Abstract
JNK3, a protein kinase of the MAPK family that is potently activated by a variety of environmental stress and pro-inflammatory cytokines, has been recognized as an important therapeutic target for several neurodegenerative diseases. However, due to the long development cycle and the cost of R&D, no related drugs have been released. By applying the CoMFA and CoMSIA methods, QSAR models that explore the structure-activity relationship of the JNK3 inhibitors are established and validated, the parameters are satisfactory (q2 = 0.774, r2 = 0.991 for CoMFA model and q2 = 0.666, r2 = 0.990 for CoMSIA model) and base on which a series of novel molecules are designed. Molecular docking is conducted to verify the potential of the new compounds, the results shows promising activity. We speculated that the series of compounds S1–S11 might have some therapeutic activity in the c-jnk3 pathway, particularly the “S8” may prove to be the best one, which provided useful guidance for the design of new and efficient subtype selective JNK3 inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a member of the mitogen-activated protein kinase (MAPK) family, the c-Jun N-terminal kinases (JNKs) regulate the serine/threonine phosphorylation of several transcription factors when activated by various stimuli such as oxidative stress, neurotoxins, cytokines, and fatty acids (Zheng et al. 2014). JNK protein plays a dual regulatory role in cell apoptosis, and stimulation of different source types, background and subtypes of JNK will make JNK play a different role: to promote cell apoptosis or inhibit cell apoptosis (Gupta et al. 1996). There are three kinds of Human source JNK isoforms: JNK1, JNK2, and JNK3 (Chambers et al. 2013; Dérijard et al. 1994; Hibi et al. 1993; Mohit et al. 1995). JNK1 and JNK2 are omnipresence in most tissues. Recent studies showed that JNK1 and JNK2 play an important role in the development of diabetes, obesity, arthritis, cancer, and heart disease (Kyriakis et al. 1994). Inhibition of JNK1 involved in the development of obesity induced insulin resistance might be an effective way of treating type-2 diabetes. Recent studies have reported that inhibition of the abnormal activation of the JNK3 pathway can protect DA neurons and thus be used in the treatment of Parkinson’s disease (Chambers et al. 2011; Kamenecka et al. 2009). JNK can directly phosphorylate epithelial cadherin 1 (E-cadherin 1, CDH1) gene in the G2 period and early mitosis, change its subcellular localization, and activate the anaphase promoting complex (anaphase-promoting complex or cyclosome, APC/C) where APC/C is the ability (Cervigni et al. 2015; Gutierrez et al. 2010), which deduced that JNK is involved in the regulation of cell cycle by indirect interaction with CDH1. The selective expression of JNK3 primarily in the brain along with findings that JNK3 knockout mice exhibit amelioration in animal models of Parkinson and Alzheimer’s disease (Chambers et al. 2011), makes the inhibition of this isoform a particularly promising therapeutic target for these degenerative diseases.
Currently, the research on inhibitors of c-jnk3 series is not clear due to the uncertainty of clinical efficacy (Ansideri et al. 2018), long period of development, high risk of the development cycle and investment funds, and the research on the mechanism of key regulation and control channels is not clear yet (Muth et al. 2017; Rech et al. 2007; Schattel et al. 2011; Zhao et al. 2006; Kamenecka et al. 2009; Koch et al. 2014; Siddiqui and Reddy 2010). Therefore, development of JNK inhibitors as therapeutic agents has gained considerable interest over the past few years. Identifying novel and potent inhibitors of JNK3 may contribute toward not only neuroprotective therapies with reduced untoward side effect but also understanding the mechanism of the therapy. Some achievements have been obtained by researchers from many groups (Li et al. 2017; Qin et al. 2013; Lange et al. 2015) among which a series of aminopyrazole derivatives drew our attention (Zheng et al. 2014). We believed that with the information based on the previous study and the developing CADD technology, further research that leads to more potent compounds could be established. The novel simulation methods are more credible in studying the structure-activity relationships, which can greatly reduce time and cost consuming in the early stage, and significantly increase the efficiency (Sharma and Ghoshal 2006; Buckingham et al. 1995; Dorsey et al. 1994). Thus the QSAR and molecular docking are introduced into our work to help us get the insight into the inhibitors of c-jnk3.
Methodology
All molecular structure modeling and statistical analysis are performed on SYBYL-X 2.0 software (Tripos, Inc., St. Louis, MO, USA), and the default parameters are used in the following calculations unless otherwise specified.
