Abstract
A novel series of 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives was synthesized from 1-aryl-1,3-diazidopropan-2-ol derivatives and diverse alkynes using copper catalyzed azide-alkyne cycloaddition in the key step. Most of synthesized compounds showed high activity against Candida spp. strains at a 0.04–0.5 μg/mL concentration range compared to Itraconazole and Fluconazole (MIC 2.56 and 1.28 μg/mL, respectively), which were used as reference compounds. A 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivative (R1 = F and R2 = cyclopropyl) displayed an outstanding selectivity against Candida albicans and Candida krusei (MIC = 0.0075 µg/mL). Moreover, Artemia salina bioassay on 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives revealed low toxicity in this kind of compounds. In addition, molecular docking studies suggest good binding affinity of halogen atoms in some 1-aryl-1,3-diazidopropan-2-ol derivatives to HEME group present in 14-alpha demethylase (CYP51), which might explain the high antifungal activity found in these compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triazoles are important heterocyclic rings, which integrate the structure of several pharmacologically active compounds from anticancer agents to antibiotics (Lass-Flörl 2011; Dismukes 1988; Saag and Dismukes 1988). A representative example of this kind of molecules is Fluconazole (1) (Richardson et al. 1985; Heeres et al. 2010), a leading drug for fungal infections treatment, which works as 14α-demethylase inhibitor at fungi cell membrane level (Sagatova et al. 2015), with favorable pharmacokinetic and pharmacodynamic characteristics that make Fluconazole an effective, powerful, safe, and low toxicity compound (Andes and van Ogtrop 1999; Rogers and Galgiani 1986). However, the emergence of new pathogens as well as resistance to Fluconazole (Dutcher 2008; Warnock et al. 1988) have promoted the development of modifications on its structure aimed to broaden the antifungal activity spectrum as well as potency increasing. Thus, several Fluconazole analogues have been designed and synthesized from the 2-aryl-1-(1,2,4-triazolyl)propan-2-ol structure presenting significant antifungal activity (Wang et al. 2014, Subhas et al. 2012; Zhang et al. 2011; Xu et al. 2009; Lebouvier et al. 2007; Silvestri et al. 2004; Heravia and Motamedi 2004; Eto et al. 2000; Dickinson et al. 1996).
On the other hand, copper-catalyzed azide-alkyne cycloaddition (CuAAC), the most important click reaction (Meldal and Tornoe 2008; Moses and Moorhouse 2007; Bock et al. 2006), represents a promissory source of biologically active compounds (Bonandi et al. 2017; Thirumurugan et al. 2013, Haider et al. 2014, Agalave et al. 2011), and especially, molecules with antifungal activity due to recent works, which have described this property in different 1,4-disubstituted 1,2,3-triazoles derived from this click reaction (Irfan et al. 2015; Aufort et al. 2008).
In this regard, CuAAC reaction has been successfully used for the preparation of Fluconazole derivative libraries, proving to be a powerful synthetic tool through a strategy based on a heterocyclic ring change where one of the 1,2,4-triazole rings was substituted by 1,2,3-triazole prepared from click chemistry obtaining derivatives from 2-aryl-1-(1,2,4-triazolyl)-3-(1,2,3-triazolyl)propan-2-ol (2), which displayed important activity against diverse human pathogenic fungi (Wang. et al. 2014; Pore et al. 2012; Zou et al. 2012, Aher et al. 2009; Pore et al. 2006).
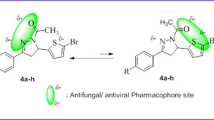
Inspired by these facts, we propose a novel approach, which would allow the synthesis of bis(1,2,3-triazolyl)propan-2-ol derivatives (3) as Fluconazole analogues with both 1,2,4-triazole rings substituted by the 1,2,3-triazole moiety (Fig. 1). This report summarizes our most recent findings in the click chemistry area with the purpose to search and develop new molecules with high antifungal activity using simplest synthetic methodologies according to click chemistry philosophy. (Scheme 1)
Materials and methods
General remarks
The starting materials were purchased from Aldrich Chemical Co. and were used without further purification. Solvents were distilled before use. Silica plates of 0.20-mm thickness were used for thin layer chromatography. Melting points were determined with a Krüss Optronic melting point apparatus and they are uncorrected. 1H and 13C NMR spectra were recorded using a Bruker Avance 300 MHz, and a Varian 500 MHz; the chemical shifts (δ) are given in ppm relative to TMS as internal standard (0.00). For analytical purposes the mass spectra were recorded on a Shimadzu GCMS-QP2010 Plus in the EI mode, 70 eV, 200 °C via direct inlet probe. Only the molecular and parent ions (m/z) are reported. IR spectra were recorded on a Bruker TENSOR 27 FT instrument.
