Abstract
Bioisosteric replacements are often tried goaling to affect the lipophilicity, polarity, and aqueous solubility of the substances, as a way to obtain therapeutically improved medicines. Also, hydrazonyl compounds are described with a wide range of pharmacological activities, having also recognized activities in antimycobacterial field. In this study, twenty-seven pyrimidinyl and pyrazinyl derivatives have been synthesized and evaluated for their antimycobacterial activity against M. tuberculosis ATTC 27294. The componds were obtained by the reaction of 2-hydrazinylpyridine, 4-hydrazinylpyridine, or 2-hydrazinylpyrazine with appropriated aromatic or heteroaromatic aldehydes. Antimycobacterial activity of the compounds was determined according to MTT assay. The most active compound, a 2-hydroxyl-5-nitrophenyl-4-pyridinylhydrazone derivative, showed good biodisponibility and nonmutagenic or tumorigenic profiles in preliminaries in silico studies, and exceptional in vitro activity, being compared with the reference drug ethambutol. This study supports that pyrimidinyl and pyrazinyl derivatives may be used for the development of new tuberculostatic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocycles’ ring structures are composed by elements other than carbon, and are able to impact strongly the physicochemical properties of the substances (Martins et al. 2015). Also, the incorporation of heterocyclic like fragments is a commonly strategy used in medicinal chemistry to design prototypes with enhanced potency and selectivity, and bioisosteric replacements are often tried goaling to affect the lipophilicity, polarity, and aqueous solubility of the substances, as a way to obtain therapeutically improved medicines (Dua et al. 2011). In order to obtain these products, modern synthetic methodologies are available, allowing rapid access to a wide variety of functionalized heterocyclic compounds of critical importance to the medicinal chemist (Taylor et al. 2016; Khanna et al. 2018; El-Sayed et al. 2018; Przybylski et al. 2018; Brahmachari 2018).
Hydrazonyl compounds are also described with a wide range of pharmacological activities, such as antibacterial, antifungal, anticonvulsant, anti-inflammatory, antimalarial, antileishmanial, and antineoplastic, having also recognized activities in antimycobacterial field (Rollas and Küçükgüzel 2007; Verma et al. 2014; Rodrigues et al. 2014; De Souza et al. 2018; Cardoso et al. 2017; Amim et al. 2016; Candéa et al. 2009.
As a part of a study goaling to investigate the importance of the nitrogen to the biological activity presented by six membered heterocycle derivatives, this manuscript present the antimicobacterial activity of componds containing the nitrogen at position 2 and 4 of the heterocycle moiety (identified as 2-pyridinylhydrazone and 4-pyridinylhydrazone derivatives), and also nitrogen dissubstituted heterocycles, defined as pyrazinylhydrazone derivatives.
Results and discussion
The leishmanicidal activities of series 2 and 4 were previously reported by our research group (De Souza et al. 2018). Concerning to 4-pyridinyl derivatives, compounds 3b, 3d, 3e, 3g, and 3h are novel, and compounds 3c and 3f were reported previously, but their spectral data have not been described in the literature.
Synthesis and characterization
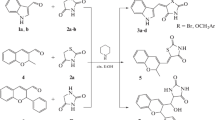
The synthesis of planned 2-pyridinylhydrazone, 2, 4-pyridinylhydrazone, 3, and 2-pyrazinylhydrazone, 4, derivatives were accomplished according to the reported procedure11 as shown in Scheme 1. It was employed, respectively, commercial 2-hydrazinylpyridine, 4-hydrazinylpyridine, or 2-hydrazinylpyrazine as starting material, and appropriated aromatic or heteroaromatic aldehydes in ethanol at room temperature for 5 min-78 h (Table 1). The compounds were obtained in 35–97% yields. It is important to mention that aromatic aldehydes containing hydroxyl, metoxyl and nitro substituents, and heteroaromatic aldehydes were selected due to their activity in different series previously study from our group being the most actives (Rodrigues et al. 2014; Coimbra et al. 2013; Nogueira et al. 2019; Cardoso et al. 2014).
