Abstract
In the search for new therapeutic alternatives for Chagas disease, a series of six aryloxy -naphthoquinone derivatives were synthesized and evaluated in vitro against Trypanosoma cruzi epimastigotes of the Tulahuén 2, INC-5, and NINOA strains. The compounds 3d and 4a showed better or similar trypanosomicidal activity than the reference drug nifurtimox. In addition, 3d and 4a also elicited better trypanosomicidal activity than nifurtimox against T. cruzi bloodstream trypomastigotes. On the other hand, 3b showed the highest selective indexes (SI values between 44 and 500, in the three T. cruzi strains). Finally, molecular docking studies suggested that these compounds could be potential trypanothione reductase inhibitors. Therefore, based on these new results, we validated that the aryloxy-naphthoquinone scaffold is essential to obtain more selective cytotoxic and trypanosomicidal compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
American trypanosomiasis (AT) is recognized by the World Health Organization (WHO) as one of the neglected tropical diseases and a main cause of morbidity and mortality with 6 to 7 million people infected and ~21,000 deaths per year in 21 countries in Central and South America (Marques Pereira and Navarro 2013; De Souza 2014; Galaviz-Silva et al. 2017). In Latin America, 25 million people live in risk infection areas, while the Pan American Health Organization reports 12,000 annual deaths related to Chagas disease (CD). The incidence of the disease is high in rural areas where environmental conditions favor the installation and breeding of triatomine insects (Marques Pereira and Navarro 2013).
AT is also known as CD, new world trypanosomiasis, South American trypanosomiasis, and Chagas-Mazza disease. It was first described by Carlos Chagas in Brazil in 1909 and it is caused by a flagellated protozoan, Trypanosoma cruzi (T. cruzi), a member of the Trypanosomatidae family, Kinetoplastida order, which affects a variety of mammals including humans (De Souza 2014; Galaviz-Silva et al. 2017).
According to the WHO, in humans, AT can be treated with benznidazole (Bnz) and nifurtimox (Nfx), which are lethal against T. cruzi. These drugs are almost 100% effective when they are administered in the acute phase of the disease, which is the initial stage of infection. Nevertheless, their effectiveness diminishes as time elapses from the start of infection. Besides, toxicity reactions and adverse effects occur with Bnz and Nfx that on many occasions cause abandonment of treatment (Heitmann et al. 2008). In addition, both drugs are mutagenics (Melo and Ferreira 1990; Jurado and Pueyo 1995).
Currently, many research groups and nongovernmental organizations are in search of various natural and/or synthetic molecules as new alternatives for pharmacotherapy for AT.
Among these compounds, naphthoquinones stands out because it presents diverse of biological properties, as in the case of α and β-lapachone derivatives, which have demonstrated activity against T. cruzi through various mechanisms of action, such as the generation of reactive oxygen species (Bombaca et al. 2019), and DNA and RNA inhibition (Pinto and De Castro 2009). They also have better activity against Candida albicans (Moraes et al. 2018). According to their origin, naphthoquinones are classified as natural and synthetic. In the case of natural naphthoquinones with trypanosomicidal activity against T. cruzi epimastigotes, we find lapachol, β-lapachone, and α-lapachone with IC50 values of 31.3, 0.21, and 24.7 µM, respectively. The above naturally obtained naphthoquinone derivatives were isolated from the bark of Bignoniaceae trees (Salas et al. 2008). Synthetic naphthoquinones have been reported as trypanosomicidal agents, for example, a series of naphthoquinone derivatives were synthesized, evaluated, and tested against Trypanosoma brucei rhodesiense trypomastigotes, T. cruzi amastigotes, and Leishmania donovani amastigotes by Bolognesi et al. in 2008. The results obtained by this research group showed that compound I (Fig. 1) had the best trypanosomicidal activity with an IC50 of 1.26 µM against the amastigote stage of Tulahuén C2C4 T. cruzi strain (Bolognesi et al. 2008). Furthermore, compound I was more powerful than Bnz; nevertheless, the selectivity index (SI) that expresses the ratio of IC50 in L6 cells (rat skeletal myoblasts) and IC50 against epimastigotes (SI = L6/IC50), expressed a low selectivity (SI < 5) which led to future research to obtain molecules with less toxicity (Bolognesi et al. 2008).
Recently, naphthoquinones were paid more consideration of trypanosomicidal agents, in this sense Tapia et al. synthesized and evaluated a series of new aryloxindol-4,9-diones against the Y strain of T. cruzi epimastigotes. Compound II (Fig. 1) presented the best IC50 with a value of 20 nM and a high SI = 625 (Selectivity index: expressed as the ratio of IC50 in J774 cells to IC50 in epimastigotes). A pharmacophoric study was performed for this kind of molecules revealed that the presence of an aromatic ring, hydrogen acceptor groups, and a hydrophobic ring are related with the biological activity (Tapia Apati et al. 2014). Subsequently, a new series of aryloxy-quinones were synthesized and evaluated against the Y strain of the T. cruzi epimastigote stage and a murine macrophage cell line. The obtained results showed that compound III displayed the best trypanosomicidal activity and selectivity with an IC50 = 20 nM and a SI = 625 (Fig. 1) (Vázquez et al. 2015).