Synthesis of 1,3-diazido-propan-2-one (5)
Typical procedure. 1,3-dichloroacetone (2.0 g, 16.0 mmol) was added to a stirred mixture of NaN3 (5.2 g, 7.98 mmol) and acetone (30 mL), and the resulting mixture was stirred at room temperature for 12–15 h. The mixture was filtered and the solvent was removed under reduced pressure. The compound was extracted with methyl tertbutyleter (2 × 30 mL), the organic layers were joined and dried over Na2SO4 and the solvent was removed under reduced pressure to yield 1,3-diazido-propan-2-one as a pale solid (2.1 g, 14.34 mmol, 91%), which was used without further purification. IR (ATR) νmax 2104, 1757 cm−1. 1H NMR: (300 MHz, CDCl3) δ = 4.086 (s, 4 H). 13C NMR: (75 MHz, CDCl3) δ = 199.9 (1C), 57.6 (2XCH2). MS [EI+] m/z (%): 140 [M]+ (50).
General procedure of synthesis of 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives
A solution of the appropriate arylmagnesium bromide (15.91 mmol, 2.0 M in THF) and CH2Cl2 (15 mL) was cooled to −75 °C. A solution of 1,3-diazido-propan-2-one (2.13 g, 15.91 mmol) in CH2Cl2 (15 mL) was added dropwise and the resulting mixture was stirred under an inert atmosphere at −75 °C for 4 h and at room temperature for 24 h. A saturated solution of NH4Cl (40 mL) was added and the mixture was stirred for 30 min. The organic layer was separated and the aqueous phase was extracted with CH2Cl2 (3 × 15 mL). The organic layers were joined and dried over Na2SO4 and the solvent was removed under reduced pressure to yield the corresponding 1,3-diazido-2-arylpropan-2-ols, which were used without purification. A solution of the corresponding alkyne (17.0 mmol) in MeOH (15 mL) was added to a solution of the appropriate 1,3-diazido-2-arylpropan-2-ol (11.43 mmol) in MeOH (10 mL). The mixture was stirred at room temperature for 5 min, a 2 N solution of NaOH (2 mL, 0.160 g, 4.0 mmol) and CuI (0.3063 g, 2.29 mmol) were successively added, and the resulting mixture was stirred at room temperature for 24 h. The solvent was removed under reduced pressure and concentrated NH4OH solution (40 mL) was added and the mixture was stirred for 2 h. The product was filtered, washed with H2O (20 mL) and cold MeOH (20 mL), and dried under reduced pressure to afford the corresponding1,3-bis-(4-cyclopropyl-1,2,3-triazol-1-yl)-2-arylpropan-2-ol derivative, which was purified by crystallization.
2-(4-Fluoro-phenyl)-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol (7)
1,3-Diazido-2-(4-fluoro-phenyl)-propan-2-ol and phenylacetylene afforded 2-(4-Fluoro-phenyl)-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol as a white solid. Yield: 4.52 g (90%). IR (ATR) νmax 3575, 3092, 1487 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 8.31 (s, 2H), 7.82 (d, J = 6.6 Hz, 4H), 7.55 (m, 2H), 7.46 (m, 4H), 7.35 (d, J = 6.3 Hz, 2H), 7.14 (t, J = 7.5 Hz, 2H), 6.47 (s, 1H), 4.98 (d, J = 14.1 Hz, 2H), 4.81 (d, J = 14.3 Hz, 2H). 13C NMR: (75 MHz, DMSO-d6) δ = 163.5759–160.3528 (C, JC-F = 241.73 Hz), 146.20 (2XC), 136.86 (C), 131.12 (2XC), 129.39 (2C), 128.49–128.60 (CH, JC-F = 8.49 Hz), 128.30 (4XCH), 125.57 (4XCH), 123.10 (2 X CH), 115.26–114.9819 (CH, JC-F = 21.02 Hz), 75.15 (C), 57.61 (2 X CH2). MS [EI+] m/z (%): 440[M]+ (10), 363 [M – C6H5]+ (100). HRMS (EI): for C25H21FN6O calcd. 440.1761, found 440.1765.
1,3-Bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-2-(4-fluorophenyl)-propan-2-ol (8)
1,3-Diazido-2-(4-fluoro-phenyl)-propan-2-ol and cyclopropylacetylene afforded 1,3-Bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-2-(4-fluoro-phenyl)-propan-2-ol as a white solid. Yield: 122.5 mg (61%). IR (ATR) νmax 3539, 3074, 1483 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 7.51 (s, 2H), 7.41 (d, J = 8.3 Hz, 2H), 7.10 (d, J = 8.3 Hz, 2H), 6.23 (s, 1H), 4.74 (d, J = 14.1, 2H), 4.69 (d, J = 14.0 Hz, 2H), 1.89 (q, 2H), 0.87 (d, J = 6.6 Hz, 2H), 0.65 (s, 2H). 13C NMR: (75 MHz, DMSO-d6) δ = 163.49–160.26 (C, JC-F = 242.19 Hz), 148.67 (2XC), 136.97 (1C), 128.52–128.41 (2XCH, JC-F = 7.94 Hz), 122.48 (2XCH), 115.13–114.85 (2XCH, JC-F = 21.0675 Hz), 75.06 (C), 57.2965 (2XCH2), 8.08 (2XCH), 6.90 (4XCH2). MS [EI+] m/z (%): 368[M]+ (23), 246 [M - C6H8N3]+ (100). HRMS (EI): for C19H21FN6O calcd. 368.1761, found 368.1759.