All these new compounds were identified by detailed spectral data, including 1H NMR, 13C NMR, high-resolution mass spectrometry and infrared spectrometry. In general, the 1H NMR spectrum showed the pyridine protons of compounds 2a-i at 6.72–8.17 and ranging from 7.19 to 13.20 for compounds 3a–i. In addition, the pyrazine protons of compounds 4a–i were observed at 7.94–8.70 ppm and the N=CH protons of all series are presented ranging from 7.27 to 8.37 ppm. The N=CH functional group is also observed in infrared spectra, exhibiting an IR band at 1557–1645 cm−1.
Anti-mycobacterial activity
The antimycobacterial activity of compounds 2, 3, and 4 are shown in Table 2. This work is a continuation of previous studies of our group, when it was observed the highlighted activities presented by 2-hydroxylphenyl and heteroaryl substituted hydrazones. The most active compound of all series, the 4-pyridinylhydrazone, 5-nitrothien-2-yl 3h, present a remarkable activity, being more active than the first line tuberculostatic drug ethambutol.
The 2-hydroxylphenyl derivatives, a-f compounds, are much more active than heteroarylic compounds in 2 and 4 series. In previous studies a role considered for the salicyldehyde hydrazones that of a chelator, and this capacity seems to be optimized when there is a nitrogen at position 2 of the main ring. Salicyldehyde derivatives are known to be able to act as tridentate O,N,N-ligands, and thereby form strong complexes with metals. It is considered that the removal of a metal vital for cell reproduction on complexation by the drug can take a major role in these drug interactions.
For compounds 3 the opposite is observed. Heteroaryl derivatives 3g-h showed an improved activity if compared with hidroxylated compounds 3a–f. The activity of the 5-nitrothienyl and 5-nitrofuranyl derivatives has been attributed to the redox potentials of such compounds (Mahmud et al. 1998; Rakesh et al. 2014; Squella et al. 2005).
The lipophilicities of 2, 3, and 4, expressed as logP values, were determined using the Data Warrior program and are listed in Table 1. Pharmacocinetic studies indicate that a good balance between permeability and aqueous solubility for a drug is indicated by a logP value between 0 and 3. The value found for the most active compound of this study, 3h, falls within this range, being 2.80.
Other compounds with remarkable activities are the 2-hydroxylphenyl derivatives 2b, 2e, 4d, and 4f, being only the pyrazinyl derivatives 4d and 4f, with logP values of 2.4991 and 1.9232, included in the ideal range. The obtained values suggest that these substances will be more soluble in water than 3h.
The toxicity of the compounds 2, 3, and 4 was also determinated using the Data Warrior program, see Table 2. The most promising compound of this study, compound 3h, as well as 2e, 4d, and 4f, were found to be nonmutagenic or tumorigenic.
Based on the results obtained for compound 3h, we can conclude that this compound has a remarkable profile and could be considered as a reasonable starting point to find new compounds with improved antitubercular activity.
Conclusion
In this study, we reported the antimycobacterial activity of 27 pyrimidinyl and pyrazinyl derivatives, which have been evaluated against M. tuberculosis ATTC 27294 using the MTT assay. Compounds 3h, 2e, 4d, and 4f displayed the best tuberculostatic profiles in all experiments. The most active compound, a 2-hydroxyl-5-nitrophenyl, 4-pyridinylhydrazone derivative 3h, showed good biodisponibility and nonmutagenic or tumorigenic profiles in preliminaries in silico studies, and exceptional in vitro activity, being compared with the reference drug ethambutol. These results suggest that this compound could be a useful starting point for further studies for new tuberculostatic drugs and confirm the potential of 4-pyridinylhydrazone derivatives as lead compounds in antituberculosis drug discovery.