In addition, it has been reported that naphthoquinone derivatives are potential inhibitors of the flavoenzyme trypanothione reductase of T. cruzi (TRTc), by enzymatic assays and molecular docking. For example, it have been reported that 3-(dibutylamino)-propylamine-1,4-naphthoquinone derivatives have a high inhibitory potential on TRTc without inhibiting the homologous enzyme human glutathione reductase, therefore the inhibition of TRTc is specific. Likewise, they highlighted that the protonable amino groups are critical for the recognition of TR (Salmon-Chemin et al. 2000). However, naphthoquinone derivatives could have more than one target for their trypanosomicidal action, according to works carried out by Pieretti and collaborators, who suggested that naphthoquinones could carry out their trypanosomicidal activity via multi-target mechanism (Pieretti et al. 2013).
Based on the aforementioned, in this work, the use of naphthoquinone as a building block for the development of new trypanosomicidal agents was considered. The naphthoquinone ring was functionalized according with three criteria, to evaluate the effect of these modifications on the trypanosomicidal effect. These modifications were: (a) the substitution of the hydrogen atom on C-3 by a bromine, due to it has been reported that this change could increase the selectivity; (b) the substitutions of methoxy group or methyl groups on aryloxy moiety, because in the previous studies only electron-withdrawing groups (nitro or halogens) were analyzed; and (c) a double substitution on C-2 and C-3 by aryloxy moiety. In addition, to elucidate the possible mechanism of action of this type of compounds, an in silico molecular docking study was carried out on T. cruzi trypanothione reductase (TcTR), a protein referred to as a pharmacological target for naphthoquinone derivatives.
Results and discussion
Chemistry
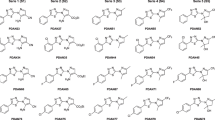
In Scheme 1 the synthetic step of compounds 3a–d and 4a,b is shown. This synthesis followed the methodology reported by Vázquez et al. 2015 with some modifications. These aryloxy-naphthoquinones were obtained in moderate to high yields (68–90%), and monosubstitution or double substitution were controlled by the respective phenol molar ratio. All compounds were purified by column chromatography and their structures were analyzed based on their spectral properties (IR, UPLC-MS, 1H NMR, and 13C NMR, see Supplementary section).
Trypanosomicidal activity against the epimastigote forms
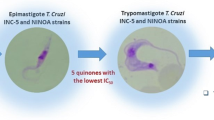
The in vitro trypanosomicidal activity of the compounds was initially tested against the epimastigote form of T. cruzi in three strains: Tulahuén 2, INC-5, and NINOA. The dose–response assay used concentrations in a range of 1–50 μM to calculate the half-maximal inhibitory concentration (IC50).
The results of the trypanosomicidal activity of aryloxy-naphthoquinone derivatives on T. cruzi epimastigotes (Tulahuén 2, INC-5, and NINOA strains) are shown in Table 1. The results showed that all compounds displayed better or equal trypanosomicidal activity than the reference drug Nfx against Tulahuén 2 strain. The compound 3b showed the best IC50 value (0.20 μM), which was 38 times greater than Nfx. In a structure–activity relationship analysis, it was seen that compounds 3a and 3c, which had methoxy groups in a phenyloxy-ring, did not increase their trypanosomicidal activity with regard to 3b. On the other hand, the presence of methyl groups in the aromatic ring and the addition of the halogen bromine to the naphthoquinone ring (3d), reduced more than twofold the trypanosomicidal effect. In the case of substitution of the halogen atom for a second aromatic ring with methoxy (4a) or methyl (4b) groups, biological activity did not improve. According to the aforementioned, the presence of only methyl groups in an aromatic ring (3b) was preferable to maintain or improve the biological effect on Tulahuén 2 strain. Regarding the comparison of halogenated compounds, it can be seen that compound 3c, which has a bromine substituent and a methoxy group, had greater trypanosomicidal activity than compound 3d with the substituents bromine and methyl groups.
In the case of the INC-5 strain, only three compounds (3b, 3d, and 4a) displayed an activity similar to the reference drug, Nfx. The three left compounds presented low activity. This means that the incorporation of a methoxy substituent in the phenolic ring in compound 3a and 3c, and the presence of a second aromatic ring with a methyl substituent, i.e., in 4b, did not favor the trypanosomicidal activity of these molecules. The presence of a methyl group improved the trypanosomicidal effect only in compounds 3b, 3d, and the presence of a second aromatic ring and a methoxy group, enhanced the trypanosomicidal activity in compound 4a. In the case of NINOA strain, as well as the INC-5 strain, the compounds did not present better biological activity than Nfx. Compound 4a showed the best trypanosomicidal activity followed by compound 3d.
