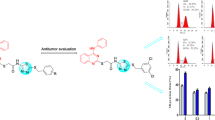
Abstract
In this study, twenty three 3,6-disubstituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives were synthesized and their antiproliferative activities in vitro were studied against SMMC-7721, HeLa, A549, and L929 by the CCK-8 assay. The bioassay results demonstrated that all tested compounds 8(a–w) exhibited antiproliferation with different degrees, and some compounds showed better effects than reference drug 5-fluorouracil. Among these screened compounds, compounds 8a, 8d, and 8l displayed significant antitumor activities in inhibiting SMMC-7721cell proliferation with IC50 values of 1.64, 1.74, and 1.61 µM, respectively. Compounds 8d and 8l were manifested highly effective biological activity versus HeLa cells with IC50 values of 2.23 and 2.84 µM, respectively. Compound 8l was found to have the highest antitumor potency against A549 cells with IC50 value of 2.67 µM. Furthermore, all compounds exhibited weaker cytotoxic effects than 5-fluorouracil on normal cell lines L929.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer has become one of the most terrible diseases around the world because of its low cure and high mortality. Therefore, the design and development of new drugs for cancer therapeutics is an important and challenging task for medicinal chemists worldwide (Anand et al. 2008; Alegaon et al. 2017; Chowrasia et al. 2017).
The heterocyclic compounds bearing 1,2,4-triazole or 1,3,4-thiadiazole moieties are of special interest to medicinal chemists because of their exceptional chemical and versatile biological properties including potential antioxidant (Menteşe et al. 2013; Padmaja et al. 2015), antimicrobial (Ceylan 2016; Li et al. 2016), antidepressant (Chelamalla et al. 2017; Khan et al. 2016), antifungal (Miniyar et al. 2017; Er et al. 2017), anticonvulsant (Kahveci et al. 2014; Harish et al. 2014), and anti-inflammatory activities (Liu et al. 2016; Banerjee et al. 2016). In particular, a great number of 1,2,4-triazole and 1,3,4-thiadiazole derivatives have been proved to show potent antitumor activities (Meti et al. 2016; Zhao et al. 2016; Bhatt et al. 2018; Vudhgiri et al. 2017). Moreover, The fused 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole moieties were found to have various biological properties, such as antioxidant (Chidananda et al. 2012), antimicrobial (Almajan et al. 2010; Cui et al. 2017), anticonvulsant (Deng et al. 2012), and anti-inflammatory (Chidananda et al. 2012; Akhter et al. 2014). Particularly, this class of compounds have received considerable attention in the last few decades, owing to their effective anticancer importance (Chowrasia et al. 2017; Xu et al. 2017; Rostom et al. 2017; Husain et al. 2013). Moreoever, disulfide derivatives are also known to display a wide spectrum of biological activities including anti-Alzheimer’s (Roldán-Peňa et al. 2017), antibacterial (Sheppard et al. 2018; Turos et al. 2008), anti-HIV-1 (Cesarini et al. 2008), antioxidant (Roldán-Peňa et al. 2017), and herbicidal properties (Li et al. 2013; Shang et al. 2012). Especially, the antitumor potential of disulfide derivatives attracts the great interest of medicinal chemists in recent years (Branowska et al. 2018; Hong et al. 2015; Rubino et al. 2017; Vale et al. 2017) (Fig. 1).
In view of the abovementioned findings, in order to screen out novel antitumor agents bearing disulfide core with high efficiency and low toxicity, hybrid compounds possessing disulfide and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole moieties will be formed, and some cells growth inhibitory effects will be examined in our research.
Results and discussion
Synthesis
As exhibited in Scheme 1, twenty three novel 1,2,4-triazole[3,4-b]-1,3,4-thiadiazole derivatives 8(a–w) were synthesized and reported for the frist time. The preparation of S-alkyl-thioisothiourea hydrochloride 2 was carried out by the reported literature method (Sirakawa et al. 1970). The 3-substitutedphenyl-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole-6-thiol 7 were obtained according to the reported procedure (Eweiss and Bahajaj 1987). Finally, the target compounds 8(a–w) were successfully gained by the reaction of intermediates 2 and compounds 7 in the presence of NaHCO3 in ethanol and water at room temperature. All newly synthesized compounds 8(a–w) were purified by silica gel column chromatography and their structures were confirmed by IR, 1H NMR, 13C NMR, and HR-ESI-MS.
Synthesis of target compounds 8(a–w). Reaction conditions and reagents: a Conc. HCl, H2O2 (30%), 0–5 °C, 8–10 h; b NH2NH2·H2O (85%), EtOH, reflux, 5–8 h; c KOH, CS2, EtOH, 0–5 °C, 4–6 h; d NH2NH2·H2O (85%), EtOH, reflux, 5–8 h; e KOH, CS2, MeOH, reflux, 10–15 h; f compound 2, EtOH, NaHCO3/H2O, rt, 4–6 h
Pharmacology evaluation
Evaluation of the antiproliferative activities in vitro for 3,6-disubstituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles 8(a–w) was carried out by utilizing the CCK-8 assay against SMMC-7721, HeLa, A549, and L929 cell lines. The inhibitory activities IC50 (μM) were expressed in Table 1.