Material and methods
Experimental
Melting points were determined using an MQAPF-302 (MicroQuímica Ltd, Santa Catarina, Brazil) apparatus. Infrared spectra were recorded on a Thermo Nicolet Nexus 6700 spectrometer as potassium bromide pellets and frequencies are expressed in cm−1. NMR spectra were were analysed using a Bruker Avance 400 operating at 400.00 MHz (1H) and 100.0 MHz (13C) in DMSO-d6. Chemical shifts are reported in ppm (δ) relative to tetramethylsilane and J-coupling in Hertz (Hz). Proton and carbon spectra were typically obtained at room temperature. High-resolution mass spectra were acquired on a Bruker compact-TOF. The progress of the reactions was monitored by thin-layer chromatography (TLC) on 2.0 cm × 6.0 cm aluminum sheets (silica gel 60, HF-254, Merck) with a thickness of 0.25 mm, using ultraviolet light irradiation. For column chromatography, Merck silica gel (70–230 or 230–400 mesh) was used.
General procedure for the synthesis of 2-pyridinylhydrazone derivatives 2a-i (GP1)
To a stirred solution of 2-hydrazinylpyridine (1.0 mmol) in ethanol (10 mL) was added the appropriate benzaldehyde (0.9 mmol), and the reaction mixture was stirred for 5 min – 78 h at room temperature. The reaction mixture was concentrated under reduced pressure, and the residue was purified by washing with cold water (3 × 10 mL), affording the 2-pyridinylhydrazone derivatives 2a–i as solid in 37–81% yields.
General procedure for the synthesis of 4-pyridinylhydrazone derivatives 3a–i (GP2)
To a stirred solution of 4-hydrazinylpyridine (1.0 mmol) in ethanol (10 mL) was added the appropriate benzaldehyde (1.0 mmol), and the reaction mixture was stirred for 20 min –24 h at room temperature. The reaction mixture was concentrated under reduced pressure, and the residue was purified by washing with cold diethyl ether (3 × 10 mL), leading to the 4-pyridinylhydrazone derivatives 3a–i as solid in 37–97% yields.
General procedure for the synthesis of 2-pyrazinylhydrazone derivatives 4a–i (GP3)
To a stirred solution of 2-hydrazinylpyrazine (1.0 mmol) in ethanol (10 mL) was added the appropriate benzaldehyde (1.05 mmol), and the reaction mixture was stirred for 2–48 h at room temperature. The reaction mixture was concentrated under reduced pressure, and the residue was purified by washing with cold water (3 × 10 mL), cold ether (3 × 10 mL), or by column chromatography purification using hexane/ethyl acetate (50 → 100%) as eluent, thus affording the 2-pyrazinylhydrazone derivatives 4a–i in 35–87% yields.
The series 2 and 4 compounds were previously synthesized by our research group, and the antileishmanial activities published (De Souza et al. 2018). The compounds of series 3 are described below.
(E)-2-((2-(pyridin-4-yl)hydrazono)methyl)phenol (3a)
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2-hydroxybenzaldehyde (122 mg, 1.0 mmol). The desired compound was obtained as amorphous colorless solid (172 mg, 81%); m.p. 261–263 °C; IR (KBr) ν 3085, 1633 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 13.92 (s, 1H, NH), 12.76 (s, 1H, H-2’ or H-4’), 10.30 (s, 1H, OH), 8.64 (s, 1H, H-2’ or H-4’), 8.32 (s, 1H, H-1’ or H-5’), 8.31 (s, 1H, H-1’ or H-5’), 7.84 (dd, J = 7.7, 1.6 Hz, 1H, H-5), 7.51 (s, 1H, N=CH), 7.28 (ddd, J = 8.3, 7.7, 1.6 Hz, 1H, H-3), 6.98 (dd, 1H, J = 8.3, 0.8 Hz, H-2), 6.89 (dt, J = 7.7, 0.8 Hz, 1H, H-4) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 156.7 (C-1), 154.5 (C-2’), 144.4 (C-4’), 140.8 (CH=NNH), 139.8 (C-3), 131.8 (C-5), 126.0 (C-6’), 119.6 (C-4), 119.3 (C-6), 116.2 (C-2), 107.8 (C-5’), 106.0 (C-1’) ppm; HRESIMS m/z: 214.0983 C12H12N3O (calcd. 214.0975).