The presence of a second aromatic ring and a methoxy substituent improved the trypanosomicidal activity in molecules against the INC-5 and NINOA strains; these structural modifications, nonetheless, did not improve the activity on Tulahuén 2 strain (Table 1).
The strains INC-5 and NINOA are Mexican strains isolated from patients and these belong to the TcI of T. cruzi and Tulahuén 2 strain, belonging to the genotype TcVI, which is a reference with high sensitivity to drugs that explains the variability of the results. The previously aforementioned results reported that there are differences in drug susceptibility in different T. cruzi strains (León-Pérez et al. 2007). In this study, it was observed that Mexican strains, which have already been shown to belong to TcI2 (I or II) (Gómez-Hernández et al. 2011), presented greater drug resistance than strains of Brazilian origin, which have a TcII type lineage.
Cytotoxicity
Cytotoxic studies were performed on murine macrophages J774.1 (Table 2). Compounds 3c and 3d were the most toxic of the series, where 3c had the lowest IC50 against macrophages. This behavior could be attributed to the presence of bromine on the quinone ring and the methoxy substituent in the aromatic ring. Interestingly, compound 3b, which does not have the mentioned constituents, was the least cytotoxic against macrophages. The results showed that all compounds present a greater cytotoxic effect than Nfx in murine macrophages; however, the SI for all the studied compounds showed a selectivity higher than four.
In the case of Tulahuén 2 strain, the SI results showed greater selectivity than the reference drug, especially 3b. Likewise, for the clinical strains (NINOA and INC-5), compound 3b presented the best SI, followed by compounds 4a and 3d. According to the SI values obtained for compounds 3d (SI = 96) and 4a (SI = 104) in Tulahuén 2 strain, we suggest that these values are acceptable according to that reported by other research groups being higher than the SI of Nfx (Nwaka and Hudson 2006; Romanha et al. 2010; Da Silva et al. 2014). The SI in NINOA strain was lower than Nfx. The same behavior occurred with INC-5 strain. When it was compared with the data obtained from the three strains, it was seen that Tulahuén 2 strain showed the greatest sensitivity to the studied compounds.
Trypanosomicidal activity against trypomastigote forms
After evaluating the trypanosomicidal effect of compounds on epimastigotes, an assay was carried out with T. cruzi blood trypomastigotes (INC-5 and NINOA strains). The blood trypomastigote stage is the infective form in mammals, mainly during the acute stage of the disease; therefore, it is important to assess the compounds activities during this stage. Only compounds 3d and 4a were considered to have the best trypanosomicidal activity over the two strains selected, with each one being representative of every evaluated series. Table 3 shows the LC50 results of 3d and 4a with both strains. Compounds 3d and 4a showed lytic activity against trypomastigotes on both strains having LC50 values similar to the reference drug Nfx. Animal experiments were performed according to Norma Oficial Mexicana (NOM-062-ZOO-1999) published on August 22, 2009 entitled Technical specifications for the production, care and use of laboratory animals.
Molecular docking analysis
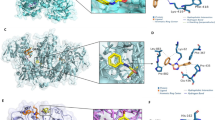
Naphthoquinones have been reported as potential trypanothione reductase (TR) inhibitors (Persch et al. 2014). Therefore, our aryloxy naphthoquinone derivatives were docked, using AutodockVina (vina), in order to assess their affinity to TR (PDB ID: 1GXF) using quinacrine as the template compound. Figure 2 highlights the structural conformation of quinacrine mustard in the active site of TcTR. Quinacrine mustard acts as a competitive inhibitor of TcTR on the hydrophobic amino acid TRY-111, which is part of the hydrophobic wall (formed by LEU-18, TRP-22, TYR-111, and MET-114) and modulates the enzyme activity of TcTR (Nwaka and Hudson 2006).
Results of the molecular docking studies on the aryloxy naphthoquinones are shown in Table 4. These six compounds showed a higher affinity than quinacrine mustard. Figure 3 shows the docking conformation of these results. The 3a interacts with the four residues of the hydrophobic wall, however unlike quinacrine mustard; TRY-111 forms a hydrogen bond to 3a. This hydrogen bond is also present with the 3c structure. This compound also interacts with TRP-22 trough stacking, these interactions sometimes have a big impact on affinity, as in 3b, the compound with the highest affinity, nevertheless for 3c this is seen to be not true. Compound 3c also form two hydrogen bonds with TRY-111 and LEU-18. In addition, its boron atom interacts with oxygen from ILE-339. For 4a, interactions are mainly hydrophobic. These interactions occur with LEU-18, TYR-111, TRP-22, ILE-339, ARG-472. In addition, a hydrogen bond is formed with ARG-472.