1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-(4-fluorophenyl)-propan-2-ol (9)
1,3-Diazido-2-(4-fluoro-phenyl)-propan-2-ol and 3,3-Dimethyl-but-1-yne afforded 1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-(4-fluoro-phenyl)-propan-2-ol as a white solid. Yield: 152.9 mg (63%). IR (ATR) νmax 3533, 3076, 1485 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 7.48 (s, 2H), 7.38 (d, J = 8.5 Hz, 2H), 7.09 d, (J = 8.3 Hz, 2H), 6.25 (s, 1H), 4.77 (d, J = 14.1, 2H), 4.64 (d, J = 14.1 Hz, 2H), 1.22 (s, 18H). 13C NMR: (75 MHz, DMSO-d6) δ = 163.52–160.29 (C, JC-F = 242.07 Hz), 156.00 (2XC), 137.08 (C), 128.48–128.37 (2XCH, JC-F = 8.2125 Hz), 121.4054 (2XCH), 115.01–114.73 (2XCH, JC-F = 21.03 Hz), 75.18 (1C), 57.23 (2XCH2), 30.69 (6XCH3), 30.65 (2XC). MS [EI+] m/z (%): 400 [M]+ (20), 262 [M – C7H12N3]+ (100). HRMS (EI): for C21H29FN6O calcd. 400.2387, found 400.2386.
2-Phenyl-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol (10)
1,3-Diazido-2-phenyl-propan-2-ol and phenylacetylene afforded 2-Phenyl-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol as a white solid. Yield: 122 mg (58%). IR (ATR) νmax 3570, 3088, 1485 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 8.24 (s, 2H), 7.79 (d, 4H), 7.52–7.42 (m, 11H), 6.37 (s, 1H), 4.89 (d, J = 14.4, 2H), 4.62 (d, J = 14.1 Hz, 2H). 13C NMR: (75 MHz, DMSO-d6) δ = 146.13 (2XC), 140.74 (C), 131.14 (CH), 129.41 (2XC), 128.42 (2XCH), 128.30 (2XCH), 128.04 (2XCH), 126.31 (2XCH), 125.55 (4xCH), 123.09 (2XCH), 75.30 (C), 57.65 (2XCH2). MS [EI+] m/z (%): 422 [M]+ (11), 264 [M – C9H8N3]+ (100). HRMS (EI): for C25H22N6O calcd. 422.1855, found 422.1857.
1,3-Bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-2-phenyl-propan-2-ol (11)
1,3-Diazido-2-phenyl-propan-2-ol and cyclopropylacetylene afforded 1,3-Bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-2-phenyl-propan-2-ol as a white solid. Yield: 167.2 mg (63%). IR (ATR) νmax 3540, 3110, 1450 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 7.50 (s, 2H), 7.43–7.30 (m, 5H), 6.19 (s, 1H), 4.76 (d, J = 13.2, 2H), 4.59 (d, J = 13.0 Hz, 2H), 1.918 (s, 2H), 0.905 (s, 2H), 0.672 (s, 2H). 13C NMR: (75 MHz, DMSO-d6) δ = 148.64 (2XC), 140.91 (1C), 128.33 (CH), 127.93 (2XCH), 126.28 (2XCH), 122.47 (CH), 75.23 (C), 57.37 (2XCH2), 8.10 (2XCH2), 6.93 (2XCH2). MS [EI+] m/z (%): 350 [M]+ (10), 228 [M – C6H8N3]+ (100). HRMS (EI): for C19H22N6O calcd. 350.1855, found 350.1859.
1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-phenyl-propan-2-ol (12)
1,3-Diazido-2-phenyl-propan-2-ol and 3,3-Dimethyl-but-1-yne afforded 1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-phenyl-propan-2-ol as a white solid. Yield: 129.9 mg (57%). IR (ATR) νmax 3516, 3095, 1465 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 7.46 (s, 2H), 7.36 (m, 2H), 7.26 (m, 3H), 6.32 (s, 1H), 4.77 (d, J = 14.1, 2H), 4.59 (d, J = 14.1 Hz, 2H), 1.22 (s, 18H). 13C NMR: (75 MHz, DMSO-d6) δ = 155.90 (2XC), 141.02 (C), 128.20 (CH), 127.82 (2XCH), 126.28 (2XCH), 121.41 (2XCH), 75.33 (C), 57.24 (2XCH2), 33.49 (C), 30.67 (6XCH3). MS [EI+] m/z (%): 382 [M]+ (10), 244 [M – C7H12N3]+ (100), 77 [C6H5]+ (70). HRMS (EI): for C21H30N6O calcd. 382.2481, found 382.2487.