All synthesized compounds 8(a–w) showed different degrees of antitumor activities, and some compounds showed better effects than positive control 5-fluorouracil against various cancer cell. The substituent groups R1 and R2 played important roles in the potency of biologically active compounds. In SMMC-7721 cells, except compound 8e bearing 4-methoxyl substituent at the phenyl ring while R1 is n-butyl group, showed moderate antitumor activity with IC50 value of 14.42 µM, the other compounds exhibited good antiproliferative effects with IC50 values ranging from 1.61 to 9.45 µM. Meanwhile, the majority of the tested compounds showed better activities than positive control 5-fluorouracil. In particular, compounds 8a, 8d, and 8l displayed significant antitumor activities against SMMC-7721 cells with IC50 values of 1.64, 1.74, and 1.61 µM, respectively. As compared with compound 8a (IC50 = 1.64 µM), which has no substituent at the phenyl ring while R1 is n-butyl group, compounds 8(b–h) all showed weaker activities against SMMC-7721 cells. In HeLa cells, compounds 8c, 8e, 8j, 8m, and 8u showed moderate antiproliferative activity with IC50 values of 12.67, 21.29, 14.25, 24.30, and 21.49 µM, respectively. The other compounds all showed good antiproliferative effects with IC50 values ranging from 2.23 to 9.71 µM, and displayed higher activities than positive control 5-fluorouracil. Among them, compounds 8d and 8l exhibited highly effective biological activity against HeLa cells with IC50 values of 2.23 and 2.84 µM, respectively. In A549 cells, except compounds 8g, 8q, and 8w, showed moderate antiproliferative activity with IC50 values of 25.26, 14.85 and 17.07 µM, respectively. The other compounds all exhibited good antitumor effects with IC50 values ranging from 2.67 to 9.07 µM, and most of the tested compounds showed better activities than positive control 5-fluorouracil. As compared with compound 8i (IC50 = 7.35 µM), which has no substituent at the phenyl ring while R1 is 2-butyl group, compounds 8(j–p) all displayed better activities against A549 cells. Among them, compound 8l possessing 4-methyl substituent at the phenyl while R1 is 2-butyl group, exhibited the best inhibitory effect against A549 cells with IC50 value of 2.67 µM. Furthermore, all compounds exhibited weaker cytotoxic effects than 5-fluorouracil on normal cell lines L929.
Conclusion
The present study comprises the synthesis of some novel 3,6-disubstituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and screening for their antiproliferative activities against SMMC-7721, HeLa, A549, and L929 cell lines via the CCK-8 assay. The preliminary investigation revealed that the tested compounds have potency of inhibition for three tumor cell lines, and the effects of substituents on anticancer efficacy have been observed. Interestingly, some compounds showed stronger antitumor effects than reference drug 5-fluorouracil, and several screened compounds, such as 8a, 8d, and 8l, displayed promising biological activities. Furthermore, all tested compounds exhibited weaker cytotoxic effects than 5-fluorouracil on the normal cell lines L929. Therefore, the pharmacological results could be helpful for improving the potency and selectivity of this class of compounds.
Material and methods
Synthesis
Unless otherwise noted, all solvents and reagents were used as received without further purification. Melting points were determined by an X-6 microscope melting point apparatus and are uncorrected. Infrared spectra were obtained in KBr pellets on a Nicolet Avatar 370 spectrometer. NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer (1H, 400 MHz; 13C, 100 MHz) using DMSO-d6 as solvent and tetramethylsilane as internal standard. Chemical shifts are given in ppm downfield from tetramethylsilane and the coupling constants (J) are in hertz (Hz). The high-resolution mass spectra were taken with a Waters Xevo G2 spectrometer. The reaction progress of some intermediates, such as compounds 5, 6, and 7, was monitored by thin layer chromatography.
General method for the synthesis of 3,6-disubstituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles 8(a–w)
S-Alkyl-thioisothiourea hydrochloride 2 (2.2 mmol) and 3-substitutedphenyl-1,2,4-triazole-[3,4-b]-1, 3,4-thiadiazole-6-thiol 7 (2.0 mmol) were dissolved in water (5 mL) and ethanol (15 mL). Then, a solution of saturated NaHCO3 (20 mL) was added dropwise with stirring for 4 h at room temperature. Solid crude products were obtained by filtration. The insoluble solid was collected and purified by silica gel column chromatography with petroleum ether/ethyl acetate (8:1, volume ratio) as eluent to afford the desired products.
3-Phenyl-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8a)
White solid, Yield 76.5%, m.p.: 92.3–93.7 °C; IR (KBr) cm−1: 2930, 1613, 1452, 1381, 1230, 685, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.18 (d, J = 7.2 Hz, 2H, Ar-H), 7.55–7.62 (m, 3H, Ar-H), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.67–11.74 (m, 2H, CH2), 1.33–1.43 (m, 2H, CH2), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 173.84, 154.47, 145.33, 130.76, 129.55 (2C), 126.23 (2C), 125.72, 39.18, 30.91, 21.34, 13.87; HR-ESI-MS m/z: calcd for C13H14N4S3 [M + H]+ 323.0465, found 323.0459.
