Abstract
The in vitro antibacterial activities of usnic acid (UA) in combination with six currently available antibiotics were evaluated through checkerboard microdilution and dynamic time-killing assays against Staphylococcus aureus and 10 clinical isolates of methicillin-resistant S. aureus (MRSA). The six antibiotics include three aminoglycosides (i.e., amikacin (AK), etimicin (EM), streptomycin (SM)), two glycopeptides (i.e., teicoplanin (TP), vancomycin (VA)) and a tetracycline (i.e., minocycline (MC)). UA alone showed MIC of 16 μg/mL against both S. aureus and MRSA strains. The checkerboard assay showed the range of fractional inhibitory indices (FICIs) as 0.156–1.500 against all the pathogens when UA was used in combination with the antibiotics. Significant bacteriostatic interactions of UA with TP and MC were observed. The enhancement of antibacterial activities against the tested pathogens were revealed by the bacteriostatic dose reduction indices (DRIs) ranges at 1–64 of UA and 1–32 of the antibiotics, especially the synergy of UA with TP by 90% and additive effects with VA by 50% isolates of MRSA strains, respectively. MC also showed 60% strains of synergy with UA. The time-killing curves further confirmed the bactericidal synergy among the combinations of UA with TP, AK, EM, and SM (1 × MIC, △LC24 = 3.406–4.344 log10CFU/mL) against one of the 10 MRSA strains, respectively. Other combinations showed additive effects or indifferences, while no antagonism occurred in all the tested combinations. The anti-MRSA potentiation is promising for further investigations in order to form a possible scenario of UA/antibiotics combinatory chemotherapy which would reduce their dosages and toxicological responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical infections caused by multidrug-resistant bacteria are the difficult issue haunting the world healthcare today, especially the problematic methicillin-resistant Staphylococcus aureus (MRSA) prevalence in medical institutions (Esposito et al. 2013; Prestinaci et al. 2015). Currently effective antibacterial agents against MRSA are confined to a few antibiotics such as the glycopeptides of teicoplanin (TE) and vancomycin (VA), the latter was once known as the last fortress of MRSA infection. However, with the wide and longtime use of these antibiotics, the MRSA susceptibility to them decreased and the minimal inhibitory concentration (MIC) increased (Bruniera et al. 2015). There has even been reported the occurrence of resistant strain of vancomycin-resistant S. aureus (VRSA) (Mcguinness et al. 2017; Walters et al. 2015). At present, there is an opposite phenomenon, that is, more and more severity of resistance spectra of pathogenic bacteria but the progress of new drug development is not satisfactory (Cole 2014). Therefore, in addition to intensify efforts to find novel anti-MRSA effective agents, the study of new substances that can increase susceptibility to currently licensed agents would be an attractive option to curb the process of resistance (Hemaiswarya et al. 2008; Segatore et al. 2012; Wagner and Ulrich-Merzenich 2009).
Usnic acid (UA; Song-Luo-Suan in Chinese) is a benzofuran derivative of lichen compounds commonly found in the genus Usnea and many other lichen species, including U. longissima Ach. (Chang-Song-Luo in Chinese) which was recorded in traditional Chinese medicines for thousands of years (NUTCM 2005) (Fig. 1). UA was first identified by Knop in 1844 and in 1948 its antibacterial activity was demonstrated (Shibata and Ukita 1948). Usnea species and other Lichens, as usnic acid producers, have long been extensively used in popular medicine for the treatment of pulmonary tuberculosis, pain relief, fever control, wounds, mycoses, sore throat, toothache, and several skin infections, whereas UA was therapeutically used as antimicrobial agent (NUTCM 2005; Cocchietto et al. 2002; Felczykowska et al. 2017). As part of our ongoing searching for the anti-MRSA potentiators from natural products when they were used in combination with conventional antibacterial agents (Zuo et al. 2014), we herein report the synergistic effects of UA on six antibacterial agents, including three aminoglycosides (i.e., amikacin (AK), etimicin (EM), streptomycin (SM)), two glycopeptides (i.e., teicoplanin (TP), vancomycin (VA)) and a tetracycline of minocycline (MC), especially the synergy of TE and MC with UA for the first time.
Materials and methods
Bacterial strains
Ten MRSA strains were obtained as previously reported (Zuo et al. 2014). ATCC 25923 (i.e., methicillin-susceptible S. aureus (MSSA)) was used as the control strain.
Antibacterial agents
UA and the six antibacterial agents were purchased from the manufacturers, i.e., UA (Xi’an Xiao-Cao Botanical Development Co., Ltd., Xi’an, China; purity: 98%). AK (Jiangsu Wuzhong Pharmaceutical Group Co., Ltd., Suzhou, China); SM (North China Pharmaceutical Co., Ltd., Shijiazhuang, China); EM (Wuxi Jiming Kexin Shanhe Pharmaceutical Co., Ltd., Wuxi China); SM (North China Pharmaceutical Co., Ltd., Shijiazhuang, China); TP (Sanofi-Aventis (Beijing) Pharmaceutical Co., Ltd., Beijing, China).VA (Eli Lilly Japan K. K., Seishin Laboratories, Japan). MC (Wyeth Pharmaceuticals LTD., Suzhou, China).