2-(4-Methoxyphenyl)-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol (13)
1,3-Diazido-2-(4-methoxy-phenyl)-propan-2-ol and phenylacetylene afforded 2-(4-Methoxy-phenyl)-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol as white solid. Yield: 133.1 mg (52%). IR (ATR) νmax 3514, 3034, 1431 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 8.30 (s, 2H), 7.83 (d, J = 6.9 Hz, 4H), 7.46 (d, J = 6.3 Hz, 4H), 7.37 (d, 6.6 Hz, 2H), 7.36 (m, 2H), 6.88 (d, J = 7.5 Hz, 2H), 6.31 (s, 1H), 4.93 (d, J = 14.6 Hz, 2H), 4.87 (d, J = 14.2 Hz, 2H) 3.74 (s, 3H). 13C NMR: (75 MHz, DMSO-d6) δ = 158.95 (C), 146.15 (2XC), 132.60 (C), 131.21 (2XC), 129.43 (4XCH), 128.32 (4XCH), 127.60 (2XCH), 125.59 (2XCH), 123.11 (2XCH), 113.73 (2XCH), 75.07 (C), 57.78 (2XCH2), 55.46 (CH3). MS [EI+] m/z (%): 252 [M]+ (20), 294 [M – C9H8N3]+ (100). HRMS (EI): for C26H24N6O2 calcd. 452.1961, found 452.1965.
1,3-Bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-2-(4-methoxyphenyl)-propan-2-ol (14)
1,3-Diazido-2-(4-methoxy-phenyl)-propan-2-ol and cyclopropylacetylene afforded 1,3-Bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-2-(4-methoxy-phenyl)-propan-2-ol as white solid. Yield: 145.8 mg (54%). IR (ATR) νmax 3520, 3067, 1432 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 7.49 (s, 2H), 7.31 (d, J = 8.1 Hz, 2H), 6.85 (d, J = 8.1 Hz, 2H), 6.09 (s, 1H), 4.69 (d, J = 14.1, 2H), 4.56 (d, J = 14.0 Hz, 2H), 3.75 (s, 3H), 1.90 (q, 2H), 0.88 (d, 4H), 0.66 (d, 4H). 13C NMR: (75 MHz, DMSO-d6) δ = 158.85 (C), 148.60 (2XC), 132.72 (C), 127.5 (2XCH), 122.45 (2XCH), 113.61 (2XCH), 74.94 (C), 55.45 (CH3) 54.43 (2XCH2), 6.92 (2XCH), 8.09 (4XCH2). MS [EI+] m/z (%): 300 [M]+ (10), 258 [M – C6H8N3]+ (100). HRMS (EI): for C20H24N6O2 calcd. 380.1961, found 380.1966.
1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-(4-methoxyphenyl)-propan-2-ol (15)
1,3-Diazido-2-(4-methoxy-phenyl)-propan-2-ol and 3,3-Dimethyl-but-1-yne afforded 1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-(4-methoxy-phenyl)-propan-2-ol as white solid. Yield: 145.8 mg (66%). IR (ATR) νmax 3526, 3067, 1432 cm−1. 1H NMR: (300 MHz, DMSO-d6) δ = 7.52 (s, 2H), 7.35 (d, 2H), 6.92 (d, 2H), 6.16 (s, 1H), 4.79 (d, J = 12.3, 2H), 4.60 (d, J = 12.5 Hz, 2H), 1.31 (s, 18H), 3.81 (s, 3H). 13C NMR: (75 MHz, DMSO-d6) δ = 159.01 (C), 156.02 (2XC), 132.96 (C), 127.57 (2XCH), 121.50 (2XCH), 113.62 (2XCH), 75.12 (C), 55.57 (CH3), 57.42 (2XCH2), 33.49 (C), 30.77 (6XCH3). MS [EI + ] m/z (%): 412 [M]+ (20), 274 [M – C7H12N3]+ (100). HRMS (EI): for C22H32N6O2 calcd. 412.2587, found 412.2589.
2-(4-Chlorophenyl)-1,3-bis-(4-phenyl-[1,2,3]triazol-1-yl)-propan-2-ol (16)
2-{4-[1-(Toluene-4-sulfonyl)-1,2,3-triazol-4-yl]butyl}isoindole-1,3-dione afforded 2-[4-(1,2,3-triazol-4-yl)butyl]isoindole-1,3-dione as white solid. Yield: 145.8 mg (84%). IR (ATR) νmax 3126, 3067, 2940, 2871, 1702 cm−1. 1H NMR: (300 MHz, CDCl3) δ = 13.14 (s, 1H), 7.84 (dd, J = 5.4, 3.0 Hz, 2H), 7.71 (dd, J = 5.3, 3.0 Hz, 2H), 7.52 (s, 1H), 3.73 (s, 2H), 2.81 (s, 2H), 1.82 – 1.70 (m, 4H). 13C NMR: (75 MHz, CDCl3) δ = 167.35(2xC), 144.58 (C), 132.78 (2xCH), 130.84 (2xC), 129.70 (CH), 122.06 (2xCH), 36.32 (CH2), 26.82 (CH2), 25.20 (CH2), 23.15 (CH2). MS [EI+] m/z (%): 456 [M]+ (30), 345 [M – C6H4Cl]+ (100). HRMS (EI): for C25H21ClN6O calcd. 456.1465, found 270.1469.
