: strategies, synthesis, and successes. J Med Chem. 2020;63:12290–12358. https://doi.org/10.1021/acs.jmedchem.0c00530
Li ED, Lin Q, Meng YQ, Zhang LY, Song PP, Li N, et al. 2,4-Disubstituted quinazolines targeting breast cancer cells via EGFR-PI3K. Eur J Med Chem. 2019;172:36–47. https://doi.org/10.1016/j.ejmech.2019.03.030
Liu L, Wang Z, Gao C, Dai H, Si X, Zhang Y, et al. Design, synthesis and antitumor activity evaluation of trifluoromethyl-substituted pyrimidine derivatives. Bioorg Med Chem Lett. 2021;51:128268 https://doi.org/10.1016/j.bmcl.2021.128268
Li ZR, Wang S, Yang L, Yuan XH, Suo FZ, Yu B, et al. Experience-based discovery (EBD) of aryl hydrazines as new scaffolds for the development of LSD1/KDM1A inhibitors. Eur J Med Chem. 2019;166:432–444. https://doi.org/10.1016/j.ejmech.2019.01.075
Rais A, Husain A, Hasan GM, Hassan MI. A review on regulation of cell cycle by extracellular matrix. Int J Biol Macromol. 2023;232:123426 https://doi.org/10.1016/j.ijbiomac.2023.123426
Wang L, Qin X, Liang J, Ge P. Induction of pyroptosis: a promising strategy for cancer treatment. Front Oncol. 2021;11:635774 https://doi.org/10.3389/fonc.2021.635774
Tibbitts J, Canter D, Graff R, Smith A, Khawli LA. Key factors influencing ADME properties of therapeutic proteins: a need for ADME characterization in drug discovery and development. MAbs. 2016;8:229–45. https://doi.org/10.1080/19420862.2015.1115937
Kono Y, Kawahara I, Shinozaki K, Nomura I, Marutani H, Yamamoto A, et al. Characterization of P-Glycoprotein Inhibitors for evaluating the effect of P-glycoprotein on the intestinal absorption of drugs. Pharmaceutics. 2021;13:388 https://doi.org/10.3390/pharmaceutics13030388
Zhang X, Ding J, Feng L, Wu H, Xu Z, Tao W, et al. Development of novel nitric oxide-releasing quinolinedione/furoxan hybrids as NQO1 inhibitors for intervention of drug-resistanthepatocellular cancer. Bioorg Chem. 2022;129:106174 https://doi.org/10.1016/j.bioorg.2022.106174
Wang Z, Liu L, Dai H, Si X, Zhang L, Li E, et al. Design, synthesis and biological evaluation of novel 2,4-disubstituted quinazoline derivatives targeting H1975 cells via EGFR-PI3K signaling pathway. Bioorg Med Chem. 2021;43:116265 https://doi.org/10.1016/j.bmc.2021.116265
Parupalli R, Akunuri R, Spandana A, Phanindranath R, Pyreddy S, Bazaz MR, et al. Synthesis and biological evaluation of 1-phenyl-4,6-dihydrobenzo[b]pyrazolo[3,4-d]azepin-5(1H)-one/thiones as anticancer agents. Bioorg Chem. 2023;135:106478 https://doi.org/10.1016/j.bioorg.2023.106478
Li J, Tang J, Wang J-P, Yang F, Liu T, Qiu W-W, et al. Design, synthesis and biological activity evaluation of 2-Mercapto-4(3H)-quinazolinone derivatives as novel inhibitors of protein tyrosine Phosphatase 1B. Heterocycles. 2012;85:1897–1911. https://doi.org/10.3987/com-12-12477
Li ED, Lin Q, Meng YQ, Zhang LY, Song PP, Li N, et al. 2,4-Disubstituted quinazolines targeting breast cancer cells via EGFR-PI3K. Eur J Med Chem. 2019;172:36–47. https://doi.org/10.1016/j.ejmech.2019.03.030
Shen C, Wang L, Wen M, Shen H, Jin J, Zhang P. Synthesis of Benzimidazo[1,2-c]quinazolines via metal-free intramolecular C–H amination reaction. Ind Eng Chem Res. 2016;55:3177–81. https://doi.org/10.1021/acs.iecr.5b04452
Krapf MK, Gallus J, Namasivayam V, Wiese M. 2,4,6-substituted quinazolines with extraordinary inhibitory potency toward ABCG2. J Med Chem. 2018;61:7952–76. https://doi.org/10.1021/acs.jmedchem.8b01011
Ge Y, Yang H, Wang C, Meng Q, Li L, Sun H, et al. Design and synthesis of phosphoryl-substituted diphenylpyrimidines (Pho-DPPYs) as potent Bruton’s tyrosine kinase (BTK) inhibitors: targeted treatment of B lymphoblastic leukemia cell lines. Bioorg Med Chem. 2017;25:765–72. https://doi.org/10.1016/j.bmc.2016.11.054
Wang X, Zhang C, Zhang X, Yan J, Wang J, Jiang Q, et al. Design, synthesis and biological evaluation of tetrahydroquinoline-based reversible LSD1 inhibitors. Eur J Med Chem. 2020;194:112243 https://doi.org/10.1016/j.ejmech.2020.112243
Romussi A, Cappa A, Vianello P, Brambillasca S, Cera MR, Dal Zuffo R, et al. Discovery of reversible inhibitors of KDM1A efficacious in acute myeloid leukemia models. ACS Med Chem Lett. 2020;11:754–9. https://doi.org/10.1021/acsmedchemlett.9b00604
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U21A20416).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, H., Yu, F., Wang, H. et al. Design, synthesis and antitumor activity evaluation of 2,4,6-trisubstituted quinazoline derivatives containing piperidine moiety. Med Chem Res 32, 2176–2195 (2023). https://doi.org/10.1007/s00044-023-03119-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03119-6