On the other hand, interactions with 3b are diverse, this compound has a π-stacking with TRP-22, establishes hydrophobic interactions with LEU-18, ILE-339, TRY-111, and TRP-22 and forms hydrogen bonds with GLU-19, ILE-339, and TYR-111. The π-stacking with TRP-22 is also present with 3d, as well as the hydrophobic interaction with ILE-339. Along with those interactions, bromine atom of 3d is interacting with SER-15 and two hydrogen bonds are formed with TYR-111 and GLU-19. Finally, 4b establishes hydrophobic interactions with LEU-18, VAL-59, ILE-107, ILE-339, and LEU-399 and forms a hydrogen bond and π-stacking interaction with TRY-111.
Based on these interactions, it seems that, just as quinacrine mustard, all the six compounds interact with the hydrophobic wall residues. This points out that additionally to their importance acting as support for the correct orientation of the natural subtract of TcTR, these amino acids also contribute with an important set of potential interactions for the binding of small molecules.
Conclusions
In the present work, new aryloxy-naphthoquinone derivatives were synthesized and evaluated in vitro against two form of T. cruzi and in three strains of them. The trypanosomicidal activity of these compounds be determined by the T. cruzi strains and these results were compared with the reference drug Nfx. The most promising naphthoquinones were 3d and 4a, due to their highest trypanosomicidal effect on INC-5 and NINOA strains than Nfx. In addition, 3d and 4a had a similar trypanosomicidal activity in a trypomastigotes ex vivo model of the same strains. Even the cytotoxicity on J774-1 cells of the compounds was higher than Nfx; 3b showed the best selectivity behavior. The results of the molecular docking on T. cruzi TR suggested that these compounds could have an inhibitory effect as a mechanism of action. Therefore, this study validates the aryloxy-naphthoquinone moiety as a privileged scaffold for the design of new molecules with trypanosomicidal activity and selectivity.
Experimental
Material and methods
Chemistry
All reagents and solvents were purchased from Sigma-Aldrich and were used without further purification. Melting points were determined on Mel-Temp capillary apparatus and are uncorrected. Solvent evaporation under vacuum was carried out with a R-100. The purity and reactions were monitored by thin-layer chromatography (TLC) performed on silica gel plates prepared with silica gel 60 (PF-245 with gypsum, Merck Japan), with a thickness of 0.25 nm. The developed chromatograms were visualized under ultraviolet light at 254–265 nm. Infrared spectra were recorded using OPUS_7.5.18 software with PLATINUM-ATR Bruker Alpha FT-IR spectrometer. NMR data were collected with Bruker Avance 500 spectrometer operating at 400 MHz (1H), and 126 MHz (13C). 1H proton NMR spectra were obtained in DMSO-d6, with TMS as an internal standard. Chemical shifts are presented on the δ scale (ppm). Multiplicities are indicated as follows: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) or br (broad).
General procedure for preparation of novel, aryloxy quinones (3a–3d to 4a–4b)
In a reaction flask, the appropriate phenol (1 mmol) and K2CO3 (3 mmol) was dissolved in DMF (10 mL). The mixture was stirred at room temperature for 20 min and the corresponding quinone (1 mmol) was added. The reaction mixture was stirred for 6–8 h at room temperature and poured in ice-cold water. The organic layer was extracted with ethyl acetate (3 × 25 mL), washed with a saturated solution of sodium sulfite and brine (3 × 25 mL). The obtained organic layer was dried with sodium sulfate and concentrated under vacuum. The product was purified by using column chromatography on silica gel and TLC. For compound 4a and 4b, appropriate phenol was used (2 mmol).
2-(3,5-Dimethoxyphenoxy)-1,4-dihydronaphthalene-1,4-dione (3a)
Yield: 85%, mp 126 °C, FT IR (υ cm−1): 3100 (CH sp2, aromatic), 2842 (C-H stretch methoxy), 1677, (C=O). 1H NMR (400 MHz, DMSO-d6) δ 3.76 (s, 6H, OCH3), 5.90 (s, 1H, H-3), 6.47 (s, 3H, H-2′, H-4′, and H-6′), 7.84–7.92 (m, 2H, H-6 and H-7), 7.93–7.99 (m, 1H, H-5), 8.04–8.11 (m, 1H, H-8). 13C NMR (101 MHz, DMSO-d6) δ 55.59 (OCH3), 98.25 (C-6′), 99.14 (C-3′ and C-4′), 113.60 (C-3), 125.62 (C-5), 126.18 (C-8), 130.86 (C-8a), 131.44 (C-4a), 133.90 (C-6 or C-7), 134.63 (C-6 or C-7), 154.44 (C-1′), 159.83 (C-2), 161.52 (C-5′ and C-2′), 179.12 (C-1), 184.39 (C-4).