Dataset
The compound structures and biological data are collected from the lectures (Zheng et al. 2014). The 3D structures of compounds are sketched using SYBYL-X, and conjugate gradient methods was applied when conducting the energy minimization under the conditions of a gradient convergence of 0.05 kcal in Gasteiger-Hückel charge. All these data forms are shown in Table 1. Compounds 22i, 26b, 26d, 8b, and 8c2 are selected as the test set randomly, and all other compounds constitute the training sets.
QSAR model
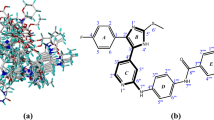
Molecular alignment is a key factor affecting CoMFA and CoMSIA model results. Because all compounds have a common structure of N-(6-methylpyridn-3-yl)-3-(4-(3-phenylureido)-1H-pyrazol-1-yl) benzamide, we treat it as the core substructure in the alignment. With the highest activity compound 26h used as template molecule, other 32 compounds are superimposed with an energy convergence criteria for 0.21 kJ/mol·A. The alignment of all compounds is shown in Fig. 1 where we can see all molecules are well overlapped.
A three-dimensional cube with the step size of 1 and 4 Å beyond the boundary of aligned compounds in every direction was introduced to calculate the descriptors of CoMFA and CoMSIA (Huang et al. 2001). As to the CoMFA (Srivastava et al. 2008), a probe (a sp3 C atom with 1e charge) is generated to compute both the electrostatic and steric fields, where the values of energy were cut under 30 kcal/mol (Xiao et al. 2008). As a CoMFA’s extension, CoMSIA method, with three more fields (hydrophobic, h-bond acceptor and h-bond donor), were calculated by an atomic probe with 1.0 Å radius and þ1.0 charge. Default value of attenuation factor of 0.3 was applied in the modeling (Klebe and Abraham 1999). The correlation of CoMFA and CoMSIA fields to the biodata were computed by the PLS (Partial least squares) method (Chambers et al. 2013). Leave-one-out analysis, where every molecule will be extracted from the database once and their biological value will be predicted based on the model derived from the rest of the compounds, was conducted to calculate the cross-validated q2. In order to further verify the prediction capacity, a parameter of the predictive correlation coefficient (r2pred) is calculated (Wang et al. 2016).
Docking analysis
Surflex-Dock in SYBYL-X was applied to study molecular docking in this work using a patented search engine and an empirical scoring function to dock ligands into a protein’s binding site (Jain 2003). The crystal structure of JNK3 was downloaded from the RCSB Protein Data Bank (PDB ID: 3TTI) (Krenitsky et al. 2012). After the preparation of the protein, our designed compounds are docked into the binding pocket. The total scores of Surflex-Dock representing binding affinities are expressed in the logarithm of the Kd value. The results were compared with the scores of current ligand to explore the credibility and validation of the model.
Results and discussion
CoMFA and CoMSIA results
The training set was constituted by 32 compounds with a wide range of pIC50 (5.00–8.00). The CoMFA obtained from the training set return us good parameters: the q2 (cross-validated correlation coefficient), with an ONC of 6, is 0.774 (>0.6); the r2 is 0.991 and STD is 0.064; F value and r2pred are also satisfactory as 373.511 and 0.995, respectively. The electrostatic field contributes 0.400 while steric fields contributes 0.600. As to CoMSIA, with three extra fields than CoMFA, several models were carried out, among which the best one consisted with four kinds of fields (steric, electrostatic, hydrophobic H-bond donor) gave us satisfactory results: the q2 of 0.666 (>0.6) and r2 of 0.990 with 6 optimized number of components; a good STD of 0.069, F value of 322.699, and r2pred of 0.996. The contributions of four fields were 0.194, 0.340, 0.321, and 0.145, respectively. The parameters of the models were shown in Table 2, and the statistics indicated that the CoMFA and CoMSIA models are capable in predicting the activities of the derivatives (Fig. 2).
Contour map analysis
CoMFA Contour map in Fig. 3 depicted the CoMFA steric and electrostatic contour plots for the most active compound 26h. The green and yellow blocks in Fig. 3a indicated steric favorable and unfavorable locations, respectively. Similarly, blue and red blocks in Fig. 3b, respectively, represented the electron donating and withdrawing sites.
As shown in Fig. 3a, the large green contour near R1 indicated where a bulky group would benefit to the activity, which can explain the higher activity of molecular 26g, 26h, 26i, and 26j than 26a, 26b, and 26c whose substitutes are spatially smaller. A small yellow contour around benzene ring suggested that a minor substituent at this location might be better. The small green contour near R2 represent a larger substituents at this site would be favored.
