Abstract
Many chemicals found in mangroves reportedly exhibit potent anticancer, antibacterial, anti-inflammatory, antioxidant, and antitumor properties. Several of such compounds include feature unique structures and display interesting pharmacological effects. Few medicinal mangrove plants from Vietnam have been characterized with regard to their chemical constituents. Aegiceras corniculatum (L.) Blanco is a mangrove shrub that exhibits activity against various types of cancer. To identify new secondary metabolites and determine the source(s) of biological activity in Vietnamese medicinal mangrove plants, the chemical constituents of A. corniculatum were isolated, and their structures were appropriately established using common spectroscopic methods (1D and 2D-NMR, IR, HR-ESI-MS), and by producing derivatives by chemical reactions. Complementarily, it is worth noting, that the anti-inflammatory effects of the isolated compounds were investigated by measuring the production of pro-inflammatory cytokines IL-12 p40, IL-6, and TNF-α in lipopolysaccharide-stimulated bone marrow-derived dendritic cells; in this sense, the target compounds 2 and 3 were potent inhibitors of cytokines TNF-α, IL-6, and IL-12 p40, indicating promising anti-inflammatory effects. Furthermore, compounds 1 and 4 strongly promoted apoptosis of B16F10 melanoma cells. It is convenient to highlight, that the obtained results suggest that saponins from A. corniculatum could be potential candidates for treating cancer and inflammatory illnesses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is the base symptom of several non-communicable diseases, which kill ~40 million people each year, 70% of all global deaths, according to the World Health Organization (Rajendran et al. 2018). Inflammation is an adaptive response to infection and tissue injury, which involves the innate and adaptive immune systems. Notably, inflammation is associated with the development and malignant progression of most cancers (Todoric et al. 2016). The development of bioactive compounds from natural products continues to be an important source of therapeutic agents for chronic inflammation and cancer (Subramaniam et al. 2019).
TNF-α is a well-characterized, pro-inflammatory cytokine that is released primarily from monocytes and macrophages upon invasion of a host by a wide variety of pathogens (Neurath 2014); it plays a crucial role in host defense and the inflammatory response. Although it has numerous beneficial roles in immunoregulation, TNF-α has been implicated in the pathogenesis of both acute and chronic inflammatory diseases. IL-6 is a particularly interesting molecule because it has both pro- and anti-inflammatory effects, and has been implicated in many inflammatory diseases in both adults and neonates (Vinh et al. 2017). IL-12 plays a central role in the initiation and regulation of cellular immunity (Vane et al. 1994). Therefore, inhibiting the expression and production of powerful cytokine mediators (e.g., IL-12 p40, IL-6, and TNF-α) by anti-inflammatory compounds is a viable preventive or therapeutic strategy and may aid in treatment of inflammatory diseases (Dinarello 2006; Vinh et al. 2019a, 2019b).
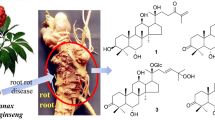
Aegiceras corniculatum (L.) Blanco is a mangrove shrub known to exhibit anticancer effects (Ding et al. 2012). It is important to note, that previous studies regarding the chemical constituents of A. corniculatum several secondary metabolites have been identified, such as: triterpenoids, flavonoids, phenols, and saponins (Roome et al. 2008). In addition, an extract of A. corniculatum was found to exhibit multiple pharmacological effects, including antioxidant, anti-inflammatory, and anticancer activities (Luo et al. 2019). However, continuous research regarding the pharmacological effects of these compounds is essential for the development of effective treatments for cancer and inflammatory diseases. With the goal of developing new anti-inflammatory therapeutic approaches to cancer, we report the isolation and structural elucidation of a new triterpenoid saponin (1), and three known compounds (2−4) from A. corniculatum leaves (Fig. 1). In addition, the anti-inflammatory and melanoma cytotoxic inhibiting effects of these compounds were evaluated.