3-(4-Cholorophenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8b)
White solid, Yield 65.2%, m.p.: 95.6–96.7 °C; IR (KBr) cm−1: 2930, 1610, 1449, 1381, 1230, 684, 505; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.19 (d, J = 8.8 Hz, 2H, Ar-H), 7.68 (d, J = 8.8 Hz, 2H, Ar-H), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.66–1.74 (m, 2H, CH2), 1.37–1.43 (m, 2H, CH2), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.32, 155.11, 143.08, 135.01, 129.06 (2C), 127.75 (2C), 125.06, 39.18, 30.90, 21.34, 13.87; HR-ESI-MS m/z: calcd for C13H13N4S3Cl [M + H]+ 357.0072, found 357.0069.
3-(4-Fluorophenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8c)
Yellow solid, Yield 60.7%, m.p.: 64.7–65.3 °C; IR (KBr) cm−1: 2932, 1613, 1450, 1382, 1230, 685, 503; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.22 (dd, J = 8.8 Hz, 2H, Ar-H), 7.46 (dd, J = 8.8 Hz, 2H, Ar-H), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.66–1.74 (m, 2H, CH2), 1.36–1.45 (m, 2H, CH2), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 164.78, 162.31, 154.48, 144.58, 128.67 (2C) (JC-F = 8.7 Hz), 122.35 (JC-F = 2.9 Hz), 116.67 (2C) (JC-F = 22.0 Hz), 39.16, 30.89, 21.33, 13.87; HR-ESI-MS m/z: calcd for C13H13N4S3F [M + H]+ 341.0386, found 341.0365.
3-(4-Methylphenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8d)
White solid, Yield 78.5%, m.p.: 83.9–84.2 °C; IR (KBr) cm−1: 2931, 1612, 1451, 1380, 1233, 682, 503; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.86 (d, J = 8.0 Hz, 2H, Ar-H), 7.36 (d, J = 8.0 Hz, 2H, Ar-H), 2.97 (t, J = 7.2 Hz, 2H, CH2), 2.37 (s, 3H, Ar-CH3), 1.64–1.74 (m, 2H, CH2), 1.33–1.41 (m, 2H, CH2), 0.87 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 160.36, 156.23, 140.85, 131.04, 130.14 (2C), 129.75, 126.52 (2C), 38.62, 30.57, 21.49, 21.28, 13.92; HR-ESI-MS m/z: calcd for C14H16N4S3 [M + H]+ 337.0635, found 337.0615.
3-(4-Methoxyphenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8e)
White solid, Yield 66.8%, m.p.: 90.3–91.6 °C; IR (KBr) cm−1: 2933, 1613, 1451, 1379, 1230, 681, 505; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.10 (d, J = 8.8 Hz, 2H, Ar-H), 7.15 (d, J = 8.8 Hz, 2H, Ar-H), 3.84 (s, 3H, OCH3), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.66–1.73 (m, 2H, CH2), 1.37–1.43 (m, 2H, CH2), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 173.48, 161.17, 154.02, 145.09, 127.88 (2C), 118.23, 114.95 (2C), 55.81, 39.20, 30.90, 21.33, 13.86; HR-ESI-MS m/z: calcd for C14H16N4OS3 [M + H]+ 353.0577, found 353.0565.
3-(4-Hydroxyphenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8f)
White solid, Yield 82.5%, m.p.: 108.5–109.4 °C; IR (KBr) cm−1: 2931, 1614, 1455, 1384, 1231, 682, 505; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.07 (s, H, OH), 8.01 (d, J = 8.8 Hz, 2H, Ar-H), 6.96 (d, J = 8.8 Hz, 2H, Ar-H), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.66–1.72 (m, 2H, CH2), 1.38–1.43 (m, 2H, CH2), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.28, 159.67, 153.90, 145.34, 127.99 (2C), 116.89, 116.29 (2C), 39.18, 30.88, 21.32, 13.87; HR-ESI-MS m/z: calcd for C13H14N4OS3 [M + H]+ 339.0422, found 339.0408.
3-(4-Tert-butylphenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8g)
White solid, Yield 79.6%, m.p.: 92.7–93.4 °C; IR (KBr) cm−1: 2935, 1613, 1451, 1380, 1230, 682, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.10 (d, J = 8.4 Hz, 2H, Ar-H), 7.61 (d, J = 8.4 Hz, 2H, Ar-H), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.63–1.73 (m, 2H, CH2), 1.36–1.45 (m, 2H, CH2), 1.32 (s, 9H, CH3), 0.88 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.65, 154.41, 153.37, 145.15, 126.31 (2C), 126.10 (2C), 123.22, 39.17, 35.09, 31.37 (3C), 30.90, 21.32, 13.87; HR-ESI-MS m/z: calcd for C17H22N4S3 [M + H]+ 379.1097, found 379.1085.