2-(4-Chlorophenyl)-1,3-bis-(4-cyclopropyl-[1,2,3]triazol-1-yl)-propan-2-ol (17)
2-{4-[1-(Toluene-4-sulfonyl)-1,2,3-triazol-4-yl]butyl}isoindole-1,3-dione afforded 2-[4-(1,2,3-triazol-4-yl)butyl]isoindole-1,3-dione as white solid. Yield: 145.8 mg (80%). IR (ATR) νmax 3126, 3067, 2940, 2871, 1702 cm−1. 1H NMR: (300 MHz, CDCl3) δ = 13.14 (s, 1H), 7.84 (dd, J = 5.4, 3.0 Hz, 2H), 7.71 (dd, J = 5.3, 3.0 Hz, 2H), 7.52 (s, 1H), 3.73 (s, 2H), 2.81 (s, 2H), 1.82–1.70 (m, 4H). 13C NMR: (75 MHz, CDCl3) δ = 167.35(2xC), 144.58 (C), 132.78 (2xCH), 130.84 (2xC), 129.70 (CH), 122.06 (2xCH), 36.32 (CH2), 26.82 (CH2), 25.20 (CH2), 23.15 (CH2). MS [EI+] m/z (%): 384 [M]+ (10), 273 [M – C6H4Cl]+ (100). HRMS (EI): for C19H21ClN6O calcd. 384.1465, found 384.1467.
1,3-Bis-(4-tert-butyl-[1,2,3]triazol-1-yl)-2-(4-chlorophenyl)-propan-2-ol (18)
2-{4-[1-(Toluene-4-sulfonyl)-1,2,3-triazol-4-yl]butyl}isoindole-1,3-dione afforded 2-[4-(1,2,3-triazol-4-yl)butyl]isoindole-1,3-dione as white solid. Yield: 145.8 mg (78%). IR (ATR) νmax 3129, 3067, 2940, 2875, 1702 cm−1. 1H NMR: (300 MHz, CDCl3) δ = 13.14 (s, 1H), 7.84 (dd, J = 5.4, 3.0 Hz, 2H), 7.71 (dd, J = 5.3, 3.0 Hz, 2H), 7.52 (s, 1H), 3.73 (s, 2H), 2.81 (s, 2H), 1.82–1.70 (m, 4H). 13C NMR: (75 MHz, CDCl3) δ = 167.35(2xC), 144.58 (C), 132.78 (2xCH), 130.84 (2xC), 129.70 (CH), 122.06 (2xCH), 36.32 (CH2), 26.82 (CH2), 25.20 (CH2), 23.15 (CH2). MS [EI+] m/z (%): 270 [M]+ (40), 104 [C7H4O]+ (100), 76 [C6H4]+ (80). HRMS (EI): for C21H29ClN6O calcd. 416.2091, found 416.2097.
In vitro antifungal activities
The antifungal activity of compounds 7–18 was screened using a reference strain of yeasts from American Type Culture Collection, Candida albicans ATCC 10231, which was included as a control strain, and four clinical isolates, which were obtained from the Maternal Perinatal Hospital Monica Pretelini Sáenz as well as the following strains provided by the microbiology laboratory of the Universidad Autónoma del Estado de México, these are clinical isolates of infections associated with health care: Candida krusei, Candida glabrata, and Candida tropicalis.
The MICs of compounds 7–18 and the control antifungal agents were determined consistently using the broth microdilution method for yeasts developed by the Clinical and Laboratory Standards Institute and published in the document M27-A3 (Rex et al. 2008). For all organisms, RPMI 1640 medium with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (pH 7.0) was used as the test medium. MICs were determined after incubation at 30 °C for 16 to 24 h and defined by the criteria of the CLSI procedures.
Toxicity evaluation of compounds using Artemia salina
The toxicity evaluation was done with the crustacean Artemia salina and the procedure was established and standardized as follows: Brine shrimp (Artemia salina Leach) eggs were purchased from a pet store. The brine shrimp eggs were hatched in shallow rectangular tray (22 × 23 cm) containing artificial seawater (3.8% sea salt solution, prepared from Instant Ocean Salt Mix, Aquatic Eco-Systems, Inc., FL) at room temperature. The eggs were sprinkled into the darken compartment and after the eggs hatched into free-swimming forms, the nauplii moved to the light side. After 48 h the phototrophic nauplii were collected. A suspension of 10 nauplii was added to each well and the covered microplate was incubated for 24 h at room temperature. The number of dead nauplii in each well was counted using a binocular microscope. For the test, salt water solution was used as positive control, whereas a potassium dichromate was used as negative control. The LD50 was determined according to the Reed–Muench method (Sam 1993; Reed and Muench 1938).
Molecular modeling
All compounds were optimized with the PBE0/3-21G method implemented in the software NWChem 6.5. In each case, was verified that the resulting structure belonged to a minimum of energy on the surface of potential energy, by means of the criterion of non-imaginary frequencies. The docking calculation was performed in the active site reported in the X-ray structure of the sterol 14-alpha demethylase (CYP51) of Candida albicans available in the Protein Data Bank, employing autodock vina 1.1.2. With the aim of perform a quantitative description of the electronic properties, some quantum chemical descriptors: Ionization Potential (IP), Electron affinity (EA), electrophilia (χ), hardness (η), and electronic chemical potential (μ) were obtained under the Khon-Sham framework and the PBE0/3-21G//PBE0/3-21++G method. Virtual Computational Chemistry Laboratory at website http://www.vcclab.org was used for the calculation of log P and Log S values of the compounds by various methods based on different theoretical procedures.