Results and discussion
Dried leaves of A. corniculatum (2.0 kg) were extracted with 80% aqueous MeOH (5L × 3 times) overnight at 50 °C. Evaporation of the solvent under reduced pressure yielded a tarry MeOH residue (200 g). The MeOH residue was resuspended in H2O and successively partitioned with n-hexane and EtOAc to produce an n-hexane extract (15.8 g), an EtOAc extract (25.6 g), and a water layer after removal of the respective solvents. The EtOAc fraction showed a strong inhibitory effect on the production of pro-inflammatory cytokines IL-12, IL-6, and TNF-α. Thus, the EtOAc fraction was selected for further separation and purification, to identify the biologically active components; in this sense, the isolation of a new saponin (1) and three known compounds (2−4, Fig. 1), namely (3β,16α,20α)-3,16,28-trihydroxyolean-12-en-29-oic acid 3-{O-β-D-glucopyranosyl (1 → 2)-O-[β-D-glucopyranosyl (1 → 4)]-α-L-arabinopyranoside} (2), aegicoroside A (3), and sakurasosaponin (4). The known compounds were identified using modern spectroscopic methods and by correlating previously reported data.
Compound 1 was isolated as a white amorphous powder, with \(\left[ {\upalpha} \right]_{\mathrm{D}}^{20}\) - 18.8 (c 0.1); in regard of its structural attribution the following discussion is provided. The elemental composition, C61H100O27, was achieved by HR-ESI-TOF-MS; this compound showed a molecular ion sodium adduct [M + Na]+ observed at m/z 1287.6350, (calcd. 1287.6344, error 0.0006 m/z units). The IR spectrum clearly showed the presence of ester (1724 cm−1), and hydroxyl (3395 cm−1) groups. The 1H-NMR spectrum of 1 (pyridine-d5) exhibited typical signals corresponding to seven tertiary methyl groups δ ppm: 0.82, 0.99, 1.06, 1.09, 1.14, 1.34, and 1.57 (each 3H, s); these signals showed correlation in the HMQC spectrum with corresponding carbon signals δ ppm: 16.9, 25.6, 17.0, 34.4, 28.4, 19.1, and 20.2, respectively. In addition, five anomeric protons were observed at δ ppm 4.87 (d, J = 7.0 Hz, H-1′) 5.84 (d, J = 7.5 Hz, H-1′′), 5.92 (brs, H-1′′′′′), 6.15 (d, J = 7.8 Hz, H-1′′′), and 6.18 (brs, H-1′′′′) (Table 1). The 13C-NMR and HSQC spectra of 1 confirmed 30 aglycon signals and 30 sugar moiety signals. The chemical shifts of the aglycon moiety in the 13C-NMR spectrum were conveniently correlated to those of sakurasosaponin in the same NMR solvent (Ohtani et al. 1993). Furthermore, the sugar proton signals of 1 were appropriately established by 1H-1H COSY and HMBC data; in this regard. COSY correlations are provided in Fig. S3 (Supplementary data). The pentasaccharide chain of 1 was identified as α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-β-D-(6′-O-methyl)-glucuronopyranoside (Table 1) (Ohtani et al. 1993). In addition, the absolute configurations, of the sugar residues provided by acid hydrolysis of 1, specified their identification as D-glucuronic acid, D-glucose, D-galactose, and L-rhamnose, in addition accompanied by TLC and GC analyses, by comparison with authentic samples. The HMBC spectrum of 1 (Figs 2 and S5) showed appropriated correlations between oxygen-base methyl protons δ ppm 3.74 (3H, s) and the carbonyl group of the glucuronic moiety at 171.8 ppm, in agreement with the presence of a methyl β-D-glucuronate moiety (Me-GlcA). Considering all the previously evidence, the structure of compound 1 was appropriately established as 3-O-[α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-β-D-(6′-O-methyl)glucuronopyranosyl]-13β,28-epoxy-3β,16α-dihydroxy-olean. To the best of our knowledge, this is the first instance of compound 1 found as a natural product.
Saponin derivatives likely induce cell death by triggering apoptosis (Luo et al. 2019). To determine whether the saponin compounds found in A. corniculatum were able to trigger apoptosis, B16F10 cells were treated with each compound (10 µM), and then analyzed using an Annexin V apoptosis assay in a time-dependent manner. Figures 3 and 4 show a clear change in the growth stage histogram in B16F10 cells after treatment. The late apoptosis ratio of B16F10 cells increased 8- or 7-fold after treatment for 6 or 24 h, respectively, compared to a DMSO control. The total late apoptotic and dead cells increased 8- or 9-fold, compared to a DMSO control. These results indicate that the compounds 1 or 4 induced apoptosis in B16F10 cancer cells at 10 µM. However, in the case of compound 4, no signal of viable cells was detected at 24 h, but only 60% of them were labeled by Annexin V and 7-ADD. Taken together, our data implied that not only apoptosis was triggered by these compounds. The involvement of other cells dead pathway such as necrosis or autophagy in this system must be examined. In addition, the signaling pathways by those compounds 1 and 4 induced apoptosis need to be further studied.