3-(3,4,5-Trimethoxyphenyl)-6-(n-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8h)
White solid, Yield 65.2%, m.p.: 106.6–107.3 °C; IR (KBr) cm−1: 2931, 1613, 1452, 1380, 1233, 685, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.48 (s, 2H, Ar-H), 3.87 (s, 6H, CH3O), 3.75 (s, 3H, CH3O), 3.09 (t, J = 7.2 Hz, 2H, CH2), 1.62–1.72 (m, 2H, CH2), 1.33–1.45 (m, 2H, CH2), 0.89 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 167.41, 153.76 (2C), 139.78, 131.96, 129.12, 121.06, 103.90 (2C), 60.67, 56.54 (2C), 39.07, 30.91, 21.32, 13.87; HR-ESI-MS m/z: calcd for C16H20N4O3S3 [M + H]+ 413.0797, found 413.0776.
3-Phenyl-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8i)
White solid, Yield 62.7%, m.p. 89.3–90.1 °C; IR (KBr) cm−1: 2930, 1612, 1451, 1380, 1231, 684, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.17 (d, J = 7.2 Hz, 2H, Ar-H), 7.55–7.63 (m, 3H, Ar-H), 3.26–3.31 (m, 1H, CH), 1.62–1.75 (m, 2H, CH2), 1.37 (d, J = 6.4 Hz, 3H, CH3), 0.99 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.14, 154.47, 145.36, 130.83, 129.60 (2C), 126.29 (2C), 125.70, 49.91, 28.83, 20.06, 11.64; HR-ESI-MS m/z: calcd for C13H14N4S3 [M + Na]+ 345.0297, found 345.0278.
3-(4-Chlorophenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8j)
White solid, Yield 82.5%, m.p.: 90.7–92.3 °C; IR (KBr) cm−1: 2931, 1612, 1450, 1381, 1229, 684, 503; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.18 (d, J = 8.8 Hz, 2H, Ar-H), 7.68 (d, J = 8.8 Hz, 2H, Ar-H), 3.26–3.31 (m, 1H, CH), 1.58–1.78 (m, 2H, CH2), 1.36 (d, J = 6.4 Hz, 3H, CH3), 0.99 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.50, 154.57, 144.39, 135.43, 129.70 (2C), 127.84 (2C), 124.52, 49.93, 28.85, 20.06, 11.65; HR-ESI-MS m/z: calcd for C13H13N4S3Cl [M + H]+ 357.0083, found 357.0069.
3-(4-Fluorophenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8k)
Yellow solid, Yield 68.6%, m.p. 62.5–63.1 °C; IR (KBr) cm−1: 2932, 1611, 1453, 1381, 1230, 684, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.22 (dd, J = 8.8 Hz, 2H, Ar-H), 7.46 (t, J = 8.8 Hz, 2H, Ar-H), 3.24–3.30 (m, 1H, CH), 1.60–1.75 (m, 2H, CH2), 1.36 (d, J = 6.8 Hz, 3H, CH3), 0.97–1.01 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 164.79, 162.32, 154.40, 144.57, 128.70 (2C) (JC-F = 8.7 Hz), 122.35 (JC-F = 3.1 Hz), 116.66 (2C) (JC-F = 22.0 Hz), 49.92, 28.84, 20.07, 11.65; HR-ESI-MS m/z: calcd for C13H13N4S3F [M + H]+ 341.0382, found 341.0365.
3-(4-Methylphenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8l)
White solid, Yield 58.3%, m.p.: 85.7–86.2 °C; IR (KBr) cm−1: 2932, 1610, 1450, 1383, 1231, 683, 503; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.86 (d, J = 8.0 Hz, 2H, Ar-H), 7.35 (d, J = 8.0 Hz, 2H, Ar-H), 3.08–3.14 (m, 1H, CH), 2.36 (s, 3H, Ar-CH3), 1.51–1.75 (m, 2H, CH2), 1.31 (d, J = 6.8 Hz, 3H, CH3), 0.93 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 160.08, 156.24, 140.46, 131.91, 130.04 (2C), 129.53, 126.40 (2C), 48.43, 28.44, 21.39, 20.11, 11.50; HR-ESI-MS m/z: calcd for C14H16N4S3 [M + H]+ 337.0631, found 337.0615.
3-(4-Methoxyphenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8m)
White solid, Yield 88.2%, m.p.: 87.8–88.6 °C; IR (KBr) cm−1: 2930, 1616, 1450, 1381, 1235, 683, 505; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.11 (d, J = 8.8 Hz, 2H, Ar-H), 7.15 (d, J = 8.8 Hz, 2H, Ar-H), 3.84 (s, 3H, OCH3), 3.25–3.32 (m, 1H, CH), 1.58–1.76 (m, 2H, CH2), 1.35 (d, J = 6.8 Hz, 3H, CH3), 0.99 (t, J = 7.6 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.63, 161.10, 154.08, 145.02, 127.87 (2C), 118.42, 114.96 (2C), 55.82, 49.90, 28.84, 20.07, 11.65; HR-ESI-MS m/z: calcd for C14H16N4OS3 [M + H]+ 353.0590, found 353.0565.