(E)-3-((2-(pyridin-4-yl)hydrazono)methyl)benzene-1,2-diol (3b):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2,3-dihydroxybenzaldehyde (138 mg, 1.0 mmol). The desired compound was obtained as amorphous white solid (220 mg, 96%); m.p. 257–259 °C; IR (KBr) ν 2930, 1634 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 12.69 (s, 1H, H-2’ or H-4’), 9.72 (s, 1H, OH), 9.32 (s, 1H, OH), 8.62 (s, 1H, H-2’ or H-4’), 8.32 (s, 1H, H-1’ or H-5’), 8.30 (s, 1H, H-1’ or H-5’), 7.28 (dd, J = 7.9, 1.5 Hz, 1H, H-5), 7.27 (s, 1H, N=CH), 6.89 (dd, J = 7.9, 1.5 Hz, 1H, H-3), 6.71 (t, J = 7.9 Hz, 1H, H-4) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 154.3 (C-1), 145.7 (C-2), 145.5 (C-2’), 144.8 (C-4’), 140.6 (C=NNH), 120.3 (C-6’), 120.3 (C-5), 119.2 (C-4), 119.2 (C-6), 117.1 (C-3), 116.3 (C-5’), 106.9 (C-1’) ppm; HRESIMS m/z: 230.0932 C12H12N3O2 (calcd. 230.0925).
(E)-4-((2-(pyridin-4-yl)hydrazono)methyl)benzene-1,3-diol (3c):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2,4-dihydroxybenzaldehyde (138 mg, 1.0 mmol). The desired compound was obtained as amorphous pale yellow solid (222 mg, 97%); m.p. 200–203 °C; IR (KBr) ν 3164, 1624 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 13.71 (s, 1H, NH), 12.51 (s, 1H, H-2’ or H-4’), 10.21 (s, 1H, OH), 10.03 (s, 1H, OH), 8.50 (s, 1H, H-2’ or H-4’), 8.25 (s, 2H, H-1’ and H-5’), 7.63 (d, J = 8.6 Hz, 1H, H-5), 7.41 (s, 1H, N=CH), 6.42 (d, J = 2.2 Hz, 1H, H-2), 6.35 (dd, J = 8.6 Hz, 2.2, 1H, H-4) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 161.2 (C-1); 158.4 (C-3); 154.0 (C-2’); 145.3 (C-4’); 140.7 (C=NNH); 139.3 (C-5); 127.7 (C-6’); 111.1 (C-6); 108.1 (C-5’); 107.5 (C-1’); 105.5 (C-4); 102.3 (C-2) ppm HRESIMS m/z: 230.0935 C12H12N3O2 (calcd. 230.0925).
(E)-2-((2-(pyridin-4-yl)hydrazono)methyl)benzene-1,4-diol (3d):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2,5-dihydroxybenzaldehyde (138 mg, 1.0 mmol). The desired compound was obtained as amorphous white solid (215 mg, 94%); m.p. 243–245 °C; IR (KBr) ν 3236, 1635 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 13.92 (s, 1H, NH), 12.69 (s, 1H, H-2’ or H-4’), 9.59 (s, 1H, OH), 9.02 (s, 1H, OH), 8.56 (s, 1H, H-2’ or H-4’), 8.32 (s, 2H, H-1’ and H-5’), 7.44 (s, 1H, N=CH), 7.22 (d, J = 2.8 Hz, 1H, H-5), 6.79 (d, J = 8.7 Hz, 1H, H-2), 6.74 (dd, J = 8.7, 2.8 Hz, 1H, H-3) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 154.4 (C-1); 149.9 (C-2’); 149.8 (C-4’); 144.6 (C-4); 140.8 (C=NNH); 139.7 (C-6’); 119.8 (C-6); 119.6 (C-3); 117.1 (C-2); 110.7 (C-5); 107.9 (C-5’); 105.8 (C-1’) ppm; HRESIMS m/z: 230.0927 C12H12N3O2 (calcd. 230.0925).