Results and discussion
Chemistry
Preliminary studies were focused to prepare 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives 3, which were obtained through a synthetic sequence that began with SN2 reaction on 1,3-dichloroacetone 4 to afford 1,3-diazidoacetone 5, which in turn was reacted with diverse Grignard reagents yielding the corresponding 1,3-diazido-2-arylpropan-2-ol derivatives 6 (Scheme 2). In the final step, CuAAC reaction of compounds 6 with the appropriate alkynes gave 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives 3 in ca 50–90% overall yields. These results are summarized in Table 1, showing yields in last CuAAC reaction step.
Biological evaluation
All compounds were characterized by the conventional spectroscopic techniques and compounds 7–18 were tested for the in vitro antifungal activity, showing activity against clinical strains of Candida spp. isolated from Maternal Perinatal Hospital Monica Pretelini Sáenz and strains provided by the Laboratory of Microbiology of UAEM, as well as control strain Candida albicans ATCC 10231. The minimum inhibitory concentrations (MIC) of compounds 7–18 against the above mentioned yeast strains are shown in Table 2, according to the microdilution method M27-A3 for yeasts described by CLSI standard protocol (Rex et al. 2008).
From in vitro evaluation, compounds 7, 9, 10, 12, 13, 15, 16, and 17 displayed inhibition of Candida albicans, Candida krusei at a concentration of 0.04 µg/mL, and inhibition of Candida glabrata and Candida tropicalis as well as control strain Candida albicans ATCC 10231 at a concentration of 0.5 µg/mL. Hence, MIC of these 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives for Candida albicans is lower than other reported for similar compounds with an activity below fluconazole and itraconazole (Salake et al. 2014).
On the other hand, compounds 8 and 14 proved to be effective for the inhibition of strains of Candida spp. and the control strain Candida albicans ATCC 10231 showing similar behavior to azoles used as control (fluconazole and itraconazole) and compared to other similar compounds (Layton-Tovar et al. 2014; Miyazaki et al. 2011).
A noteworthy feature is that compound 8 exhibits an inhibitory effect on strains of Candida albicans and Candida krusei below 0.0075 µg/mL, whereas antifungal activity against other Candida strains is similar, and in some cases, lower (Candida glabrata, Candida tropicalis, MIC = 2.56 µg/mL), which can be attributed to a combination of the effect of cyclopropyl groups and fluorine atom presents in this molecule. This property suggests a high selectivity of compound 8 inhibiting these kind of Candida strains.
Furthermore, Artemia salina bioassay was used to assess toxicity of compounds 7–18 according to procedures described by the Wiwat (Woradulayapinij et al. 2005) and Camper (Turker and Camper 2002) groups, and corresponding LD50 values of the 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives were calculated by Reed-Muench statistical method (Sam 1993; Reed and Muench 1938). From this analysis, which included Fluconazole, Itraconazole, DMSO, and RPMI 1640 with MOPS, together with the controls Saltwater (negative) and potassium dichromate (positive), only potassium dichromate (positive control) and DMSO were toxic to Artemia salina, and 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives 7–18 proved to be non-toxic in this assay, as well as RPMI 1640 broth supplemented with MOPS, itraconazole, and fluconazole showed no toxicity to Artemia salina. Thus, this study also reveals that studied compounds are relatively harmless to environment (Carballo et al. 2002).
Molecular docking studies
Initial theoretical studies included a geometrical optimization. All the molecular optimized structures show a folded conformation among aromatic rings displaying the formation of two possible intramolecular hydrogen bond interactions, the first interaction occurs between the –OH hydrogen with the N3 triazole electron rich zone, and a second interaction between the C5 triazole hydrogen and the oxygen of the tertiary alcohol. These interactions provide some rigidity to the system and an arrangement, which suggests a less interaction of the tertiary alcohol with the receptor. Some molecular optimized structures are represented in Fig. 1.
In addition, Fig. 2 shows the docking calculation results for the 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives with best (compound 8) and worst (compound 15) biological activity, in both cases a direct interaction of the heterocyclic rings to HEME group in 14-alpha demethylase (CYP51) was observed. Other important interactions also take place through the halogen atoms 4-substituted in phenyl ring with HEME moiety, and through π–π interactions for the compound 15 with lowest biological activity. This good binding affinity of halogen atoms could be related with the high antifungal activity in compounds 7, 8, 9, 16, 17, and 18, and would explain an improvement in the antifungal activity when fluorine atoms are used. For compound 8, we observed an additional interaction of 1,2,3-triazole ring with Met508, which in turn interacts with cyclopropyl group that was also seen to form interactions with Pro230 and Leu121.