Cytotoxicity of compounds 1, and 4 on B16F10 cells. B16F10 cells treated with either DMSO (negative control), mitomycin C (MMC, positive control), compounds 1 or 4 for 24 h. Then the cytotoxic effect was assessed by MTT assay. Data represent of three independent experiments performed in triplicate. (P value between compound 1 and DMSO/MMC is 0.0040/0.0346; P value between compound 4 and DMSO/MMC is 0.0043/0.0378, respectively, *P < 0.05; **P < 0.01)
Treatment with compounds 1 (AC-1) and 4 (AC-4) induced apoptosis in B16F10 cells. Muse Annexin V apoptosis assay was used to analyse the ratio of cellular apoptosis in B16F10 cells after treatment with 10 µM of compounds 1 or 4 for 6, and 24 h, respectively. The data recorded when more than 60% of cells was dead are shown. Total late apoptotic and dead cell ratio was shown in the bar graph. Data were obtained from three independent experiments. (P value between compounds 1 or 4, and DMSO is 0.0064 or 0.0039, respectively, **P < 0.01)
Regarding the anti-inflammatory effects of compounds isolated from A. corniculatum, compound 2 significantly inhibited TNF-α, IL-6, and IL-12 p40 production in lipopolysaccharide-stimulated bone marrow-derived dendritic cells; its IC50 values were 2.38 ± 0.31, 5.12 ± 0.58, and 2.37 ± 0.46 μM, respectively (Table 2). Compound 3 was particularly potent, with IC50 values of 0.40 ± 0.05, 0.79 ± 0.16, and 1.58 ± 0.38 μM, respectively. SB203580, an inhibitor of cytokine suppressive binding protein/p38 kinase, was used as a positive control. SB203580 inhibited TNF-α, IL-6, and IL-12 production with IC50 values of 7.20 ± 0.08, 3.50 ± 0.01, and 5.00 ± 0.02 μM, respectively. Therefore, compounds 2 and 3 significantly inhibited the production of multiple pro-inflammatory cytokines (Fig. 5).
Effect of compounds 2 and 3 on IL-12p40, IL-6, and TNF-α production in LPS-stimulated BMDCs. The data were presented as inhibition rate (%) compared to the value of vehicle-treated DCs. SB203580 was used as positive control. Data are representative of three independent experiments. *P < 0.05, **P < 0.01 versus compound untreated BMDCs in the presence of LPS
Secondary metabolites reportedly play important roles in controlling inflammatory response pathways. Oleanane triterpene saponins are structurally diverse and often exhibit noteworthy bioactivity. These are generally abundant in both classical medicinal herbs and marine organisms and have been shown to possess various pharmacological effects including anticancer, antidiabetic, anti-cardiovascular, anti-inflammatory, antioxidant, and antitumor activities (Vinh et al. 2019a). In particular, triterpene saponins have been identified as the primary constituents responsible for the biological activities of Panax ginseng (Vinh et al. 2017), Gynostemma pentaphyllum (Ky et al. 2010), Glycyrrhiza uralensis (Song et al. 2014), and Gymnema sylvestre (Pham et al. 2018). Our study indicates that saponins 1 and 4 strongly promoted the apoptosis of B16F10 melanoma cells. Moreover, saponins 2 and 3 were potent inhibitors of pro-inflammatory cytokines TNF-α, IL-6, and IL-12 p40 in lipopolysaccharide-stimulated bone marrow-derived dendritic cells. In conclusion, the findings of this study demonstrate that several triterpene saponins found in the leaves of A. corniculatum might be useful for the treatment of inflammatory diseases and other related ailments.
Material and methods
General experimental procedures
The optical rotation values were recorded with a Jasco P-1020 polarimeter with MeOH as the solvent. FT-IR spectra were obtained on a JASCO Report 100 infrared spectrophotometer. All NMR spectra were measured at Bruker Avance III 600 spectrometers with TMS as the internal standard. The HR-ESI-MS were obtained from an Agilent 6530 Accurate-Mass Q-TOF LC/MS system. Gas chromatography spectra were recorded on a Shidmazu-2010 spectrometer (Shimadzu, Kyoto, Japan). Silica gel (70–230, 230–400 mesh, Merck, Whitehouse Station, NJ), YMC RP-18 resins (75 µm, Fuji Silysia Chemical Ltd., Kasugai, Japan) were used as absorbents in the column chromatography. Thin-layer chromatography (TLC) plates (silica gel 60 F254 and RP-18 F254, 0.25 µm, Merck) were purchased from Merck KGaA (Darmstadt, Germany). Spots were detected under UV radiation (254 and 365 nm) and by spraying the plates with 10% H2SO4 followed by heating with a heat gun. All solvents used were provided by SK chemicals Korea. All chemicals and reagents were purchased from Sigma-Aldrich.