3-(4-Hydroxyphenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8n)
White solid, Yield 78.4%, m.p.: 103.4–104.2 °C; IR (KBr) cm−1: 2932, 1611, 1450, 1382, 1231, 683, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.08 (s, H, OH), 8.01 (d, J = 8.4 Hz, 2H, Ar-H), 6.96 (d, J = 8.4 Hz, 2H, Ar-H), 3.25–3.30 (m, 1H, CH), 1.61–1.76 (m, 2H, CH2), 1.36 (d, J = 6.4 Hz, 3H, CH3), 0.99 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 173.36, 159.87, 153.55, 145.63, 128.10 (2C), 116.55, 116.34 (2C), 49.89, 28.83, 20.07, 11.66; HR-ESI-MS m/z: calcd for C13H14N4OS3 [M + H]+ 339.0426, found 339.0408.
3-(4-Tert-butylphenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8o)
White solid, Yield 78.6%, m.p.: 83.4–84.1 °C; IR (KBr) cm−1: 2932, 1615, 1452, 1382, 1233, 684, 503; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.08 (d, J = 8.4 Hz, 2H, Ar-H), 7.62 (d, J = 8.4 Hz, 2H, Ar-H), 3.25–3.30 (m, 1H, CH), 1.60–1.76 (m, 2H, CH2), 1.36 (d, J = 7.2 Hz, 3H, CH3), 1.33 (s, 9H, CH3), 0.98 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 173.81, 154.09, 153.55, 145.39, 126.32 (2C), 126.15 (2C), 122.95, 49.89, 35.09 (3C), 31.34, 28.84, 20.06, 11.65; HR-ESI-MS m/z: calcd for C17H22N4S3 [M + H]+ 379.1099, found 379.1085.
3-(3,4,5-Trimethoxyphenyl)-6-(2-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8p)
White solid, Yield 70.3%, m.p. 114.6–115.3 °C; IR (KBr) cm−1: 2931, 1613, 1451, 1385, 1233, 685, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.48 (s, 2H, Ar-H), 3.88 (s, 6H, CH3O), 3.75 (s, 3H, CH3O), 3.28 (t, J = 7.2 Hz, 1H, CH), 1.60–1.78 (m, 2H, CH2), 1.37 (d, J = 6.4 Hz, 3H, CH3), 0.99 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 173.87, 153.76 (2C), 145.16, 139.83, 129.11, 121.02, 103.96 (2C), 60.67, 56.56 (2C), 49.64, 28.83, 20.04, 11.61; HR-ESI-MS m/z: calcd for C16H20N4O3S3 [M + H]+ 413.0797, found 413.0776.
3-Phenyl-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8q)
White solid, Yield 81.4%, m.p. 105.1–106.3 °C; IR (KBr) cm−1: 2931, 1610, 1451, 1380, 1232, 683, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.8 (d, J = 7.2 Hz, 2H, Ar-H), 7.55–7.62 (m, 3H, Ar-H), 3.01 (d, J = 6.8 Hz, 2H, CH2), 1.93–2.03 (m, 1H, CH), 1.01 (d, J = 6.4 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.44, 154.62, 145.15, 130.65, 129.52 (2C), 126.20 (2C), 125.88, 48.38, 28.24, 21.66 (2C); HR-ESI-MS m/z: calcd for C13H14N4S3 [M + H]+ 323.0480, found 323.0459.
3-(4-Chlorophenyl)-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8r)
White solid, Yield 79.8%, m.p. 101.1–102.3 °C; IR (KBr) cm−1: 2930, 1612, 1450, 1381, 1230, 685, 503; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.18 (d, J = 8.8 Hz, 2H, Ar-H), 7.68 (d, J = 8.8 Hz, 2H, Ar-H), 3.01 (d, J = 6.8 Hz, 2H, CH2), 1.94–2.01 (m, 1H, CH), 1.01 (d, J = 6.8 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.29, 154.78, 144.45, 135.45, 129.76 (2C), 127.9 (2C), 124.54, 48.37, 28.23, 21.65 (2C); HR-ESI-MS m/z: calcd for C13H13N4S3Cl [M + H]+ 357.0077, found 357.0069.
3-(4-Fluorophenyl)-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8s)
Yellow solid, Yield 67.5%, m.p. 78.3–79.2 °C; IR (KBr) cm−1: 2930, 1609, 1451, 1381, 1229, 684, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.22 (dd, J = 8.8 Hz, 2H, Ar-H), 7.45 (dd, J = 8.8 Hz, 2H, Ar-H), 3.01 (d, J = 6.8 Hz, 2H, CH2), 1.91–2.02 (m, 1H, CH), 1.01 (d, J = 6.8 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 164.83, 162.35, 154.64, 144.14, 128.78 (2C) (JC-F = 8.8 Hz), 122.17 (JC-F = 2.8 Hz), 116.66 (2C) (JC-F = 22.1 Hz), 48.37, 28.23, 21.67 (2C); HR-ESI-MS m/z: calcd for C13H13N4S3F [M + H]+ 341.0378, found 341.0365.
