Abstract
DFT calculations were performed on nine flavones and flavonols to explain their high antioxidant activity and variations in their activity. Conformational analysis showed that only flavonols with 3-OH directed toward the B-ring are nonplanar; however, flavones and resulted radicals are planar. Hydroxyl group eligible for dissociation is the one with ortho OH directed toward it; otherwise, one of B-ring (in 2′ or 4′-position) hydroxyl group. There are two main factors responsible for stabilizing the resulted radicals and lowering the bond dissociation energy and hence there were found well correlated with the experimental activity. First, driving force resulted from the conversion of nonplanar flavonols to planar radicals accompanied by resonance toward the carbonyl group and H-bond formation with 3-OH and second, radical stabilization by H-bond with ortho hydroxyl group with resonance toward carbonyl or pyrone oxygen. All resonance and H-bonds were confirmed by spin density, bond length, and molecular orbital calculations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoids are well known to be widely distributed in many fruits and vegetables. They reached more than 4000 flavonoids and constitutes about half of the polyphenols found in nature (Ignat et al. 2011). They showed various biological activities including anti-inflammatory, oestrogenic, microbial, and allergic activities in addition to protection against many chronic diseases e.g., atherosclerosis, diabetes, cancer, and Alzheimer, as well as inhibition of lipid peroxidation and DNA damage; these diverse activities are believed to be attributed to their high antioxidant activity (Panche et al. 2016; Verma and Pratap 2010; Heim et al. 2002; Rice-Evans 2001; Rice-Evans et al. 1996), where some flavonols e.g., quercetin and myricetin were found to have higher antioxidant activity than vitamin E (Hopia and Heinonen 1999; Rice-Evans et al. 1995) and some flavonoids have high redox potential comparable with trolox (Jovanovic et al. 1994), a known commercial antioxidant with high antioxidant activity.
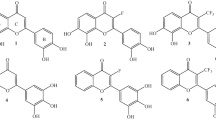
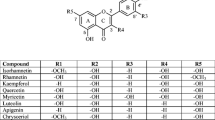
Flavones and flavonoids share similar structure of 2-phenyl benzopyrone system (C6–C3–C6), which is characterized by having three aromatic rings, two of them (benzopyrone system) are fused (rings A and C), i.e., planar system, while the third ring (B) is attached at C2 by a single bond allowing a possible rotation. Flavonols differ from flavones only in having more a 3-OH group. The antioxidant activity of these compounds is mainly attributed to the number of hydroxyl groups and presence of 3-OH group, 2,3-double bond conjugated to the carbonyl group and catecholic moiety on either ring A or B (Verma and Pratap 2010; Seyoum et al. 2006; Cai et al. 2006). However, there is still in the literature much controversy concerning conformational analysis of these molecules e.g., the conformation of the hydroxyl groups and the coplanarity of the B-ring (Cai et al. 2014; Aparicio 2010; Russo et al. 2000; Cornard et al. 1997); in addition, correlation between the structural features of these compounds with their mechanisms and antioxidant activity, SAR or QSAR, are still not clear. Therefore, the aim of this study was to get better insight on the origin and mechanism of the high antioxidant activity of flavones and flavonols (Table 1); therefore, conformation analysis and various radical stabilization resonance mechanisms in addition to correlation of these conformations and mechanisms with the experimental antioxidant activity reported in the literature were examined. Variations in the antioxidant activity of the examined compounds were rationalized in accordance with the mentioned analysis and mechanisms using DFT calculations. Quantitative structure-activity relationship (QSAR) of the experimental antioxidant activity was also formulated.
Computational methods
DFT calculations
Gaussian 09 program was used in all DFT calculations at the level of DFT/B3LYP with a basis set 6-31-G (d,p), while CambridgeSoft Chem 3D program of ChemOffice 12 software was used for model visualization and finding the bond lengths and the dihedral angles between the benzopyrone ring system and each of the 3-O–H bond (ϕ1 C2–C3–O3–H3) and 2-phenyl plane (ϕ2 O1–C2–C1′–C2′). Radicals were generated by removing a hydrogen atom from the desired OH group. Calculations of flavones and flavonols were executed at a restricted closed shell level, while calculations of radicals were performed at an unrestricted open shell level in the gas-phase at temperature 298.15 K and pressure 1 atm. For all species, the energy was minimized and the lowest conformation was used in all calculations without any geometry or symmetry constraints and harmonic vibrational frequencies were computed to characterize saddle points and the zero-point energies for thermochemical correction of electronic thermal enthalpies. Spin density was calculated by frequency calculation and placed on the models; spin densities of hydrogen atoms are summed into those of heavy atoms where the sum of spin densities of all atoms is one.