(E)-5-methyl-2-((2-(pyridin-4-yl)hydrazono)methyl)phenol (3e):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2-dihydroxy-4-methylbenzaldehyde (136 mg, 1.0 mmol). The desired compound was obtained as amorphous white solid (216 mg, 95%); m.p. 290–292 °C; IR (KBr) ν 3191, 1635 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 13.98 (s, 1H, NH), 12.81 (s, 1H, H-2’ or H-4’), 10.24 (s, 1H, OH), 8.61 (s, 1H, H-2’ or H-4’), 8.30 (s, 2H, H1’ and H-5’), 7.71 (d, J = 8.0 Hz, 1H, H-5), 7.48 (s, 1H, N=CH), 6.81 (s, 1H, H-2), 6.71 (d, J = 8.0 Hz, 1H, H-4), 2.27 (s, 3H, CH3) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 156.8 (C-1); 154.4 (C-3); 144.8 (C-2’); 142.1 (C-4’); 140.8 (C=NNH); 139.7 (C-5); 126.1 (C-6’); 120.5 (C-4); 117.1 (C-6); 116.6 (C-2); 107.9 (C-5’); 105.9 (C-1’); 21.2 (CH3) ppm; HRESIMS m/z: 228.1138 C13H14N3O (calcd. 228.1137).
(E)-4-nitro-2-((2-(pyridin-4-yl)hydrazono)methyl)phenol (3f):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2-hydroxy-5-nitrobenzaldehyde (167 mg, 1.0 mmol). The desired compound was obtained as amorphous white solid (245 mg, 95%); m.p. 294–296 °C; IR (KBr) ν 1635, 1519, 1336 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 12.93 (s, 1H, H-2’ or H-4’), 8.65 (d, J = 2.9 Hz, 1H, H-5), 8.61 (s, 1H, H-2’ or H-4’), 8.38 (s, 1H, H-1’ or H-5’), 8.36 (s, 1H, H-1’ or H-5’), 8.18 (dd, J = 9.1, 2.9 Hz, 1H, H-3), 7.58 (s, 1H, N=CH), 7.21 (d, J = 9.1 Hz, 1H, H-2) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 162.1 (C-1); 154.7 (C-4); 141.6 (C=NNH); 139.9 (C-2’); 139.9 (C-4’); 126.8 (C-5); 121.3 (C-3); 120.5 (C-6’); 120.5 (C-6); 116.8 (C-2); 108.1 (C-5’); 106.5 (C-1’) ppm; HRESIMS m/z: 259.0822 C12H11N4O3 (calcd. 259.0826).
(E)-4-(2-((5-nitrofuran-2-yl)methylene)hydrazinyl)pyridine (3g):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 5-nitrofuran-2-carbaldehyde (141 mg, 1.0 mmol). The desired compound was obtained as amorphous yellow solid (162 mg, 70%); m.p. 280–282 °C; IR (KBr) ν 1643, 1534, 1346 cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 14.20 (s, 1H, NH), 13.20 (s, 1H, H-2’ or H-4’), 8.45 (s, 1H, H-1’ or H-5’), 8.43 (s, 1H, H-1’ or H-5’), 8.30 (s, 1H, H-2’ or H-4’), 7.83 (d, J = 4.0 Hz, 1H, H-3), 7.41 (s, 1H, N=CH), 7.38 (d, J = 4.0 Hz, 1H, H-4) ppm; 13C-NMR (100 MHz, DMSO-d6): δ = 154.8 (C-2); 152.0 (C-5); 151.0 (C-2’); 151.0 (C-4’); 141.2 (C=NNH); 134.9 (C-6’); 116.1 (C-3); 116.1 (C-4); 114.7 (C-5’); 108.2 (C-1’) ppm; HRESIMS m/z: 233.0677 C10H9N4O3 (calcd. 233.0670).