Biological material
The plant material of A. corniculatum was harvested at Quang Ninh Province, Vietnam in July 2016, and were identified by Prof. Young Ho Kim. The voucher specimens (ĐTCB-HSB 15) have been deposited at the herbarium of Institute of Marine Biochemistry, VAST, Vietnam.
Extraction and isolation
The dried leaves of A. corniculatum (2 kg) were extracted three times (each 5L) with MeOH by sonication for 1 h. The MeOH extract was concentrated under reduced pressure to obtain a residue (200 g). This residue was suspended in water (3 L) and successively partitioned with n-hexane (4 × 3 L) and EtOAc (3 × 3 L) to give: n-hexane (H, 15 g), EtOAc (E, 25 g) and a water layer after removal of the solvents.
The EtOAC fraction (E, 25 g) was separated by VLC using gradient concentrations of MeOH in CH2Cl2 (from 0 to 100%) to give six fractions (E1 to E6). Fraction E4 (9 g) was separated into four subfractions (E4.1–E4.4) using silica gel column chromatography (CC) with CH2Cl2−MeOH−H2O (3.5:1:0.07, v/v). Subfraction E4.3 (3 g) was separated by YMC RP-18 and Sephadex LH-20 CC using as the eluent acetone−H2O (1:1, v/v) and further purified by silica gel CC with CH2Cl2−MeOH−H2O (2.5:1:0.1, v/v) to afford compounds 1 (30 mg), and 4 (200 mg). Repeating the same steps as for subfraction E4.3, compounds 2 (39 mg), and 3 (47 mg) were obtained from subfraction E4.4 (2 g).
Physical and spectroscopic data of isolated compounds
3-O-[α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-β-D-(6′-O-methyl)glucuronopyranosyl]-13β,28-epoxy-3β,16α-dihydroxy-olean (1). White amorphous powder, \(\left[ {\upalpha} \right]_{\mathrm{D}}^{20}\) - 18.8 (c 0.1, MeOH). IR (KBr) νmax 3395, 2972, 1724, and 1044 cm−1. 1H (C5D5N, 600 MHz) and 13C-NMR (C5D5N, 150 MHz) data are given in the Table 1; HR QTOF MS m/z 1287.6350 [M + Na]+ (calcd for C61H100NaO27+, 1287.6344).
(3β,16α,20α)-3,16,28-trihydroxyolean-12-en-29-oic acid 3-{O-β-D-glucopyranosyl (1 → 2)-O-[β-D-glucopyranosyl (1 → 4)]-α-L-arabinopyranoside} (2). White amorphous powder, \(\left[ {\upalpha} \right]_{\mathrm{D}}^{20}\) -3.00 (c 0.6, MeOH). IR (KBr) νmax 3400, 2970, 1730, and 1070 cm−1. 1H (C5D5N, 600 MHz) and 13C-NMR (C5D5N, 150 MHz) data, see Figs S7–S11; HR QTOF MS m/z 967.4861 [M + H]+ (calcd for C47H76NaO19+ 967.4847).
Aegicoroside A (3). White amorphous powder, \(\left[ {\upalpha} \right]_{\mathrm{D}}^{20}\) - 34.8 (c 0.1, MeOH). IR (KBr) νmax 3349, 2931, 1306, and 1079 cm−1. 1H (C5D5N, 600 MHz) and 13C-NMR (C5D5N, 150 MHz) data, see Figs S12–S15; HR QTOF MS m/z 1109.5496 [M + Na]+ (calcd for C54H86NaO22+, 1109.5503).
Sakurasosaponin (4). White amorphous powder, \(\left[ {\upalpha} \right]_{\mathrm{D}}^{20}\) + 44.8 (c 0.1, MeOH). IR (KBr) νmax 3400, 2950, 1730, and 1077 cm−1. 1H (C5D5N, 600 MHz) and 13C-NMR (C5D5N, 150 MHz) data, see Figs S16–S21; HR QTOF MS m/z 1249.6217 [M-H]− (calcd for C6H97O27−, 1249.6223).