In Fig. 3b, the blue and red denoted regions which were favored and disfavored, respectively. The large blue contour above R1 indicated that electron-donating groups at this site would be beneficial. The blue contour close to the point of the benzene ring site also advise us that electron-donating groups at this site may be helpful in improving the activity. Another red contour near the carbonyl revealed electron-donating groups at this position was detrimental to the potency.
With the compound 26h still as the template molecule, the CoMISA contours maps were shown in Fig. 4. Figure 4a, b depicted the steric and electrostatic contours of CoMSIA, respectively, which are similar to CoMFA ones in Fig. 3. As to the hydrophobic contours (Fig. 4c), yellow and white blocks emphasized the hydrophobic and hydrophilic favored regions, respectively. The cyan and purple blocks in H-bond donor contour maps (Fig. 4d) indicated favorable and unfavorable to H-bond donor, respectively.
Near the substituent R1, there is a blue region near the site. This indicates that if the substituent in the region is increased, the hydrophilicity will be helpful to enhance the activity, and there is also a huge yellow contour around the benzene ring position and R2 site which shows that hydrophobic groups play a large role in this region. That is why the compound 8c2 has the higher activity than 8b.
The hydrogen bond donor contour maps of the CoMSIA model with compound 26h were depicted in Fig. 4d. A purple contour around the R1 indicated that H-bond acceptor groups at this site would be harmful to the potency. This agrees with the fact that most active compounds had a non-polar methyl group at this site which could not form a H-bond with the CLC chloride channel receptor. As seen from Fig. 4d, the large purple contour above the benzene ring site, suggesting that a H-bond acceptor group at this site would be favorable. A cyan contour near the R2 showed that a H-bond donor at this site may benefit the potency. This also consisted with the observations that 8c2 (acting as H-bond donor) compounds showed stronger inhibitory activities than 8b.
Summary of the structure-activity relationships
The structure-activity relationships revealed by the present study were summarized in Fig. 5. The bulky, electron-withdrawing and steric groups at R1 are crucial to enhance the activity, on the other hand, the bulky and hydrophobic groups to R2 may improve the potency. By introducing electron-donating groups to the meta-position of R1 may also be beneficial to the activity. In addition, the hydrogen donor was also preferred in the red zone.
Designing novel derivatives
Based on QSAR revealed by this study above, we selected compound 26h (PIC50 = 7.678) as lead compound, and 11 new potent molecules (S1–S11) were designed. These molecules were superposed to the alignment as the test set, then their activities on JNK-3 inhibition were calculated using 3D-QSAR models we built previously. The chemical structures as well as calculated activities are demonstrated in Table 3.
Molecular docking analysis
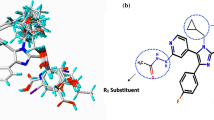
Figure 6 showed the docking pose of compound 26h in JNK3 (Koch et al. 2014). The hydroxyl groups acted as hydrogen bond donor and acceptor by forming a H-bond with Gin75 and Arg227-Arg230, respectively, matching the corresponding CoMSIA hydrogen bond donor and acceptor contour maps. Moreover, a cavity located beneath the pyrazol ring where steric contour map hints us a bulky group favored verified our results. The reason why the molecule 26h showed higher docking score than most other structures may commentate in following results. As shown in Fig. 6, 3-NH on the side chain R1 of 26h formed H-bond with the carbonyl group of Arg227-Arg230. Main core 4 carbonyl of 26h formed H-bond with the -NH group of Gln75. The formation of hydrogen bonds with key amino acid groups may also explain the reason why 26h is higher than other structures and the chlorobenzene group in the end region exerts force against the strong molecules of the docking protein. The detail review of docking results proves the binding effects of 26 h are stronger than that of the most other structures on C-JNK3 Protein, further speculating JNK3 (3TTI) is the target of prediction for compound 26h groups with active pockets of C-JNK3 inhibitors.