The bond dissociation energy of all hydroxyl groups in molecules (BDEO–H) was calculated as the difference between the electronic thermal enthalpy of the parent compound (Hp) and the sum of the electronic thermal enthalpy of resulted radical (HR) and hydrogen atom (HH) according to the following equation:
Where hydrogen atom enthalpy (HH) was −0.4979 Hartree, correction factor suggested by the program manual to eliminate systematic error in thermal energies of frequency calculations is 0.9804 and the conversion factor from Hartree to kcal/mol unit is 627.51.
Statistical analysis
Regression analyses were executed by SPSS program (version 16). QSAR equations were validated by Pearson correlation coefficient (R), standard error of the estimate (SE), the number of data point (N), the least significant difference (p), and the 95% confidence intervals (in parentheses) for each regression coefficient.
Results and discussion
Conformational analysis and structural features
Results presented in Table 2 and Figs 1 and 2 indicated that the presence and direction of the 3-OH group defined by the dihedral angle (ϕ1 C2–C3–O3–H) along with substitutions on the B-ring determine the coplanarity of the B-ring with the benzopyrone rings measured by the dihedral angle (ϕ2 O1–C2–C1′–C2′). All the examined flavones (absence of 3-OH) were found planar. On the other hand, in flavonol, the 3-OH can be directed toward the B-ring or pointed to the carbonyl group. Flavonols with 3-OH directed toward the B-ring (Km, Qu) or having 2′-OH (Mo) are nonplanar with dihedral angle (ϕ2) −33.9°, −33.7°, and 38.6°, respectively, while those with the 3-OH directed away from the B-ring (toward the carbonyl group) and having no 2′-OH are planar (Ga, My); the effect of 3-OH conformation on the coplanarity of the B-ring was previously observed for anthocyanidins (Ali and Ali 2017). The nonplanarity of Mo despite its 3-OH is directed away from the B-ring can be attributed to the repulsion between the 2′-OH and pyrone oxygen (O1) atoms. In all examined compounds and their radicals, the 5-OH is directed away from the carbonyl group i.e., toward H6, while the 7-OH is pointed to H8. Radicals also follow the same rules, where the 3-OH in all flavonol radicals are directed toward the carbonyl group even in Km and Qu, which have 3-OH directed toward the B-ring, allowing complete planarity of the radicals except for Mo, which still has the repulsion caused by the 2′-oxygen atom, is nonplanar but moved toward planarity from dihedral angle (ϕ2) 38.6° in parent compound to only 8.5° in 2′-radical (Fig. 2). Flavone radicals, as expected, are also planar. These results explain also the formation of 2′-radical rather than 4′-radical in Mo since the latter radical has lower planarity with dihedral angle (ϕ2) 23.1° compared with 8.5° in the former radical. Moving toward planarity in Km, Qu, and Mo radicals is also manifested by increasing the π-bond characters of C2–C1′ bond, where its bond length decreased from 1.472, 1.473, and 1.468 Å in parent flavonols to 1.440, 1.446, and 1.443 Å in their radicals, respectively. It is well known that planar structure maximizes the p-orbital overlap and thus enhances the stability of the resulted radicals (Aparicio 2010; Leopoldini et al. 2011).
Each of luteolin, quercetin, and morin has two conformers because of the rotation of B ring 180°. The two conformers of luteolin and quercetin have energy deference (ΔG) < 0.4 kcal/mol indicating that both conformers of each compound are practically energetically similar; similar result for luteolin was obtained by Leopoldini et al. (2004). However, the more stable conformer of morin has less energy by 1.17 kcal/mol (Fig. 1). Energy profile generated by AM1 calculations showed also that Qu is not planar and has two minimum energy at dihedral angle 27° and 153° with maximum energy at 90° (Russo et al. 2000; Cornard et al. 1997); on the other hand, energy profile produced by density function calculation for My showed minimum energy at 0° (planar) with maximum energy at 90° (Sadasivama and Kumaresanb 2011); Ap and Lu showed also planarity at the same level of calculation (Leopoldini et al. 2004). On the other hand, previous DFT calculations showed nonplanarity of flavones with dihedral angle ~20° (Aparicio 2010) and planarity of flavonols (Aparicio 2010) e.g., Qu (Cai et al. 2014). The controversy between the planar and nonplanar structure of Qu is attributed to the low energy difference (0.5 kcal/mol) between the two conformers (Russo et al. 2000) along with using different levels of calculations, basis sets, and used software. This little difference accounts also for the small dihedral angle (5–7°) shown by X-ray crystallography for the compact crystal state of Qu (Jin et al. 1990; Rossi et al. 1986).