Acid hydrolysis and sugar identification
Compound 1 (3.0 mg) was dissolved in 3 mL of 10% HCl 1M-dioxan (1:1, v/v) and then heated at 60 °C for 2 h. After the solvent was removed in vacuo, the residue was partitioned successively with CH2Cl2 and H2O to give the aglycon and the sugar, respectively. The aqueous phase was concentrated to provide a residue. After drying, the residue was dissolved in anhydrous pyridine (100 µL) and then mixed with a pyridine-d5 solution of 0.1 M L-cysteine methyl ester hydrochloride (100 µL). The reaction mixture was heated at 60 °C for 2 h, and 0.1 mL of trimethylsilylimidazole solution was added, followed by heating at 60 °C for 1.5 h. The dried product was partitioned with n-hexane and H2O (0.1 mL, each), and the organic layer was analyzed by gas-liquid chromatography (GC): Column: column SPB-1 (0.25 mm × 30 m); detector FID, column temp 210 °C, injector temperature 270 °C, detector temperature 300 °C, carrier gas He. The absolute configuration of the monosaccharide for compound 1 was confirmed to be D-glucuronic acid, D-glucose, D-galactose, and L-rhamnose, respectively (Thao et al. 2016; Vinh et al. 2017).
In vitro anti-tumoral activity of isolated compounds
MTT viability assay
5 × 104 cells were plated on 96-well plates. After seeding, cells were treated with compounds (10 µM). MMC (10 µM) was treated as a positive control and DMSO (0.1%) was used as a negative control. The cells were cultured for 24 h and the viability of cells was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were then solubilized in 100 μl 0.04 N HCl isopropanol. The absorbance reading was obtained using a SpectraMax ABS Plus Microplate Reader (Molecular devices, CA, USA). The amount of MTT dye reduction was calculated on the basis of the difference between the absorbances at 570 nm. The cell viability in treated cells was expressed as the amount of dye reduction relative to that in DMSO treated control cells. In addition, the cytotoxicity of compounds 1-4 toward BMDCs was evaluated using MTT colorimetric assay at the concentration of 25 µM (Ali et al. 2017). Compounds 1 and 4 showed cytotoxicity toward BMDCs, while the other compounds 2 and 3 displayed no notable cytotoxicity.
Apoptotic assay
The percentage of cells that actively underwent apoptosis was analyzed using Annexin V-phycoerythrin-based immunofluorescence according to the user guide of the Muse Annexin V & Dead Cell Kit. The cells were incubated in six-well plates (5 × 105 cells/well) overnight. The cells were then treated with DMSO, or 1 (10 µM) for 6 h, or 4 (10 µM) for 24 h. Adherent and floating cells were collected, washed in cold phosphate-buffered saline (PBS) twice, and then stained with the MuseAnnexin V & Dead Cell reagent. Apoptosis was identified using a Muse Cell Analyzer (Millipore Corporation, Hayward, CA, USA).
In vitro anti-inflammatory activity of isolated compounds
Cell cultures and measurement of cytokine production
To grow BMDCs, wild-type 6-week-old female C57BL/6 mice bone marrow was used as previously described (Ali et al. 2017). All animal procedures were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of Jeju National University, Jeju, South Korea (#2016-0059). Briefly, bone marrow cells were allowed to differentiate in RPMI 1640 (BD, Grand Island, NY) medium containing granulocyte-macrophage colony-stimulating factor for DCs generation. For BMDCs, on day 6 of incubation the cells were harvested and seeded in 48-well plates at a density of 1 × 105 cells/0.5 mL, and then treated with the compound for 1 h before stimulation with LPS. Supernatants were harvested 18 h after stimulation. Concentrations of murine IL-12 p40, IL-6, and TNF-α in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) (BD PharMingen, San Jose, CA).
Statistical analysis
All assays were repeated in at least three independent experiments. Statistical significance was indicated by one-way ANOVA or T-test and was performed using Graph Pad Prism ver. 5.01 (GraphPad Software, San Diego, CA, USA).