Media
Standard Mueller–Hinton agar and broth (MHA and MHB, Tianhe Microbial Agents Co., Hang Zhou, China) were used as bacterial culture media. MHB was used for all susceptibility testing and time-kill experiments. Colony counts were determined using MHA plates.
Susceptibility testing
MICs of the antibacterial agents used alone were determined by conventional broth microdilution techniques with starting inoculums of 5 × 105 CFU/mL and incubated at 35 °C according to CLSI guidelines (CLSI 2012; Zuo et al. 2014). They were determined in duplicate.
Synergy testing
Potential anti-MRSA synergy was measured by fractional inhibitory concentration (FIC) indices (FICI) with checkerboard method and by time-killing curves as previously reported (Zuo et al. 2014). The FIC of a combination was calculated through dividing the MIC of UA (or antibiotics) in the combination by the MIC of UA (or the antibiotics) alone, and the FICI was obtained by adding the FIC of UA and that of antibiotics. The FICI results were interpreted as follows: FICI ≤ 0.5, synergy; 0.5 < FICI ≤ 1, additivity; and 1 < FICI ≤ 2, indifference (or no effect) and FICI > 2, antagonism (Hu et al. 2002). In the killing curves, synergy was defined as ≥2 log10 CFU/mL increase in killing at 24 h of the combination (i.e., △LC24 ≥ 2 log10 CFU/mL), in comparison with the killing by the most active single drug. Additivity was defined as a 1–2 log10 CFU/mL increase in kill with the combination in comparison with the most active single agent. Indifference was defined as ±1log10 CFU/mL killing or growth. Combinations that resulted in >1log10 CFU/mL bacterial growth in comparison with the least active single agent were considered to represent antagonism (Chin et al. 2008). All experiments were performed in triplicate.
Results and discussion
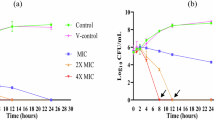
The MICs of UA and the six conventional antibiotics against MSSA (n = 1) and MRSA (n = 10) used alone and in combination are collected in Table 1. The combinations resulted in various degrees of bacteriostatic potentiation effects of UA and the antibiotics. Figure 2 shows the corresponding time-killing curves in the proposed six combinations of UA with the antibiotics at the concentration of their respective MICs against a representative strain of MRSA.
Time-kill curves of the synergistic effect of the combination at 1 × MIC (alone) concentrations of usnic acid (UA) with teicoplanin (TP) (a), vancomycin (VA) (b), amikacin (AK) (c), etimicin (EM) (d), streptomycin (SM) (e), and minocycline (MC) (f) against one of the 10 clinical MRSA strains. The viable cells counts reduced 3.406 (a), 1.836 (b), 3.742 (c), 3.415 (d), 4.344 (e) and 1.557 (f), respectively
As Table 1 shows, the MICs of UA alone against MSSA and MRSA were tested equally as 16 μg/mL, respectively. The results are different from the previous reports which might be due to the different bacterial strains and methods used (Segatore et al. 2012; Felczykowska et al. 2017). In the interaction of UA with the antibiotics against MSSA strain, UA was assayed as MICs range of 0.25–16 μg/mL, it showed synergy in combination with TP and AK, additivity with SM and MC, and indifference with VA and EM, respectively. The FICIs ranged 0.188–1.125, together with the dose reduction indices (DRIs, the times of MIC decreased of UA or a single antibiotic after combining use) from 1 to 64 times of UA and 1 to 8 times of the antibiotics.
For the potentiation on MRSA strains, bacteriostatic interaction of UA with the antibiotics showed FICIs range of 0.156–1.500. The minimal FICI of a combination means the best synergistic effect. Therefore, UA in combination with TP showed synergy against 90% (nine strains) of MRSA (FICI = 0.156–0.500). It also showed additive effects with VA by 50% strains of MRSA (FICI = 0.516–1.500) and synergy with MC against 60% MRSA strains (FICI = 0.375–1.031) (Table 1). As a whole, the combinations caused the DRIs ranged to be 1–64 times of UA and 1–32 times of the antibiotics, with 50% of the values (DRI50) as 4–64 and 2–8 times, respectively. The MIC50 of UA reduced from 16 to the range of 0.25–4 μg/mL, together with the MIC50 of the antibiotics reducing from 1 to 0.25 (TP), 1 to 0.5 (VA), 16 to 4 (MC), 16 to 8 (EM), 64 to 32 (AK) and 128 to 64 (SM), respectively (Table 1). It is also noted that the DRIs of UA were generally greater than those of the antibiotics in Table 1, for example, UA was resulted in as great as 64 times of its MIC decreasing from 16 to 0.25 μg/mL (i.e., DRI = 16/0.25 = 64) when it was used in combination with MC, whereas the DRI of MC showed only the greatest of 32 times, i.e., its MIC could reduce from 8 to 0.25 μg/mL (DRI = 8/0.25 = 32) (Table 1).