2-(2,4-Dimethylphenoxy)-1,4-dihydronaphthalene-1,4-dione (3b)
Yield: 90%, mp 120 °C, FT IR (υ cm−1): 2920 (CH sp2, aromatic), 1680, (C=O). 1H NMR (400 MHz, DMSO-d6) δ 2.12 (s, 3H, Ar–CH3), 2.31 (s, 2H, Ar-CH3), 5.56 (s, 1H, H-3), 7.06 (d, J = 8.2 Hz, 1H, H-6′), 7.14 (dd, J = 8, 1.6 Hz, 1H, H-5′), 7.20 (d, J = 1.6 Hz, 1H, H-3′), 7.83–7.91 (m, 2H, H-6 and H-7), 7.93–7.96 (m, 1H, H-5), 8.05–8.12 (m, 1H, H-8).
13C NMR (101 MHz, DMSO-d6) δ 15.17 (Ar-CH3), 20.40 (Ar-CH3), 111.92 (C-3), 120.77 (C-6′), 125.60 (C-5), 126.19 (C-8), 128.35 (C-5′), 129.15 (C-4′), 130.96 (C-4a), 131.47 (C-8a), 132.35 (C-3′), 133.84 (C-2′), 134.60 (C-6 or C-7), 135.90 (C-7 or C-6), 148.34 (C-1′), 159.65 (C-2), 179.08 (C-1), 184.25 (C-4).
2-Bromo-3-(3,5-dimethoxyphenoxy)-1,4-dihydronaphthalene-1,4-dione (3c)
Yield: 87%, mp 133 °C, FT IR (υ cm−1): 3056 (CH sp2, aromatic), 2839 (C–H stretch methoxy), 1679, (C=O). 1H NMR (400 MHz, DMSO-d6) δ 3.71 (s, 6H, OCH3), 6.26 (t, J = 2.1 Hz, 1H, H-4′), 6.36 (d, J = 2.1 Hz, 2H, H-2′ and H-6′), 7.85–7.93 (m, 2H, H-6 and H-7), 7.96–8.01 (m, 1H, H-8), 8.10 (m, 1H, H-5). 13C NMR (101 MHz, DMSO-d6) δ 55.50 (OCH3), 95.13 (C-3′ and C-6′), 95.38 (C-4′), 126.52 (C-8), 126.81 (C-5), 128.51 (C-2), 130.67 (C-4a), 131.42 (C-8a), 134.28 (C-6 or C-7), 134.44 (C-6 or C-7), 154.63 (C-3), 157.77 (C-1′), 161.24 (C-2′ and C-5′), 177.30 (C-1), 178.41 (C-4).
2-Bromo-3-(2,4-dimethylphenoxy)-1,4-dihydronaphthalene-1,4-dione (3d)
Yield: 87%, mp 150 °C, FT IR (υ cm−1): 2917 (CH sp2, aromatic), 1680, (C=O). 1H NMR (400 MHz, DMSO-d6) δ 2.23 (s, 3H, Ar-CH3), 2.31 (s, 3H, Ar-CH3), 6.84 (s, 2H, H-5′ and H-6′), 7.09 (s, 1H, H-3′), 7.83–7.90 (m, 2H, H-6 and H-7), 7.92–7.97 (m, 1H, H-5), 8.10 (m, 1H, H-8). 13C NMR (101 MHz, DMSO-d6) δ 15.84 (Ar-CH3), 20.16 (Ar-CH3), 115.08 (C5′ or C-6′), 126.02 (C-3), 126.53 (C-5), 126.83 (C-8), 127.19 (C-6′ or C-5′), 130.55 (C-8a), 131.20 (C-4a), 131.70 (C-3′), 132.46 (C-1′), 134.32 (C-6 or C-7), 134.52 (C-7 or C-6), 152.76 (C-2′ and C-4’), 155.78 (C-2), 177.44 (C-4), 178.36 (C-1).
2,3-Bis (3,5-dimethoxyphenoxy)-1,4-dihydronaphthalene-1,4-dione (4a)
Yield: 68%, mp 159 °C, FT IR (υ cm−1): 2945 (CH sp2, aromatic), 2841 (C-H stretch methoxy), 1670, (C=O). 1H NMR (400 MHz, DMSO-d6) δ 3.67 (s, 12H, OCH3), 6.18 (t, J = 2.1 Hz, 2H, H-4′ and H-4′′), 6.32 (d, J = 2.1 Hz, 4H, H-3′, H-3′′, H-6′, and H-6′′), 7.84–7.92 (m, 2H, H-6, and H-7), 8.01 (m, 2H, H-5, and H-8). 13C NMR (101 MHz, DMSO-d6) δ 55.39 (OCH3), 95.19 (C-3′, C-3′′, C-6′ and C-6′′), 95.24 (C-4′ and C-4′′), 125.95 (C-5 and C-8), 131.17 (C-4a and C-8a), 134.15 (C-6 and C-7), 145.86 (C-2 and C-3), 158.39 (C-1′ and C-1′′), 160.97 (C-2′, C-2′′, C-5′ and C-5′′), 180.05 (C-1 and C-4).