Bond dissociation energy and radical stabilization
Bond dissociation energy of all hydroxyl groups in each compound is listed in Table 2 in ascending order. The first BDE varies from 57.58 (Qu) to 83.73 (Ch) kcal/mol and accounts for the observed antioxidant activity variations as will be discussed in Section 3.3. The lowest BDE and the stable phenoxyl radical in each flavone and flavanol are determined by the presence of a catechol structure in either ring A or B and the presence of hydroxyl substituents (4′ or 2′) on B-ring. If there is a catechol structure (Ba, Lu, Qu, and My), the hydroxyl group with ortho OH directed toward it showed the lowest BDE i.e., 6-OH in Ba and 4′-OH in Lu, Qu, and My. In the absence of catecholic moiety, a hydroxyl group on B-ring in 4′ (Ap and Km) or 2′ (Mo) position is dehydrogenated to form a radical stabilized by resonance flows toward the carbonyl group through forming a double bond on C2–C1′ bond, indicated by shortening the bond length, to support a planar conformation. In the absence of both factors, the lowest BDE is observed for 3-OH (Ga) or 7-OH (Ch) bond, where the formed radical is stabilized by resonance toward pyrone oxygen (Ga) or the carbonyl group (Ch). Radicals formed on 7-OH (Ch), 4′-OH (Ap, Lu, Km, Qu, My) and 2′-OH (Mo) undergo resonance toward the carbonyl group, while 6- and 3-radicals in Ba and Ga, respectively, resonate toward the pyrone oxygen. Stabilizing resonances of all radicals are indicated by spin density distribution and HOMO as well as bond length and dihedral angle (ϕ2) as illustrated in Figs 1, 2, and S1. 6-Ba radical, for example, as presented in Fig. 1, shows spin density distribution on alternative atoms in rings A and C including the pyrone oxygen; HOMO of the radical which is expected to carry the unpaired electron covers also the same atoms confirming resonance towards the pyrone oxygen. On the other hand, Lu radical shows the spin distribution and HOMO cover rings B and C in the direction of the carbonyl group. In addition, in both radicals, the radical oxygen forms a double bond with a neighboring carbon atom as indicated by shortening the C-O bond from 1.37 Å in parent compound to 1.26 and 1.25 Å in their radicals, respectively, confirming the initiation of the resonance; while the C2–C1′ bond shortened only in Lu radical.
Catecholic radicals (e.g., Ba, Lu, and Qu) are also stabilized by forming a H-bonding with the ortho hydroxyl group with bond length 1.94, 1.99, and 1.98 Å, respectively, which are shortened, i.e., strengthened, in radicals than in parent compounds (Figs 1, 2). The role of H-bonding in stabilizing catechol radicals than it does in parent catechols was previously suggested (Ali and Ali 2018, 2015; Zhang et al. 2003; Foti et al. 2002). In addition, in flavonol radicals where the resonance flows toward the carbonyl group and there is spin residue on the carbonyl oxygen (Km, Mo, Qu, and My), there is another H-bond is formed between the 3-OH and carbonyl oxygen since the 3-OH is directed in all radicals toward the carbonyl group. Interestingly, even in flavonols with 3-OH directed to the B-ring in parent flavonols (Km, Qu), the 3-OH in their 4′-radical is redirected toward the carbonyl group to enhance radical stabilization by H-bonding especially the 5-OH is directed toward H6 in all radicals and parent compounds and hence cannot form H-bond with the carbonyl group as depicted in Fig. 2, wherein Qu the 3-OH is pointed toward the B-ring, while in its 4′-radical the 3-OH converts its direction forming H-bond with carbonyl oxygen with bond length 1.85 Å; on the other hand, the 3-OH in parent Mo is already directed toward the carbonyl group forming H-bond (1.91 Å), which is shortened in its 4′-radical to 1.83 Å (Fig. 2). It was observed previously that the H-atom donation from hydroxyl groups on B-ring has a priority over those on rings A or C (Sadasivama and Kumaresanb 2011).