References
Ali I, Manzoor Z, Koo J-E, Kim J-E, Byeon S-H, Yoo E-S, Kang H-K, Hyun J-W, Lee N-H, Koh Y-S (2017) 3-Hydroxy-4, 7-megastigmadien-9-one, isolated from Ulva pertusa, attenuates TLR9-mediated inflammatory response by down-regulating mitogen-activated protein kinase and NF-κB pathways. Pharm Biol 55:435–440
Dinarello CA (2006) The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev 25:307–313
Ding L, Dahse H-M, Hertweck C (2012) Cytotoxic alkaloids from Fusarium incarnatum associated with the mangrove tree Aegiceras corniculatum. J Nat Prod 75:617–621
Ky PT, Huong PT, My TK, Anh PT, Van Kiem P, Van Minh C, Cuong NX, Thao NP, Nhiem NX, Hyun J-H (2010) Dammarane-type saponins from Gynostemma pentaphyllum. Phytochemistry 71:994–1001
Luo H, Hao E, Tan D, Wei W, Xie J, Feng X, Du Z, Huang C, Bai G, Hou Y (2019) Apoptosis effect of Aegiceras corniculatum on human colorectal cancer via activation of FoxO signaling pathway. Food Chem Toxicol 134:110861
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14:329–342
Ohtani K, Mavi S, Hostettmann K (1993) Molluscicidal and antifungal triterpenoid saponins from Rapanea melanophloeos leaves. Phytochemistry 33:83–86
Pham HTT, Hoang MC, Ha TKQ, Dang LH, Tran VO, Nguyen TBT, Lee CH, Oh WK (2018) Discrimination of different geographic varieties of Gymnema sylvestre, an anti-sweet plant used for the treatment of type 2 diabetes. Phytochemistry 150:12–22
Rajendran P, Chen YF, Chen YF, Chung LC, Tamilselvi S, Shen CY, Day CH, Chen RJ, Viswanadha VP, Kuo WW (2018) The multifaceted link between inflammation and human diseases. J Cell Physiol 233:6458–6471
Roome T, Dar A, Ali S, Naqvi S, Choudhary MI (2008) A study on antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective actions of Aegiceras corniculatum (stem) extracts. J Ethnopharmacol 118:514–521
Song W, Si L, Ji S, Wang H, Fang XM, Yu LY, Li RY, Liang LN, Zhou D, Ye M (2014) Uralsaponins M–Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J Nat Prod 77:1632–1643
Subramaniam S, Selvaduray KR, Radhakrishnan AK (2019) Bioactive compounds: natural defense against cancer? Biomolecules 9:758
Thao NP, Luyen BTT, Vinh LB, Lee JY, Kwon YI, Kim YH (2016) Rat intestinal sucrase inhibited by minor constituents from the leaves and twigs of Archidendron clypearia (Jack.) Nielsen. Bioorg Med Chem Lett 26:4272–4276
Todoric J, Antonucci L, Karin M (2016) Targeting inflammation in cancer prevention and therapy. Cancer Prev Res 9:895–905
Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA (1994) Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci 91:2046–2050
Vinh LB, Lee Y, Han YK, Kang JS, Park JU, Kim YR, Yang SY, Kim YH (2017) Two new dammarane-type triterpene saponins from Korean red ginseng and their anti-inflammatory effects. Bioorg Med Chem Lett 27:5149–5153
Vinh LB, Jang H-J, Phong NV, Cho KW, Park SS, Kang JS, Kim YH, Yang SY (2019a) Isolation, structural elucidation, and insights into the anti-inflammatory effects of triterpene saponins from the leaves of Stauntonia hexaphylla. Bioorg Med Chem Lett 29:965–969
Vinh LB, Jang H-J, Phong NV, Dan G, Cho KW, Kim YH, Yang SY (2019b) Bioactive triterpene glycosides from the fruit of Stauntonia hexaphylla and insights into the molecular mechanism of its inflammatory effects. Bioorg Med Chem Lett 29:2085–2089
Acknowledgements
This work was the financial support by College of Pharmacy, Chungnam National University, Republic of Korea.
Author contributions:
Isolation and structure elucidation, L.B.V, N.V.P, G.D, and H.L.T.A; Analysis of active compounds: L.B.V, G.D, and H.L.T.A; Research idea, S.Y.Y, and Y.H.K; Biological assay, D.T.V.A, I.A, and Y.S.K. All authors have read and agreed to the published version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vinh, L.B., Phong, N.V., Ali, I. et al. Identification of potential anti-inflammatory and melanoma cytotoxic compounds from Aegiceras corniculatum. Med Chem Res 29, 2020–2027 (2020). https://doi.org/10.1007/s00044-020-02613-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02613-5