(E)-4-(2-((5-nitrothiophen-2-yl)methylene)hydrazinyl)pyridine (3h):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 5-nitrothiophene-2-carbaldehyde (157 mg, 1.0 mmol). The desired compound was obtained as amorphous white solid (201 mg, 81%); m.p. 288–290 °C; IR (KBr) ν 1639, 1522, 1329 cm−1; 1H NMR (500 MHz, DMSO-d6): δ = 14.11 (s, 1H, NH), 13.11 (s, 1H, H-2’ or H-4’), 8.52 (s, 1H, H-2’ or H-4’), 8.42 (s, 1H, H-1’ or H-5’), 8.41 (s, 1H, H-1’ or H-5’), 8.16 (d, J = 4.3 Hz, 1H, H-3), 7.65 (d, J = 4.3 Hz, 1H, H-4), 7.41 (s, 1H, N=CH) ppm; 13C-NMR (125 MHz, DMSO-d6): δ = 154.6 (C-5); 151.1 (C-2); 145.7 (C-2’); 145.7 (C-4’); 141.2 (C=NNH); 140.4 (C-6’); 130.6 (C-3); 130.6 (C-4); 130.1 (C-5’); 130.1 (C-1’) ppm; HRESIMS m/z: 249.0450 C10H9N4O2S (calcd. 249.0441).
(E)-2-((2-(pyridin-4-yl)hydrazono)methyl)pyridine (3i):
GP2 was followed using 4-hydrazinylpyridine (109 mg, 1.0 mmol), ethanol (10 mL), and was added 2-pyridinecarboxaldehyde (107 mg, 1.0 mmol). The desired compound was obtained as amorphous yellow solid (74 mg, 37%); m.p. 250–252 °C; IR (KBr) ν 1645cm−1; 1H NMR (400 MHz, DMSO-d6): δ = 8.60 (m, 1H, H-5), 8.33 (s, 1H, H-2’ or H-4’), 8.32 (s, 1H, H-2’ or H-4’), 8.14 (s, 1H, N=CH), 8.03 (d, J = 4.8 Hz, 1H, H-2), 7.86 (t, J = 7.6 Hz, 1H, H-4), 7.38 (ddd, J = 7.6, 4.8, 1.1 Hz, 1H, H-3), 7.20 (s, 1H, H-1’ or H-5’), 7.19 (s, 1H, H-1’ or H-5’) ppm; 13C-NMR (125 MHz, DMSO-d6): δ = 153.3 (C-6); 152.1 (C-2); 149.4 (C-2’); 146.4 (C-4’); 143.2 (C=NNH); 136.7 (C-4); 123.8 (C-3); 123.8 (C-6’); 119.7 (C-5); 119.7 (C-5’); 107.4 (C-1’) ppm; HRESIMS m/z: 199.0982 C11H11N4+ (calcd. 199.0979).
Anti-mycobacterial activity
The antimycobacterial activities of compounds 2, 3, and 4 were assessed against M. tuberculosis ATCC 27294 using Mosman’s MTT (3-(4,5-dimethylthylthiazol-2yl)-2,5-dimethyl tetrazolium bromide; Merck) microcultured tetrazolium assay (Mosmann 1983). Briefly, the cells were plated in flat-bottom 96 well plates (2.5 × 106 cells/well/100 μL) cultured for 24 h in a controlled atmosphere (CO2 5% at 37 °C), and nonadherent cells were washed by gentle flushing with RPMI 1640 supplemented with fetal bovine serum (10%, Sigma) and gentamicin (25 μg/mL). Adherent cells were infected or not with BCG (2.5 × 106 UFC/well/100 μL) cultured in the presence of medium alone, Tween 20 (3%) (live and dead controls, respectively), or different concentrations of compounds (1.0, 10.0, and 100 μg/mL) in a triplicate assay. After 48 h, stock MTT solution (5 mg/mL of saline; 20 mL/well) was added to the culture and 4 h later, the plate was centrifuged for 2 min at 2800 rpm, supernatant was discharged, and dimethyl sulfoxide (DMSO) (100 μL/well) was added to formazan crystals solubilization, and the absorbance was ready at 540 nm in a plate reader (Biorad – 450).