2,3-Bis(2,4-dimethylphenoxy)-1,4-dihydronaphthalene-1,4-dione (4b)
Yield: 65%, mp 117 °C, FT IR (υ cm−1): 2919 (CH sp2, aromatic), 1667, (C=O). 1H NMR (400 MHz, DMSO-d6) δ 1.97 (s, 6H, Ar-CH3), 2.18 (s, 6H, Ar-CH3), 6.80 (dd, J = 8, 0.8 Hz, 2H, H-5′ and H-5′′), 6.89 (d, J = 8.2 Hz, 2H, H-6′ and H-6′′), 6.93 (d, J = 0.8 Hz, 2H, H-3′ and H-3′′), 7.88 (br s, 2H, H-6 and H-7), 8.00 (br s, 2H, H-5 and H-8). 13C NMR (101 MHz, DMSO) δ 15.36 (Ar-CH3), 20.10 (Ar-CH3), 115.08 (C-6′ and C-6′′), 125.85 (C-5 and C-8), 125.97 (C-4′ and C-4′′), 126.94 (C-5′ and C-5′′), 130.85 (C-4a and C-8a), 131.22 (C-3′ and C-3′′), 131.82 (C-1′ and C-1′′), 134.23 (C-6 and C-7), 145.91 (C-2 and C-3), 153.00 (C-2′ and C-2′′), 180.26 (C-1 and C-4).
Biological activity in vitro on epimastigotes
T. cruzi Tulahuén 2, NINOA, and INC-5strain epimastigotes were grown at 28 °C in an axenic medium (BHI), as previously described, complemented with 5% fetal bovine serum. Epimastigotes from a 10-day-old culture (stationary phase) were inoculated into 50 mL of fresh culture medium to reach an initial concentration 1 × 106. Cell growth was monitored by measuring the absorbance of the culture at 600 nm in an Elisa Epoch reader (Epoch 2 Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) every day. Before inoculation, the media was supplemented with a given amount of the drug from a stock solution in DMSO (25 mM). The final DMSO concentration in the culture medium never exceeded 0.4% and the control was run in the presence of 0.4% DMSO and in the absence of drugs. The percentage of growth inhibition (% GI) and IC50 values (50% inhibitory concentrations), and parasite growth was followed in the absence (control), and in the presence, of a range of concentrations of the corresponding drug. On day 5, the absorbance of the culture was measured and related to the control. The IC50 value was determined as the concentration of drug needed to reduce the absorbance ratio by 50%. The experiments of each compound were carried out in triplicate and Nfx was used as reference compound (Álvarez et al. 2014).
Ex vivo biological activity towards T. cruzi trypomastigotes
CD1 female mice, 6–8 weeks old, were infected with T. cruzi bloodstream trypomastigotes of INC-5 and NINOA strains. The course of infection continued for 4–6 weeks. At the peak of parasitemia, blood was obtained by cardiac puncture using sodium heparin as an anticoagulant. The blood was adjusted to 1 × 106 bloodstream trypomastigotes/mL. In each well of the 96-well plate, 90 µL of infected blood and 10 µL of the new aryloxy 1,4-naphthoquinone or reference drug dilutions were seeded with each well reaching a final volume of 100 µL. Each assay was performed in triplicate. Initially, all compounds were tested at 50 µg/mL. Afterwards, the compounds with a lysis percentage >50 were tested at four concentrations, 50 µM, 20 µM, 10 µM, and 5 µM, to obtain a lysis concentration of 50% (LC50). As a negative control of lysis, wells with untreated blood trypomastigotes were used, and as a positive control, wells with the reference drugs were used (Nfx and Bnz). The microplates were incubated at 4 °C for 24 h. Bloodstream trypomastigotes were quantified by the Brener-Pizzi method, 5 µL of blood was placed on a slide and covered with a coverslip 18 × 18 mm. The mobile protozoa were counted in 15 fields at ×40 magnification using an optical microscope. The percentage lysis was determined by comparing the remaining trypomastigotes against the negative control. LC50 was determined with the Probit statistical tool (Chacón-Vargas et al. 2017).
Measurement of cell cytotoxicity
Cell cytotoxicity was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay for the evaluation of mitochondrial function (Mosmann 1983). In a 96-well plate for cell culture, 5 × 104 of the J774.1 cell line of murine macrophages (ATCC, USA) was incubated at 37 °C with 4% CO2 for 24 h. Later, the synthesized compounds were added at decreasing concentrations from 100 to 1.25 µM, with three independent repetitions. Removing the previously used culture medium and adding 5 µL of the dilutions of the compounds, the incubation of the macrophages along with the compounds was allowed for 24 h. After incubation, the treatment was removed and washed three times with PBS to ensure the evacuation of the compounds and dead cells. Then, 50 μL (5 mg/mL) of MTT (Sigma-Aldrich) and 200 μL of DMEM free of phenol red (Sigma-Aldrich) were added, leaving it to act for 4 h at 37 °C; at that time, the MTT and DMEM were removed and replaced by 200 μL DMSO (Sigma-Aldrich) to dissolve the formazan crystals, incubating for 30 min. The plate reading was at a wavelength of 570 nm in an Elisa Epoch reader (Epoch 2 Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA)).