The discussed controversy of the conformational analysis of flavones and flavonols has also impact on the calculated BDE. Previous DFT calculations showed also that 4′-OH bond has the lowest BDE in Ap, Lu, Km, and Qu (Vagánek et al. 2012) as well as My (Sadasivama and Kumaresanb, 2011); similar results were obtained for Ap, Lu (Leopoldini et al. 2004), and Qu (Lespade and Bercion 2012). However, Cai et al. 2014 found using DFT that 3′-OH has the lowest BDE, while Seyoum et al. 2006 utilizing lower level of calculations showed that 3-OH in Ga, Km, Mo, and Qu has the lowest BDE.
SAR and QSAR
Literature antioxidant activity of the examined flavones and flavonols as expressed by TEAC value is listed in Table 2 (Cai et al. 2006). According to the hydrogen atom transfer (HAT) mechanism, the antioxidant activity of phenolic compounds depends mainly on lowering the BDE of the hydroxyl group and the stability of the resulted radical upon dissociation. Radical stabilization can be modeled by the spin density on the phenoxyl oxygen where the lower spin density, the more delocalization of the unpaired electron, and the better stability. Therefore, the experimental antioxidant activity was found fairly correlating with either BDE or spin density as presented by Eqs. 1 and 2, respectively.
R = 0.795, n = 9, SE = 0.89, p = 0.005.
R = 0.677, n = 9, SE = 1.08, p = 0.023.
The two other mechanisms that are also frequently used, but at less extent, to explain the antioxidant activity of phenolic and flavonoid compounds are single electron transfer-proton transfer and sequential proton loss-electron transfer (SPLET) mechanisms; the two mechanisms are best modeled by the ionization potential (IP) and the electron transfer enthalpy (ETE), respectively (Chen et al. 2015; Lespade and Bercion 2012). In contrary to BDE, IP and ETE are sensitive to solvation and thus were calculated in water and reported previously for the examined compounds, except Ba (Lespade and Bercion 2012); however, stepwise regression analyses of each of IP and ETE with TEAC value (Table 2) at the confidence level 95% were found not correlated which gives more support for the HAT mechanism. It was reported that most polyphenols act through HAT mechanism since electron transfer generally requires higher energy (Leopoldini et al. 2011). SPLET mechanism becomes more important at the alkaline condition where the polyphenols present mainly in the anionic form (Lespade and Bercion 2012).
According to the conformational analysis and radical stabilization discussed above, it can be concluded that there are two processes that can enhance the antioxidant activity of flavones and flavonols; first process, conversion of nonplanar parent compound (Km, Qu, and Mo) to a planar radical with resonance through the carbonyl group and redirection of the 3-OH from the direction of the B-ring to toward the carbonyl group (Km, Qu) or removing the steric effect of 2′-OH by dehydrogenation (Mo), in addition to enhancing double bond character of C2–C1′ bond and forming a H-bond between the 3-OH and carbonyl oxygen. This planarity stabilization process provides a good driving force for the hydrogen atom donation and antioxidant activity; it is donated by (Pl) factor. The second process, stabilization of the formed radical on either ring A or B by a hydrogen bonding formation with an ortho hydroxyl group (catecholic structure); the catechol H-bond formation is abbreviated by (Cat) factor.
The relative stability provided by each process can be obtained by calculating the difference in free energy (ΔG) of hydrogen bond dissociation of two isomers where only one of them provides a certain stabilization process. Qu and Mo are positional isomers differ only in the position of the second hydroxyl group on ring B (3′- or 2′-, respectively). Both isomers possess the planarity factor (Pl) but Qu possesses also the catecholic factor (Cat). Therefore, the difference between their dissociation free energy, 50.078 and 71.241 kcal/mol, respectively (Table 2), yields catechol stabilization energy (ΔGCat = −21.164 kcal/mol). Km and Lu are also positional isomers; Km is nonplanar and possesses the planarity factor, while the Lu is planar but possesses the catecholic factor. Hence, using the following equation and values in Table 2 yields planarity stabilization energy (ΔGPl).
where ΔGKm and ΔGLu are the dissociation free energy of Km and Lu, respectively.