References
Alptüzün V, Parlar S, Taşlı H, Erciyas E (2009) Synthesis and antimicrobial activity of some pyridinium salts. Molecules 14:5203–5215
Amim RS, Firmino GSS, Rego ACPD, Nery AL, Da Silva SAG, Figueroa-Villar JD, Resende JALC, De Souza MVN, Pessoa CO, Lessa JA (2016) Cytotoxicity and leishmanicidal activity of isoniazid-derived hydrazones and 2-pyrazineformamide thiosemicarbazones. J Braz Chem Soc 27:769–777
Berge DG (1983) Synthesis of new 2-pyridylhydrazones and 2-quinolylhydrazones containing 2-thiophene or 2-furan groups. J Chem Eng Data 28:431–432
Brahmachari G (2018) Synthesis of biologically relevant heterocycles in aqueous media. Asian J Org Chem 7:1982–2004
Candéa ALP, Ferreira ML, Pais KC, Cardoso LNF, Kaiser CR, Henriques MGMO, Lourenço MCS, Bezerra FAFM, De Souza MVN (2009) Synthesis and antitubercular activity of 7-chloro-4-quinolinylhydrazones derivatives. Bioorg Med Chem Lett 19:6272–6274
Cardoso LNF, Nogueira TCM, Rodrigues FAR, Oliveira ACA, Luciano MCS, Pessoa CO, De Souza MVN (2017) N-acylhydrazones containing thiophene nucleus: a new anticancer class. Med Chem Res 26:1605–1608
Cardoso LNF, Bispo MLF, Kaiser CR, Wardell JL, Wardell SMSV, Lourenço MCS, Bezerra FAFM, Soares RPP, Rocha MN, De Souza MVN (2014) Anti-tuberculosis evaluation and conformational study of N -acylhydrazones containing the thiophene nucleus. Arch Pharm 347:432–448
Case FH (1976) Preparation of new 2-pyridyl and pyrazinylhydrazones containing ferroin group. J Chem Eng Data 21:124–125
Coimbra ES, Antinarelli LMR, Da Silva AD, Bispo MLF, Kaiser CR, De Souza MVN (2013) 7-chloro-4-quinolinylhydrazones: a promising and potent class of antileishmanial compounds. Chem Biol Drug Des 81:658–665
Cushman M, Nagarathnam D, Gopal D, Geahlen RL (1991) Synthesis and evaluation of new protein-tyrosine kinase inhibitors. Part 2. Phenylhydrazones. Bioorg Med Chem Lett 1:215–218
De Souza MVN, Coimbra ES, Antinarelli LMR, Crispi MA, Nogueira TCM, Pinheiro AC (2018) Synthesis, biological activity, and mechanism of action of 2-pyrazyl and pyridylhydrazone derivatives, new classes of antileishmanial agents. ChemMedChem 13:1387–1394
Dua R, Shrivastava S, Sonwane SK, Srivastava SK (2011) Pharmacological significance of synthetic heterocycles scaffold: a review. Adv Biol Res 5:120–144
El-Sayed TH, Aboelnaga A, El-Atawy MA, Hagar M (2018) Ball milling promoted N-heterocycles synthesis. Molecules 23:1348
Lions F, Martin KV (1958) Sexadentate chelate compounds.1X. JACS 80:3858–3865
Hegarty AF, Moroney PJ, Scott FL (1973) A change from rate-determining bromination to geometric isomerisation of pyridylhydrazones. J Chem Soc Perkin Trans 2:1466–1471
Kakemi K, Uno T, Arita T, Nakano H, Shimada I, Ikegami Y, Kitazawa S (1961) Synthesis of pyrazinoic acid derivatives. I. Derivatives of pyrazinoic acid, aminopyrazine, pyrazinohydrazide, and pyrazinecarboxaldehyde. Yakugaku Zasshi 81:1609–1614
Khanna P, Khanna L, Thomas SJ, Asiri AM, Panda SS (2018) Microwave assisted synthesis of spiro heterocyclic systems: a review. Curr Org Chem 22:67–84