Molecular docking
Molecular docking studies were performed using AutoDockVina 1.1.2 (Trott and Olson 2010). The crystal structure of TcTR bound to the inhibitor quinacrine mustard (PDB ID: 1GXF) was obtained from the Protein Data Bank (PDB). Next, the protein structure was extracted from the crystal, missing side chains were repaired, and all hydrogens were added with the Dock Prep tool of UCSF Chimera (Morris et al. 2009). Then, the script prepare_receptor4.py from MGTools 1.5.6 (Morris et al. 2009) was used to add AutoDock atom types and the charge to the prepared protein structure. Six aryloxy-naphthoquinones (Fig. 4) were docked in the coordinates: X = 17.733, Y = 13.499, and Z = −1.197, according to the binding site of quinacrine mustard in the crystal complex (PDB ID: 1GXF). Before performing the docking, aryloxy-naphthoquinone structures were drawn using MarvinSketch (MarvinSketch. 2014) and were minimized with the Open Babel tool (O’Boyle et al. 2011). In addition, the MGTools script, prepare_ligand4.py was employed to assign charge and atom types from Autodock to each ligand structure.
References
Álvarez G, Varela J, Márquez P, Gabay M, Arias Rivas CE, Cuchilla K, Echeverría GA, Piro OE, Chorilli M, Leal SM, Escobar P, Serna E, Torres S, Yaluff NIG, Vera de Bilbao, González M, Cerecetto H (2014) Optimization of antitrypanosomatid agents: identification of nonmutagenic drug candidates with in vivo activity. J Med Chem 57:3984–3999
Bolognesi ML, Lizzi F, Perozzo R, Brunc R, Cavallia A (2008) Synthesis of a small library of 2-phenoxy-1,4-naphthoquinone and 2-phenoxy-1,4-anthraquinone derivatives bearing anti-trypanosomal and anti-leishmanial activity. Bioorg Med Chem Lett 18:2272–2276
Bombaca AC, Viana PG, Santos ACC, Silva TL, Rodrigues ABM, Guimaraes ACR, Goulart MOF, Da Silva EN,Jr, Menna-Barreto RFS(2019) Mitochondrial dysfunction and ROS production are essential for anti- Trypanosoma cruzi activity of β-lapachone-derived naphthoimidazoles Free Radic Biol Med 130:408–418
Chacón-Vargas KF, Nogueda-Torres B, Sánchez-Torres LE, Erick Suarez-Contreras E, Villalobos-Rocha JC, Torres-Martinez Y, Lara-Ramirez EE, Giulia F, Krauth-Siegel RL, Bolognesi ML, Monge A, Rivera G (2017) Trypanocidal activity of quinoxaline 1,4-di-N-oxide derivatives as Trypanothione Reductase inhibitors. Molecules 22:220–237
Da Silva Jr EN, Jardim Guilherme AM, Menna-Barreto Rubem FS, De Castro Solange L (2014) Anti-Trypanosoma cruzi compounds: our contribution for the evaluation and insights on the mode of action of naphthoquinones and derivatives. J Braz Chem Soc 25:1780–1798
De Souza W (2014) Trypanosoma cruzi-host cell interaction. Front Immunol 5:1–2
Galaviz-Silva L, Mercado-Hernández R, Zárate-Ramos JJ, Molina-Garza ZJ (2017) Prevalence of Trypanosoma cruzi infection in dogs and small mammals in Nuevo León, México. Rev Argent Microbiol 49:216–223
Gómez-Hernández C, Rezende-Oliveira K, Nogueira Nascentes GA, Rocha Batista L, Borges Kappel H, Martinez-Ibarra JA, Trujillo Contreras F, Lages-Silva E, Ramírez LE (2011) Molecular characterization of Trypanosoma cruzi Mexican strains and their behavior in the mouse experimental model. Rev da Soc Brasileira de Med Trop 44:684–690
Heitmann GI, Jercic LMI, Jofré ML, Muñoz C, del VP, Noemí HI, San Martín VAM, Sapunar PJ, Torres HM, Zulantay AI (2008) Chagas disease in immune compromised patients. Rev Chil Infectol 25:289–292
Jurado J, Pueyo C (1995) Role of classical nitroreductase and O-acetyltransferase on the mutagenicity of nifurtimox and eight derivatives in Salmonella typhimurium. Environ Mol Mut 26:86–93
León-Pérez F, Gómez-Garcia L, Alejandre-Aguilar R, López R, Monteón VM (2007) Mexican Trypanosoma cruzi isolates: in vitro susceptibility of epimastigotes to anti-Trypanosoma cruzi drugs and metacyclic forms to complement-mediated lysis. Vector Borne Zoonotic 7:330–336
Marques Pereira PC, Navarro EC (2013) Challenges and perspectives of Chagas disease. J Venom Anim Toxins incl Trop Dis 19:1–17
MarvinSketch (version 6.2.2) (2014) Calculation module developed by ChemAxon, http://www.chemaxon.com/products/marvin/marvinsketch/.