It is consistently reported in the literature (Cai et al. 2006; Heim et al. 2002; Tabart et al. 2009) that My has lower antioxidant activity (TEAC value 1.31, 3.10, and 1.50 mM) than that of Qu (TEAC value 4.42, 4.70, and 1.80 mM), respectively, even though My has the same structure of Qu with even one more hydroxyl group in the 5′-position; however, no explanation was provided. The above discussion can account for the observed unexpected activities of My and Qu since both of them possess the catecholic stabilization factor but, as calculated, Qu has the 3-OH directed toward the B-ring and thus the molecule is not planar with dihedral angle (ϕ2′) 33.7° which gives upon dissociation the planar 4′-radical (dihedral angle 0.0°) providing also a driving force of the planarity factor (Pl) manifested by lower BDE (57.58 kcal/mol) and higher antioxidant activity (TEAC 4.42 mM). On the other hand, My, despite having more hydroxyl group, is planar, the 3-OH pointed to the carbonyl group (Fig. 2), and hence lacks the Pl which rationalizes its higher BDE (70.20 kcal/mol) and lower activity (TEAC 3.10 mM). This observation indicates that the position and conformation of the O-H groups are more important in determining the antioxidant activity than their total number in the molecule.
Most reported QSAR studies on flavones and flavonols were performed generally on flavonoids and phenolic compounds and used general variables as lipophilicity (Yang et al. 2001), oxidation potential (Yang et al. 2001; Heijnen et al. 2002), structural features i.e., number of hydroxyl groups and presence of 2,3-double bond or catechol moiety (Amić et al. 2003, Lien et al. 1999), molar volume, dipole moment, electron density (Srivastava et al. 2012), HOMO, LUMO (Rasulev et al. 2005, Lien et al. 1999), heat of formation (Lien et al. 1999), topological, and connectivity indices (Farkas et al. 2004) and 3D (CoMFA) properties (Lumbiny et al. 2013) rather than depending on mechanisms and conformational analysis. Since, the experimental antioxidant activity was found dependent mainly on the above mentioned two processes, namely conversion toward planarity (Pl) and stabilization of the resulted radical by ortho hydroxyl group (Cat), the experimental antioxidant activity as expressed by TEAC value was found correlated with the sum of free energy of these two factors (ΔGPl and ΔGCat) as presented by Eq. 3.
Indicating that the sum of both factors explains 84.8% of the variations in the observed antioxidant activity. In addition, BDE was found well correlated with the sum of the two factors and hence they are the main source of radical stabilization as expressed by the following correlation (4).
The obtained good correlations not only supports the importance of the suggested factors (Pl and Cat) in determining radical stabilization and the antioxidant activity of flavones and flavonols but also validates our conformational analysis and radical stabilization calculations.
Conclusion
Conformational analysis of flavones and flavonols showed that flavonols with 3-OH directed toward the B-ring (Km, Qu, and Mo) are not planar, while those with 3-OH directed toward the carbonyl group (Ga and My) in addition to all examined flavones and their resulted radicals are planar. DFT calculations indicated that the hydroxyl group with lowest BDE is determined as follows: if there is a catechol structure (Ba, Lu, Qu, and My), the hydroxyl group with ortho OH directed toward it showed the lowest BDE i.e., 6-OH in Ba and 4′-OH in Lu, Qu, and My; otherwise, a hydroxyl substituents on B-ring (in 2′ or 4′-position) is dissociated. Radical stabilization resonance takes place toward either the carbonyl group or the pyrone oxygen. Experimental antioxidant activity was found consistent with our results where activity was well correlated with the driving forces resulted from, first, conversion from nonplanar conformation of parent compound to a planar radical (Km, Qu, and Mo) with H-bond formation between carbonyl and 3-OH groups and, second, an H-bond formation between catecholic radical and neighboring hydroxyl group.