Mahmud NP, Garrett SW, Threadgill MD (1998) The 5-nitrofuran-2-ylmethylidene group as a potential bioreductively activated prodrug system for diol-containing drugs. Anticancer Drug Des 13:655–662
Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, Baptista PV, Fernandes AR (2015) Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 20:16852–16891
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nogueira TCM, Cruz LS, Lourenço MCS, De Souza MVN (2019) Design, synthesis and anti-tuberculosis activity of hydrazones and N-acylhydrazones containing vitamin B6 and different heteroaromatic nucleus. Lett Drug Des Disco 16:792–798
Przybylski P, Pyta-Klich K, Pyta K, Janas A (2018) Cascade reactions as efficient and universal tools for construction and modification of 6-, 5-, 4- and 3-membered sulfur heterocycles of biological relevance. Tetrahedron 74:6335–6365
Rakesh, Bruhn DF, Scherman MS, Woolhiser LK, Madhura DB, Maddox MM, Singh AP, Lee RB, Hurdle JG, McNeil MR, Lenaerts AJ, Meibohm B, Lee RE (2014) Pentacyclic nitrofurans with in vivo efficacy and activity against nonreplicating mycobacterium tuberculosis. PLoS ONE 9:e8790
Rodrigues FAR, Bomfim IS, Cavalcanti BC, Pessoa CO, Wardell JL, Solange SMSV, Pinheiro AC, Kaiser CR, Nogueira TCM, Low JN, Gomes LR, de Souza MVN (2014) Design, synthesis and biological evaluation of (E)-2-(2-arylhydrazinyl)quinoxalines, a promising and potent new class of anticancer agents. Bioorg Med Chem Lett 24(3):934–939
Rollas S, Küçükgüzel ŞG (2007) Biological activities of hydrazone derivatives. Molecules 12:1910–1939
Sandbhor U, Padhye S, Billington D, Rathbone D, Franzblau S, Anson CE, Powell AK (2002) Metal complexes of carboxamidrazone analogs as antitubercular agents. 1. Synthesis, X-ray crystal-structures, spectroscopic properties and antimycobacterial activity against Mycobacterium tuberculosis H(37)Rv. J Inorg Biochem 90:127–136
Sondhi SM, Jain S, Dinodia M, Kumar A (2008) Synthesis of some thiophene, imidazole and pyridine derivatives exhibiting good anti-inflammatory and analgesic activities. Med Chem 4:146–154
Squella JA, Bollo S, Núñez-Vergara LJ (2005) Recent Developments in the Electrochemistry of Some Nitro Compounds of Biological Significance. Curr Org Chem 9:565–581
Syeda N, Srinivasan VR (1965) Some fused S-triazoles derived from aza-heterocyclic hydrazones. Indian J Chem 3:162–167
Taylor AP, Robinson RP, Fobian YM, Blakemore DC, Jones LH, Fadeyi O (2016) Modern advances in heterocyclic chemistry in drug discovery. Org Biomol Chem 14:6611–6637
Verma G, Marella A, Shaquiquzzaman M, Akhtar M, Rahmat Ali M, Alam MM (2014) A review exploring biological activities of hydrazones. J Pharm Bioallied Sci 6:69–80
Acknowledgements
We are grateful to the Brazilian agency CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico) for fellowships and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pinheiro, A.C., Nogueira, T.C.M., Pereira, G.E. et al. Synthesis and antimycobacterial evaluation of pyridinyl- and pyrazinylhydrazone derivatives. Med Chem Res 29, 1662–1668 (2020). https://doi.org/10.1007/s00044-020-02592-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02592-7