The geometric results provided by a scoring function usually are not enough for the elucidation of the enzyme inhibition phenomena, for this reason we analyzed some electronic descriptors, which are gathered in Table 3: Ionization Potential (IP), Electron affinity (EA), electrophilia (χ), hardness (η), and electronic chemical potential (μ). However, the direct correlation between obtained values with experimental data did not provide additional information that could support a plausible explanation. Similarly, no conclusive results have been found from analysis of some physicochemical properties calculations summarized in Table 4 except that an increase in the hydrophilic character of compounds containing cyclopropyl groups has been identified.
To the best of our knowledge, these are the first examples of Fluconazole analogues where all 1,2,4-triazole rings were changed by 1,2,3-triazoles obtaining a new family of compounds with high antifungal activity, which would open a new trend in the design and synthesis of antifungal compounds
Conclusions
In summary, a small library of 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives analogues to Fluconazole was prepared through click chemistry approach as key reaction through a short synthetic sequence, which required three steps using commercially available starting materials. The compounds evaluated in this investigation presented an important activity to inhibit hospital strains of Candida spp. and control strain Candida albicans ATCC 10231. Moreover, most of these compounds resulted not toxic to Artemia salina, which suggests promising applications as drugs. These elements allow to propose 1,1-diaryl-2-(1,2,3)triazol-1-yl-ethanol derivatives to subsequent biological assays in order to consider them as possible drugs. The simplicity of synthetic methods and antifungal activities found in some compounds suggests that this kind of Fluconazole analogues will enjoy a widespread application.
References
Agalave SG, Maujan SR, Pore VS (2011) Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem Asian J 6:2696–2718
Aher NG, Pore VS, Mishra NN, Kumar A, Shukla PK, Sharma A, Bhat MK (2009) Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg Med Chem Lett 19:759–763
Andes D, van Ogtrop M (1999) Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother 43:2116–2120
Aufort M, Herscovici J, Bouhours P, Moreau N, Girard C (2008) Synthesis and antibiotic activity of a small molecules library of 1,2,3-triazole derivatives. Bioorg Med Chem Lett 18:1195–1198
Bock VD, Hiemstra H, van Maarseveen JH (2006) CuI-catalyzed alkyne–azide “Click” cycloadditions from a mechanistic and synthetic perspective. Eur J Org Chem 51–68
Bonandi E, Christodoulou MS, Fumagalli G, Perdicchia D, Rastelli G, Passarella D (2017) The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov Today 22:1572–1581
Carballo JL, Hernández-Inda ZL, Pérez P, García-Grávalos MD (2002) A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol 2:17, http://www.biomedcentral.com/1472-6750/2/17
Dickinson RP, Bell AS, Hitchcock CA, Narayanaswami S, Ray SJ, Richardson K, Troke PF (1996) Novel antifungal 2-aryl-i-(ih-1,2,4-triazol-1-yl)butan-2-ol derivatives with high activity against Aspergillus fumigatus. Bioorg Med Chem Lett 6:2031–2036
Dismukes WE (1988) Azole antifungal drugs: old and new. Ann Intern Med 109:177–179
Dutcher R (2008) Candidemia: optimizing the dose of fluconazole. US Pharm 33:HS14–HS19
Eto H, Kaneko Y, Sakamotoa T (2000) New antifungal 1,2,4-triazoles with Difluoro(heteroaryl)methyl moiety. Chem Pharm Bull 48:982–990
Haider S, Alam MS, Hamid H (2014) 1,2,3-Triazoles: scaffold with medicinal significance. Inflamm Cell Signal 1:e95
Heeres J, Meerpoel L, Lewi P (2010) Conazoles. Molecules 15:4129–4188
Heravia MM, Motamedi R (2004) Synthesis of some new propanol derivatives analogous to fluconazole. Phosphorus Sulfur 179:2329–2334
Irfan M, Aneja B, Yadava U, Khan SI, Manzoor N, Daniliuc CG, Abid M (2015) Synthesis, QSAR and anticandidal evaluation of 1,2,3-triazoles derived from naturally bioactive scaffolds. Eur J Med Chem 93:246–254
Lass-Flörl C (2011) Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs 71:2405–2419
Layton-Tovar CF, Cuevas-Yañez E, Velasco-Montejo BE, Mendieta-Zerón H (2014) High susceptibility of Candida albicans ATCC 10231 to tetrahydrofuranosyl-1,2,3-triazoles obtained by click chemistry. Rev Boliv Quím 31:15–21
Lebouvier N, Pagniez F, Duflos M, Le Pape P, Na YM, Le Baut G, Borgne M (2007) Synthesis and antifungal activities of new fluconazole analogues with azaheterocycle moiety. Bioorg Med Chem Lett 17:3686–3689
Meldal M, Tornoe CW (2008) Cu-catalyzed azide-alkyne cycloaddition. Chem Rev 108:2952–3015