References
Ali HM, Ali IH (2015) QSAR and mechanisms of radical scavenging activity of phenolic and anilinic compounds using structural, electronic, kinetic, and thermodynamic parameters. Med Chem Res 24:987–998. https://doi.org/10.1007/s00044-014-1174-y
Ali HM, Ali IH (2017) A DFT and QSAR study of the role of 3-, 5-, 7- and 4’-hydroxyl groups in charge and unpaired-electron resonance of anthocyanidins and their radicals. Med Chem Res 26:2666–2674. https://doi.org/10.1007/s00044-017-1964-0
Ali HM, Ali IH (2018) Energetic and electronic computation of the two-hydrogen atom donation process in catecholic and non-catecholic anthocyanidins. Food Chem 243:145–150. https://doi.org/10.1016/j.foodchem.2017.09.120
Amić D, Davidović -Amić D, Beṧlo D, Trinajstić N (2003) Structure-radical scavenging activity relationships of flavonoids. Croatica Chem Acta 76:55–61
Aparicio S (2010) A systematic computational study on flavonoids. Int J Mol Sci 11:2017–2038
Cai W, Chen Y, Xie L, Zhang H, Hou C (2014) Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur Food Res Technol 238:121–128
Cai Y-Z, Sun M, Xing J, Luo Q, Corke H (2006) Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci 78:2872–2888
Chen Y, Xiao H, Zheng J, Liang G (2015) Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: an experimental and theoretical evaluation. PLoS ONE 10:e0121276. https://doi.org/10.1371/journal.pone.0121276
Cornard JP, Merlin JC, Boudet AC, Vrielynck L (1997) Structural study of quercetin by vibrational and electronic spectroscopies combined with semiempirical calculations. Biospectroscopy 3:183–193
Farkas O, Jakus J, Héberger K (2004) Quantitative structure—antioxidant activity relationships of flavonoid compounds. Molecules 9:1079–1088
Foti MC, Barclay LRC, Ingold KU (2002) The role of hydrogen bonding on the H-atom-donating abilities of catechols and naphthalene diols and on a previously overlooked aspect of their infrared spectra. J Am Chem Soc 124:12881–12888
Heijnen CGM, GRMM Haenen, Oostveen RM, Stalpers EM, Bast A (2002) Protection of flavonoids against lipid peroxidation: the structure activity relationship revisited. Free Radic Res 36:575–581
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Hopia A, Heinonen M (1999) Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. J Am Oil Chem Soc 76:139–144
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Jin GZ, Yamagata Y, Tomita KI (1990) Structure of quercetin dihydrate. Acta Crystallogr C 46:310–313
Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG (1994) Flavonoids as antioxidants. J Am Chem Soc 116:4846–4851
Lien EJ, Ren S, Bui H-H, Wang R (1999) Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic Biol Med 26:285–294
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem A 108:92–96
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306
Lespade L, Bercion S (2012) Theoretical investigation of the effect of sugar substitution on the antioxidant properties of flavonoids. Free Radic Res 46:346–358
Lumbiny BJ, Hui Z, Islam MA (2013) Antiaging, antioxidant flavonoids; synthesis, antimicrobial screening as well as 3D QSAR CoMFA models for the prediction of biological activity. J Asiat Soc Bangladesh 39:191–199
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5. https://doi.org/10.1017/jns.2016.41
Rasulev BF, Abdullaevb ND, Syrovb VN, Leszczynski J (2005) A quantitative structure-activity relationship (qsar) study of the antioxidant activity of flavonoids. QSAR Comb Sci 24:1056–1065
Rice-Evans C (2001) Flavonoid antioxidant. Curr Med Chem 8:797–807
Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 22:375–383
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rossi M, Rickles LF, Halpin WA (1986) The crystal and molecular structure of quercetin: a biologically active and naturally occurring flavonoid. Bioorg Chem 14:55–69
Russo N, Toscano M, Uccella N (2000) Semiempirical molecular modeling into quercetin reactive site: structural, conformational, and electronic features. J Agric Food Chem 48:3232–3237
Sadasivama K, Kumaresanb R (2011) Antioxidant behavior of mearnsetin and myricetin flavonoid compounds—A DFT study. Spectrochim Acta Part A 79:282–293
Seyoum A, Asres K, El-Fiky FK (2006) Structure–radical scavenging activity relationships of flavonoids. Phytochemistry 67:2058–2070
Srivastava AK, Gupta R, Srivastava R, Mishra DK (2012) Application of electron density and molar volume as descriptors in qsar study of the antioxidant activity of flavonoids. Int J Pharm Sci Res 3:2648–2654
Tabart J, Kevers C, Pincemail J, Defraigne J-O, Dommes J (2009) Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem 113:1226–1233
Vagánek A, Rimarčík j, Lukeš V, Klein E (2012) On the energetics of homolytic and heterolytic OAH bond cleavage in flavonoids. Comput Theor Chem 991:192–200
Verma AK, Pratap R (2010) The biological potential of flavones. Nat Prod Rep 27:1571–1593
Yang B, Kotani A, Arai K, Kusu F (2001) Estemation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci 17:599–604
Zhang H-Y, Sun Y-M, Wang X-L (2003) Substituent effects on O-H bond dissociation enthalpies and ionization potentials of catechols: a DFT study and its implications in the rational design of phenolic antioxidants and elucidation of structure-activity relationships of phenolic antioxidants. Eur J Chem 9:502–508
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ali, H.M., Ali, I.H. Structure-antioxidant activity relationships, QSAR, DFT calculation, and mechanisms of flavones and flavonols. Med Chem Res 28, 2262–2269 (2019). https://doi.org/10.1007/s00044-019-02452-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02452-z