Melo ME, Ferreira LC (1990) Screening the mutagenic activities of commonly used antiparasitic drugs by the Simultest, a simplified Salmonella/microsome plate incorporation assay. Rev do Inst de Med Trop de Sao Paulo 32:269–274
Moraes DC, Curvelo JAR, Anjos CA, Moura KCG, Pinto MCFR, Portela MB, Soares RMA (2018) β-lapachone and a-nor-lapachone modulate Candida albicans viability and virulence factors. J Myc Méd 28:314–319
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp Chem 16:2785–2791
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63
Nwaka S, Hudson A (2006) Innovative lead discovery strategies for typical diseases. Nat Rev Drug Discov 5:941–955
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Chem Inform 3:1–14
Persch E, Bryson S, Todoroff NK, Eberle C, Thelemann J, Dirdjaja N, Kaiser M, Weber M, Derbani H, Brun R, Schneider G, Pai EF, Krauth-Siegel RL, Diederich F (2014) Binding to large enzyme pockets: small-molecule inhibitors of trypanothione reductase. ChemMedChem 9:1880–1891
Pieretti S, Haanstra JR, Mazet M, Perozzo R, Bergamini C, Prati F, Fato R, Lenaz G, Capranico G, Brun R, Bakker BM, Michels PAM, Scapozza L, Bolognesi ML, Cavalli A (2013) Naphthoquinone derivatives exert their antitrypanosomal activity via a multi-target mechanism. PLoS Negl Trop Dis 7:1–12
Pinto AV, De Castro SL (2009) The trypanocidal activity of naphthoquinones: a review. Molecules 14:4570–4590
Romanha AJ, Castro SL, Soeiro M, de N, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave WA, de Andrade Z (2010) In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem do Inst Oswaldo Cruz 105:233–238
Salas C, Tapia Apati R, Ciudad K, Armstrong V, Orellana M, Kemmerling U, Ferreira J, Maya DJ, Morello A (2008) Trypanosoma cruzi: activities of lapachol and α- and β-lapachone derivatives against epimastigote and trypomastigote forms. Bioorg Med Chem 16:668–674
Salmon-Chemin L, Lemaire A, De Freitas S, Deprez B, Sergheraert C, Davioud-Charvet E (2000) Parallel synthesis of a library of 1,4-naphthoquinones and automated screening of potential inhibitors of trypanothione reductase from Trypanosoma cruzi. Bioorg Med Chem Lett 10:631–635
Tapia Apati R, Salas CO, Vázquez K, Espinosa-Bustos C, Soto-Delgado J, Varela J, Birriel E, Cerecetto H, González M, Paulino M (2014) Synthesis and biological characterization of newaryloxyindole-4,9-diones as potent trypanosomicidal agents. Bioorg Med Chem Lett 24:3919–3922
Trott O, Olson AJ (2010) AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comp Chem 31:455–461
Vázquez K, Espinosa-Bustos C, Soto-Delgado J, Tapia Apati R, Varela J, Birriel E, Segura R, Pizarro J, Cerecetto H, González M, Paulino M, Salas CO (2015) New aryloxy-quinone derivatives as potential anti-Chagasic agents: synthesis, trypanosomicidal activity, electrochemical properties, pharmacophore elucidation and 3D-QSAR analysis. RSC Adv 5:65153–65166
Acknowledgements
We wish to express our gratitude to the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-20190295) for their financial support. Gildardo Rivera and Benjamín Nogueda-Torres hold a scholarship from the “Comisión de Operación y Fomento de Actividades Académicas” (COFAA-IPN) and the “Programa de Estímulos al Desempeño de los Investigadores” (EDI-IPN). Karina Vázquez thanks to the Proyecto “Programa para el Desarrollo Profesional Docente, para el Tipo Superior (PRODEP) DSA/103.5/16/10510 for their financial support and Professor Jose Carlos Espinoza Hicks of the Universidad Autonoma de Chihuahua.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
González, A., Becerra, N., Kashif, M. et al. In vitro and in silico evaluations of new aryloxy-1,4-naphthoquinones as anti-Trypanosoma cruzi agents. Med Chem Res 29, 665–674 (2020). https://doi.org/10.1007/s00044-020-02512-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02512-9