Miyazaki M, Horii T, Hata K, Watanabe NA, Nakamoto K, Tanaka K, Shirotori S, Murai M, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M (2011) In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 55:4652–4658
Moses ME, Moorhouse AD (2007) The growing applications of click chemistry. Chem Soc Rev 36:1249–1262
Pore VS, Aher NG, Kumar M, Shukla PK (2006) Design and synthesis of fluconazole/bile acid conjugate using click reaction. Tetrahedron 62:11178–11186
Pore VS, Jagtap MA, Agalave SG, Pandey AK, Siddiqi MI, Kumar V, Shukla PK (2012) Synthesis and antifungal activity of 1,5-disubstituted-1,2,3-triazole containing fluconazole analogues. Med Chem Commun 3:484–488
Reed RJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Hyg 27:493–497
Rex JH, Alexander BD, D. Arthington-Skaggs AB, Brown SD, Chaturvedi V, Ghannoum MA, Espinel-Ingroff A, Knapp CC, Ostrosky-Zeichner L, Pfaller MA, Sheehan DJ, Walsh TJ (2008) National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of yeasts; Approved Standard-Third Edition. M27-A3vol. 28 Clinical and Laboratory Standards Institute, Wayne
Richardson K, Brammer KW, Marriott MS, Troke PF (1985) Activity of UK-49,858, a Bis-Triazole Derivative, Against Experimental Infections with Candida albicans and Trichophyton mentagrophytes. Antimicrob Agents Chemother 27:832–835
Rogers TE, Galgiani JN (1986) Activity of Fluconazole (UK 49,858) and Ketoconazole against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother 30:418–422
Saag MS, Dismukes WE (1988) Azole antifungal agents: emphasis on new Triazoles. Antimicrob Agents Chemother 31:1–8
Sagatova AA, Keniya MV, Wilson RJ, Monk BC, Tyndall JDA (2015) Structural insights into binding of the antifungal drug fluconazole to Saccharomyces cerevisiae lanosterol 14α-demethylase. Antimicrob Agents Chemother 59:4982–4989
Salake AB, Chothe AS, Nilewar SS, Khilare M, Rutuja S, Meshram RS, Pandey AA, Kathiravan MK (2014) Design, synthesis, and evaluations of antifungal activity of novel phenyl(2H-tetrazol-5-yl)methanamine derivatives. J Chem Biol 7:29–35
Sam TW (1993) Toxicity testing using the brine shrimp: Artemia salina. In: Colegate SM (ed.) Bioactive Natural Product, Detection, Isolation, and Structural Determination. CRC Press, New York, p 441–456
Silvestri R, Artico M, La Regina G, Di Pasquali A, De Martino G, D’Auria FD, Nencioni L, Palamara AT (2004) Imidazole Analogues of Fluoxetine, a Novel Class of Anti-Candida Agents. J Med Chem 47:3924–3926
Subhas S, Veliyath SK, Mahendra Kumar CB (2012) Review on substituted 1,2,4-triazoles as potent antifungal and antibacterial agents. Int J Res Pharm Sci 3:326
Thirumurugan P, Matosiuk D, Jozwiak K (2013) Click chemistry for drug development and diverse chemical−biology applications. Chem Rev 113:4905–4979
Turker AU, Camper ND (2002) Biological activity of common mullein, a medicinal plant. J Ethnopharmacol 82:117–125
Wang S, Zhang L, Jin Y, Tang JH, Su H, Yu S, Ren H (2014) Synthesis and evaluation of some substituted heterocyclic fluconazole analogues as antifungal agents. Asian J Chem 26:2362–2364
Wang Y, Xu K, Bai G, Huang L, Wu Q, Pan W, Yu S (2014) Synthesis and antifungal activity of novel triazole compounds containing piperazine moiety. Molecules 19:11333–11340
Warnock DW, Burke J, Cope NJ, Johnson EM, von Fraunhofer NA, Williams EW (1988) Fluconazole resistance in candida glabrata. Lancet 332:1310
Woradulayapinij W, Soonthornchareonnon S, Wiwat C (2005) In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J Ethnopharmacol 101:84–89
Xu L, Muller MR, Yu X, Zhu BQ (2009) Improved chiral synthesis of ravuconazole. Synth Commun 39:1611–1625
Zhang YY, Mi JL, Zhou CH, Zhou XD (2011) Synthesis of novel fluconazoliums and their evaluation for antibacterial and antifungal activities. Eur J Med Chem 46:4391–4402
Zou Y, Zhao Q, Liao J, Hua H, Yu S, Chai X, a, Xu M, Wu Q (2012) New triazole derivatives as antifungal agents: Synthesis via click reaction, in vitro evaluation and molecular docking studies. Bioorg Med Chem Lett 22:2959–2962
Acknowledgements
This work was supported by COMECYT (fellowship for AZH) and ASCILA (fellowship for DDCC). Financial support from CONACYT is also gratefully acknowledged. The authors would like to thank Signa S.A. de C. V. for some kindly donated solvents and reagents and to N. Zavala, A. Nuñez, and L. Triana for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zambrano-Huerta, A., Cifuentes-Castañeda, D.D., Bautista-Renedo, J. et al. Synthesis and in vitro biological evaluation of 1,3-bis-(1,2,3-triazol-1-yl)-propan-2-ol derivatives as antifungal compounds fluconazole analogues. Med Chem Res 28, 571–579 (2019). https://doi.org/10.1007/s00044-019-02317-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02317-5