3-(4-Methylphenyl)-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8t)
White solid, Yield 63.2%, m.p.: 90.4–91.2 °C; IR (KBr) cm−1: 2931, 1614, 1451, 1382, 1231, 680, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.86 (d, J = 8.0 Hz, 2H, Ar-H), 7.36 (d, J = 8.0 Hz, 2H, Ar-H), 2.87 (d, J = 6.8 Hz, 2H, CH2), 2.37 (s, 3H, Ar-CH3), 1.97–2.06 (m, 1H, CH), 0.97 (d, J = 6.8 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 160.30, 156.26, 140.84, 131.95, 130.12 (2C), 129.11, 126.47 (2C), 48.18, 27.73, 21.82, 21.44 (2C); HR-ESI-MS m/z: calcd for C14H16N4S3 [M + H]+ 337.063, found 337.0615.
3-(4-Methoxyphenyl)-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8u)
White solid, Yield 83.6%, m.p.: 85.6–86.3 °C; IR (KBr) cm−1: 2932, 1612, 1453, 1383, 1233, 680, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.12 (d, J = 8.8 Hz, 2H,Ar-H), 7.15 (d, J = 8.8 Hz, 2H, Ar-H), 3.84 (s, 3H, OCH3), 3.01 (d, J = 6.8 Hz, 2H, CH2), 1.94–2.01 (m, 1H, CH), 1.01 (d, J = 6.8 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 173.15, 161.07, 154.32, 144.72, 127.88 (2C), 118.50, 114.96 (2C), 55.82, 48.36, 28.22, 21.67 (2C); HR-ESI-MS m/z: calcd for C14H16N4OS3 [M + H]+ 353.0596, found 353.0565.
3-(4-Hydroxyphenyl)-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8v)
White solid, Yield 70.5%, m.p.: 105.7–106.3 °C; IR (KBr) cm−1: 2933, 1612, 1450, 1382, 1232, 685, 504; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 10.09 (s, H, OH), 8.01 (d, J = 8.8 Hz, 2H, Ar-H), 6.96 (d, J = 8.8 Hz, 2H, Ar-H), 3.01 (d, J = 6.8 Hz, 2H, CH2), 1.94–2.01 (m, 1H, CH), 1.01 (d, J = 6.8 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.19, 159.89, 153.75, 145.60, 128.11 (2C), 116.53, 116.38 (2C), 48.38, 28.22, 21.68 (2C); HR-ESI-MS m/z: calcd for C13H14N4OS3 [M + H]+ 339.0429, found 339.0408.
3-(4-Tert-butylphenyl)-6-(i-butyldisulfanyl)-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazole (8w)
White solid, Yield 82.6%, m.p. 88.2–89.7 °C; IR (KBr) cm−1: 2931, 1614, 1450, 1383, 1231, 683, 505; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 8.10 (d, J = 8.4 Hz, 2H, Ar-H), 7.61 (d, J = 8.4 Hz, 2H, Ar-H), 3.01 (d, J = 6.8 Hz, 2H, CH2), 1.93–2.03 (m, 1H, CH), 1.32 (s, 9H, CH3), 1.02 (d, J = 6.4 Hz, 6H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 174.14, 154.47, 153.42, 145.12, 126.31 (2C), 126.12 (2C), 123.14, 48.37, 35.10, 31.37 (3C), 28.23, 21.66 (2C); HR-ESI-MS m/z: calcd for C17H22N4S3 [M + H]+ 379.1085, found 379.1085.
Tumor cell growth inhibitory assay
The cell lines (SMMC-7721, HeLa, A549, and L929) were cultured in proper medium in a 5% CO2 at 37 °C during the experiment. The inhibition (IC50) of the selected cells proliferation by target compounds 8(a–w) and reference drug was measured by our previous method as described in the literature (Xuan et al. 2015).
References
Akhter MW, Hassan MZ, Amir M (2014) Synthesis and pharmacological evaluation of 3-diphenylmethyl-6-substituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles: a condensed bridgehead nitrogen heterocyclic system. Arab J Chem 7:955–963
Alegaon SG, Parchure P, Araujo LD, Salve PS, Alagawadi KR, Jalalpure SS, Kumbaret VM (2017) Quinoline-azetidinone hybrids: synthesis and in vitro antiproliferation activity against Hep G2 and Hep 3B human cell lines. Bioorg Med Chem Lett 27:1566–1571
Almajan GL, Barbuceanu SF, Bancescu G, Saramet I, Saramet G, Draghici C (2010) Synthesis and antimicrobial evaluation of some fused heterocyclic [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Eur J Med Chem 45:6139–6146
Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 25:2097–2116
Banerjee AG, Das N, Shengule SA, Sharma PA, Srivastava RS, Shrivastava SK (2016) Design, synthesis, evaluation and molecular modelling studies of some novel 5,6-diphenyl-1,2,4-triazin-3(2H)-ones bearing five-member heterocyclic moieties as potential COX-2 inhibitors: a hybrid pharmacophore approach. Bioorg Chem 69:102–120
Bhatt P, Kumar M, Jha A (2018) Synthesis, docking and anticancer activity of azo-linked hybrids of 1,3,4-thia-/oxadiazoles with cyclic imides. Mol Divers 22:827–840