With the same parameter that we optimized to dock the compound 26h, the designed compounds, S1–S11, were also placed into the receptor. As shown in Table 4, the total score of compounds S3, S4, S7, S8, and S9 is quite high, among which compound S8 even showed a total score higher than 9. Despite the high total scores of compounds S5 and S11, their CScore and global_CScore which is <4 limited their approval of potency. The positive correlation of QSAR predicted activity and the docking scores of compounds S1–S11 were verified by aligning their results. Comparing with 26h, the reason that compound S8 has a higher score, as shown in Fig. 7, may be due to the larger contact area between the receptor binding pocket and substituent R1 and R2, In addition, the key hydrogen bonds between S8 and JNK3 are quite similar but firmer than those between 26h and JNK3, especially the H-bond between Arg227, Arg230, Gin75, and ligand showed essential all the time. The interactions and binding mode of our compounds are associated with the mechanism research of the JNK-3 dynamic study (Mishra and Günther 2018) which indicated the conformation rearrangement of hinge region caused by inhibitors may be the key factor.
Conclusion
In this study, we have established CoMFA model (q2 = 0.774, r2 = 0.991) and CoMSIA model (q2 = 0.666, r2 = 0.990) with good correlative and predictive abilities from the c-Jun N-terminal kinase 3 (JNK3) inhibitors. The CoMFA and CoMSIA contour maps provided information to summarize the structure–activity relationship and characterize the structural features affecting the neuronal recovery activity. In addition, good prediction ability, experimental observation group compounds indicate that these models can be used to predict the accord with the IC50 value, and based on which, 11 new molecules are predicted to reach same or better activity. Further validation is conducted through the docking study. The results indicate that our series of compounds are promising as c-jnk3 inhibitors, suggesting that the models can be helpful for the development of discovering novel inhibitors of JNK3.
References
Ansideri F, Macedo JT, Eitel M, El-Gokha A, Zinad DS, Scarpellini C, Laufer SA (2018) Structural Optimization of a Pyridinylimidazole scaffold: shifting the selectivity from p38α mitogen-activated protein kinase to c-Jun N-terminal kinase 3. ACS Omega 3(7):7809–7831
Buckingham J, Glen RC, Hill AP, Hyde RM, Martin GR, Robertson AD, Woollard PM (1995) Computer-aided design and synthesis of 5-substituted tryptamines and their pharmacology at the 5-HT1D receptor: discovery of compounds with potential anti-migraine properties. J Med Chem 38(18):3566–3580
Cervigni RI, Bonavita R, Barretta ML, Spano D, Ayala I, Nakamura N, Colanzi A (2015) JNK2 controls fragmentation of the Golgi complex and the G2/M transition through phosphorylation of GRASP65. J Cell Sci 128(12):2249–2260
Chambers JW, Howard S, LoGrasso PV (2013) Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J Biol Chem 288(2):1079–1087
Chambers JW, Pachori A, Howard S, Ganno M, Hansen Jr D, Kamenecka T, Cherry L (2011) Small molecule c-jun-N-terminal kinase inhibitors protect dopaminergic neurons in a model of Parkinson’s disease. Acs Chem Neurosci 2(4):198–206
Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76(6):1025–1037
Dorsey BD, Levin RB, McDaniel SL, Vacca JP, Guare JP, Darke PL, Schleif WA (1994) L-735,524: the design of a potent and orally bioavailable HIV protease inhibitor. J Med Chem 37(21):3443–3451
Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 15(11):2760–2770
Gutierrez GJ, Tsuji T, Chen M, Jiang W, Ze’ev AR (2010) Interplay between Cdh1 and JNK activity during the cell cycle. Nat Cell Biol 12(7):686
Hibi M, Lin ANNING, Smeal T, Minden A, Karin M (1993) Identification of an oncoprotein-and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Gene dev 7(11):2135–2148
Huang X, Liu T, Gu J, Luo X, Ji R, Cao Y, Jiang H (2001) 3D-QSAR model of flavonoids binding at benzodiazepine site in GABAA receptors. J Med Chem 44(12):1883–1891
Jain AN (2003) Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J Med Chem 46(4):499–511