Branowska D, Ławecka J, Sobiczewski M, Karczmarzyk Z, Wysocki W, Wolińska E, Olender E, Mirosław B, Perzyna A, Bielawska A, Bielawski K (2018) Synthesis of unsymmetrical disulfanes bearing 1,2,4-triazine scaffold and their in vitro screening towards anti-breast cancer activity. Mon Chem 149:1409–1420
Cesarini S, Spallarossa A, Ranise A, Schenone S, Bruno O, Colla PL, Casula L, Collu G, Sanna G, Loddo R (2008) Parallel one-pot synthesis and structure–activity relationship study of symmetric formimidoester disulfides as a novel class of potent non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg Med Chem 16:6353–6363
Ceylan S (2016) Synthesis and biological evaluation of new Mannich and Schiff bases containing 1,2,4-triazole and 1,3,4-oxadiazole nucleus. Med Chem Res 25:1958–1970
Chelamalla R, Akena V, Manda S (2017) Synthesis of N′-arylidene-2-(5-aryl-1H-1, 2, 4-triazol- 3-ylthio)acetohydrazides as antidepressants. Med Chem Res 26:1359–1366
Chidananda N, Poojary B, Sumangala V, Kumari NS, Shetty P, Arulmoli T (2012) Facile synthesis, characterization and pharmacological activities of 3,6-disubstituted 1,2,4-triazolo[3,4-b][1,3,4] thiadiazoles and 5, 6-dihydro-3,6-disubstituted-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Eur J Med Chem 51:124–136
Chowrasia D, Karthikeyan C, Choure L, Sahabjada, Gupta M, Arshad M, Trivedi P (2017) Synthesis, characterization and anticancer activity of some fluorinated 3,6-diaryl-[1,2,4]triazolo[3,4-b][1,3, 4]thiadiazoles. Arab J Chem 10:S2424–S2428
Cui P, Li XL, Zhu MY, Wang BH, Liu J, Chen H (2017) Design, synthesis and antimicrobial activities of thiouracil derivatives containing triazolo-thiadiazole as SecA inhibitors. Eur J Med Chem 127:159–165
Deng XQ, Dong ZQ, Song MX, Shu B, Wang SB, Quan ZS (2012) Synthesis and anticonvulsant activities of some triazolothiadiazole derivatives. Arch Pharm Chem Life Sci 345:565–573
Er M, Ergüven B, Tahtaci H, Onaran A, Karakurt T, Ece A (2017) Synthesis, characterization, preliminary SAR and molecular docking study of some novel substituted imidazo[2,1-b][1,3,4]thiadiazole derivatives as antifungal agents. Med Chem Res 26:615–630
Eweiss NF, Bahajaj AA (1987) Synthesis of heterocycles. Part VII synthesis and antimicrobial activity of some 7H-s-triazolo[3,4-b][1,3,4] thiadiazine and s-triazolo[3,4-b] [1,3,4]thiadia-zole derivatives. J Heterocycl Chem 24:1173–1182
Harish KP, Mohana KN, Mallesha L (2014) Synthesis of new 2,5-disubstituted-1,3,4-thiadiazole derivatives and their in vivo anticonvulsant activity. Russ J Bioorg Chem 40:97–105
Hong S, Shin Y, Jung M, Ha MW, Park Y, Lee YJ, Shin J, Oh KB, Lee SK, Park HG (2015) Efficient synthesis and biological activity of Psammaplin A and its analogues as antitumor agents. Eur J Med Chem 96:218–230
Husain A, Shaharyar MRM, Siddiqui AA, Mishra R (2013) Benzimidazole clubbed with triazolo-thiadiazoles and triazolo-thiadiazines: new anticancer agents. Eur J Med Chem 62:785–798
Kahveci B, Menteşe E, Akkaya E, Yılmaz F, Doğan IS, Özel A (2014) Synthesis of some novel 1,2,4-triazol-3-one derivatives bearing the salicyl moiety and their anticonvulsant activities. Arch Pharm Chem Life Sci 347:449–455
Khan I, Tantray MA, Hamid H, Alam MS, Kalam A, Dhulap A (2016) Synthesis of benzimidazole based thiadiazole and carbohydrazide conjugates as glycogen synthase kinase-3b inhibitors with antidepressant activity. Bioorg Med Chem Lett 26:4020–4024
Li ZS, Wang WM, Lu W, Niu CW, Li YH, Li ZM, Wang JG (2013) Synthesis and biological evaluation of nonsymmetrical aromatic disulfides as novel inhibitors of acetohydroxyacid synthase. Bioorg Med Chem Lett 23:3723–3727
Li BC, Zhang DW, Zhang YM, Jiang D, Li S, Lei W, Wang HY, Lin F (2016) Synthesis and evaluation of novel benzene-ethanol bearing 1,2,4-triazole derivatives as potential antimicrobial agents. Med Chem Res 26:44–51
Liu DC, Gong GH, Wei CX, Jin XJ, Quan ZS (2016) Synthesis and anti-inflammatory activity evaluation of a novel series of 6-phenoxy-[1,2,4]triazolo[3,4-a]phthalazine-3-carboxamide derivatives. Bioorg Med Chem Lett 26:1576–1579
Menteşe E, Karaali N, Yılmaz F, Ülker S, Kahveci B (2013) Microwave-assisted synthesis and biological evaluation of some benzimidazole derivatives containing a 1,2,4-triazol ring. Arch Pharm Chem Life Sci 346:556–561
Meti GY, Kamble AA, Kamble RR, Somagond SM, Devarajegowda HC, Kumari S, Kalthur G, Adiga SK (2016) Synthesis, anti-proliferative and genotoxicity studies of 6-chloro-5-(2-substituted-ethyl)-1,3-dihydro-2H-indol-2-ones and 6-chloro-5-(2-chloroethyl)-3-(alkyl/ary-2-ylidene)indo-lin-2-ones. Eur J Med Chem 121:221–231
Miniyar PB, Mahajan AA, Mokale SN, Shah MU, Kumar AS, Chaturbhuj GU (2017) Triazole hybrids as new type of anti-fungal agents. Arab J Chem 10:295–299
Padmaja A, Pedamalakondaiah D, Sravya G, Reddy GM, Kumar MVJ (2015) Synthesis and antioxidant activity of a new class of sulfone/sulfonamide-linked bis(oxadiazoles), bis(thiadiazoles), and bis(triazoles). Med Chem Res 24:2011–2020
Roldán-Peňa JM, Alejandre-Ramos D, López Ó, Maya I, Lagunes I, Padrón JM, Peňa-Altamira LE, Bartolini M, Monti B, Bolognesi ML, Fernández-Bolaňos JG (2017) New tacrine dimers with antioxidant linkers as dual drugs: anti-Alzheimer’s and antiproliferative agents. Eur J Med Chem 138:761–773
Rostom SAF, Badr MH, El Razik HAA, Ashour HMA (2017) Structure-based development of novel triazoles and related thiazolotriazoles as anticancer agents and Cdc25A/B phosphatase inhibitors. Synthesis, in vitro biological evaluation, molecular docking and in silico ADME-T studies. Eur J Med Chem 139:263–279
Rubino S, Busà R, Attanzio A, Alduina R, Stefano VD, Girasolo MA, Orecchio S, Tesoriere L (2017) Synthesis, properties, antitumor and antibacterial activity of new Pt(II) and Pd(II) complexes with 2,20-dithiobis(benzothiazole) ligand. Bioorg Med Chem 25:2378–2386
Shang J, Wang WM, Li YH, Song HB, Li ZM, Wang JG (2012) Synthesis, crystal structure, in vitro acetohydroxyacid synthase inhibition, in vivo herbicidal activity, and 3D-QSAR of new asymmetric aryl disulfides. J Agric Food Chem 60:8286–8293
Sheppard JG, Frazier KR, Saralkar P, Hossain MF, Geldenhuys WJ, Long TE (2018) Disulfiram-based disulfides as narrow-spectrum antibacterial agents. Bioorg Med Chem Lett 28:1298–1302
Sirakawa K, Aki O, Tsujikawa T (1970) S-alkylthioisothioureas. I. Chem Pharm Bull 18:235–242
Turos E, Revell KD, Ramaraju P, Gergeres DA, Greenhalgh K, Young A, Sathyanarayan N, Dickey S, Lim D, Alhamadsheh MM, Reynolds K (2008) Unsymmetric aryl-alkyl disulfide growth inhibitors of methicillinresistant staphylococcus aureus and bacillus anthracis. Bioorg Med Chem 16:6501–6508
Vale N, Ferreira A, Fernandes I, Alves C, Araújo MJ, Mateus N, Gomes P (2017) Gemcitabine anti-proliferative activity significantly enhanced upon conjugation with cell-penetrating peptides. Bioorg Med Chem Lett 27:2898–2901
Vudhgiri S, Koude D, Veeragoni DK, Misra S, Prasad RBN, Jala RCR (2017) Synthesis and biological evaluation of 5-fatty-acylamido-1,3,4-thiadiazole-2-thioglycosides. Bioorg Med Chem Lett 27:3370–3373
Xu QL, Sun ML, Bai ZS, Wang YT, Wu Y, Tian HQ, Zuo DY, Guan Q, Bao K, Wu YL, Zhang WG (2017) Design, synthesis and bioevaluation of antitubulin agents carrying diaryl-5,5-fused-heterocycle scaffold. Eur J Med Chem 139:242–249
Xuan LN, Wang P, Zhang K, Shi YP, Liu YM, Zhu T, Chen BQ (2015) Synthesis and in vitro antiproliferative activity of novel benzisoselenazolone derivatives. Med Chem Res 24:543–552
Zhao PL, Chen P, Li Q, Hu MJ, Diao PC, Pan ES, You WW (2016) Design, synthesis and biological evaluation of novel 3-alkylsulfanyl-4-amino-1,2,4-triazole derivatives. Bioorg Med Chem Lett 26:3679–3683
Acknowledgements
We are grateful to the Tianjin Municipal Natural Science Foundation (18JCYBJC94900) and Training Project of Innovation Team of Colleges and Universities in Tianjin (TD13-5020) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
About this article
Cite this article
Liu, XJ., Liu, HY., Wang, HX. et al. Synthesis and antitumor evaluation of novel fused heterocyclic 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives. Med Chem Res 28, 1718–1725 (2019). https://doi.org/10.1007/s00044-019-02409-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02409-2