Kamenecka T, Jiang R, Song X, Duckett D, Chen W, Ling YY, Cameron MD (2009) Synthesis, biological evaluation, X-ray structure, and pharmacokinetics of aminopyrimidine c-jun-N-terminal kinase (JNK) inhibitors. J Med Chem 53(1):419–431
Klebe G, Abraham U (1999) Comparative molecular similarity index analysis (CoMSIA) to study hydrogen-bonding properties and to score combinatorial libraries. J Comput Aid Mol Des 13(1):1–10
Koch P, Gehringer M, Laufer SA (2014) Inhibitors of c-Jun N-terminal kinases: an update. J Med Chem 58(1):72–95
Krenitsky VP, Nadolny L, Delgado M, Ayala L, Clareen SS, Hilgraf R, Wright J (2012) Discovery of CC-930, an orally active anti-fibrotic JNK inhibitor. Bioorg Med Chem Lett 22(3):1433–1438
Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Woodgett JR (1994) The stress-activated protein kinase subfamily of c-Jun kinases. Nature 369(6476):156
Lange A, Günther M, Büttner FM, Zimmermann MO, Heidrich J, Hennig S, Koch P (2015) Targeting the gatekeeper MET146 of C-Jun N-terminal kinase 3 induces a bivalent halogen/chalcogen bond. J Am Chem Soc 137(46):14640–14652
Li S, Fan J, Peng C, Chang Y, Guo L, Hou J, Xiao G (2017) New molecular insights into the tyrosyl-tRNA synthase inhibitors: CoMFA, CoMSIA analyses and molecular docking studies. Sci Rep 7(1):11525
Mishra P, Günther S (2018) New insights into the structural dynamics of the kinase JNK3. Sci Rep 8(1):9435
Mohit AA, Martin JH, Miller CA (1995) p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron 14(1):67–78
Muth F, El-Gokha A, Ansideri F, Eitel M, Döring E, Sievers-Engler A, Laufer SA (2017) Tri-and tetrasubstituted pyridinylimidazoles as covalent inhibitors of c-Jun N-terminal kinase 3. J Med Chem 60(2):594–607
Qin LT, Liu SS, Xiao QF, Wu QS (2013) Internal and external validtions of QSAR model: Review. Environ Chem 32(7):1205–1211
Rech JC, Yato M, Duckett D, Ember B, LoGrasso PV, Bergman RG, Ellman JA (2007) Synthesis of potent bicyclic bisarylimidazole c-jun N-terminal kinase inhibitors by catalytic C−H bond activation. J Am Chem Soc 129(3):490–491
Schattel V, Hinselmann G, Jahn A, Zell A, Laufer S (2011) Modeling and benchmark data set for the inhibition of c-Jun N-terminal kinase-3. J Chem Inf Model 51(3):670–679
Sharma P, Ghoshal N (2006) Exploration of a binding mode of benzothiazol-2-yl acetonitrile pyrimidine core based derivatives as potent c-Jun N-terminal kinase-3 inhibitors and 3D-QSAR analyses. J Chem Inf Model 46(4):1763–1774
Siddiqui MA, Reddy PA (2010) Small molecule JNK (c-Jun N-terminal kinase) inhibitors. J Med Chem 53(8):3005–3012
Srivastava V, Kumar A, Mishra BN, Siddiqi MI (2008) Molecular docking studies on DMDP derivatives as human DHFR inhibitors. Bioinformation 3(4):180
Wang Z, Chang Y, Han Y, Liu K, Hou J, Dai C, Chen W (2016) 3D-QSAR and docking studies on 1-hydroxypyridin-2-one compounds as mutant isocitrate dehydrogenase 1 inhibitors. J Mol Struct 1123:335–343
Xiao A, Zhang Z, An L, Xiang Y (2008) 3D-QSAR and docking studies of 3-arylquinazolinethione derivatives as selective estrogen receptor modulators. J Mol Model 14(2):149–159
Zhao H, Serby MD, Xin Z, Szczepankiewicz BG, Liu M, Kosogof C, Pederson T (2006) Discovery of potent, highly selective, and orally bioavailable pyridine carboxamide c-Jun NH2-terminal kinase inhibitors. J Med Chem 49(15):4455–4458
Zheng K, Iqbal S, Hernandez P, Park H, LoGrasso PV, Feng Y (2014) Design and synthesis of highly potent and isoform selective JNK3 inhibitors: SAR studies on aminopyrazole derivatives. J Med Chem 57(23):10013–10030
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 81573294, 81773593, 81872759), the Excellent Young Teachers Program of Guangdong Provincial Colleges and Universities (YQ 2015061) and the Pearl River S&T Nova Program of Guangzhou (201610010100). We thank the software support from Dr. Junxia Zheng of the Guangdong University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yanda Liu, Yewei Xie
Rights and permissions
About this article
Cite this article
Liu, Y., Xie, Y., Liu, Y. et al. Insights into the c-Jun N-terminal kinase 3 (JNK3) inhibitors: CoMFA, CoMSIA analyses and molecular docking studies. Med Chem Res 28, 1796–1805 (2019). https://doi.org/10.1007/s00044-019-02416-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02416-3