Bactericidal interaction of UA with the six antibiotics in Fig. 2 further showed the enhancement of dynamic killing effects after combining uses on the whole, among which four combinations showed synergy as demonstrated by their kill of △LC24 > 2 log10 CFU/mL, i.e., the combinations of UA with SM (4.344), AK (3.742), EM (3.415) and TP (3.406), and the rest two combinations showed additive effects as △LC24 of 1.836 and 1.557 tested for UA with VA and MC, respectively (Chin et al. 2008).
UA was previously assayed of its bacteriostatic interaction with five antibacterial agents, i.e., clindamycin, erythromycin, gentamicin, levofloxacin, and oxacillin (Segatore et al. 2012). We present here further new results of interaction with another six antibiotics, including the dynamic time-killing experiment of each proposed combinations (Fig. 2). Furthermore, as the combined anti-MRSA MICs of MC (i.e., 0.25–4, n = 10, Table 1) reduced to the susceptible grade (S) following the MIC Interpretive Criteria (i.e., MIC ≤ 4 μg/mL against MSSA) from the Clinical and Laboratory Standards Institute (CLSI 2012), we might as well say that UA in combination with MC caused the MRSA resistance (i.e., MC used alone against MRSA with the MICs of ranged 8–16 μg/mL) to this antibiotic to be reversed, which is the best results we would have expected. And this is apt to be confirmed by larger MRSA strains and more antibiotics as well. Therefore, our results revealed new antibiotic synergistic effects of UA in comparison with those of the reported results.
The mechanisms of interactions are still not yet studied, however, UA was reported as inhibitors of RNA and DNA synthesis and bacterial biofilm formation (Francolini et al. 2004; Maciag-Dorszynska et al. 2014; Nithyanand et al. 2015), and there could as well be speculated possibly involved in the interfering with the pathogens’ cell membrane, inhibition of the β-lactamase or efflux pump (Hemaiswarya et al. 2008; Wagner and Ulrich-Merzenich 2009), which should be confirmed in the future investigation.
UA exhibits a wide range of biological properties, e.g., antibacterial, antifungal, and antimitotic activities. It is a classic natural antimicrobial agent which has been reported inhibition against gram-positive bacteria, including S. aureus, Enterococcus faecalis, and E. faecium (Shibata and Ukita 1948; Cocchietto et al. 2002; Araujo et al. 2015). It also showed effects on MRSA and VRE (vancomycin-resistant enterococci), together with antituberculous and anticancer activities (Elo et al. 2007; Ferraz-Carvalho et al. 2016; Felczykowska et al. 2017). However, its adverse effects limited its application (Moreira et al. 2013). It was reported of the hepatotoxicity associated with use of a dietary supplement containing UA (Sanchez et al. 2006; Lu et al. 2011). Meanwhile, the severely adverse effects of the antibiotics such as nephrotoxicity and ototoxicity which were observed in the treatment with VA or an aminoglycosides, usually presenting as tinnitus, but was attributed to elevated serum concentrations found in patients with renal failure. Rapid infusion of vancomycin has been associated with the “red man,” or “red neck,” syndrome. This syndrome, which is characterized by a combination of erythema, pruritus, hypotension, and angioedema, is a histamine-like response to rapid infusion (Levine 2006). The previous data also suggested that higher-dose vancomycin regimens were associated with a higher likelihood of vancomycin related nephrotoxicity (Lodise et al. 2008).
As the adverse effects are associated with their dosages, reduced MICs of both UA and its combined antibiotics meant reduced the potential therapeutic dosages which would in turn beneficial in reducing the toxicity and adverse effects of the agents, and also beneficial in relieving the selective pressure attributable to the occurrence of resistant pathogens.
Conclusions
We have demonstrated novel efficient interactions of UA, a classic antimicrobial agent derived from the genus Usnea and many other lichen species, with the six antibiotics AK, EM, SM, TP, VA, and MC against both MSSA and MRSA isolates. UA synergistically enhanced the in vitro anti-MRSA efficacy of these antibiotics and vice versa, especially of which the MICs of the two glycopeptides (TP and VA) were reduced and the MRSA resistance to MC was reversed.
The significant enhancement of bacteriostatic activities by UA against the tested pathogens were revealed by the MICs reduction times (i.e., DRIs) ranges at 1–64 of UA and 1–32 of the antibiotics, especially the synergy of UA with TP by 90% and additive effects with VA by 50% isolates of MRSA strains, respectively. MC also showed 60% strains of synergy with UA. The time-killing curves further confirmed the bactericidal synergy among the combinations of UA with TP, AK, EM, and SM (1 × MIC, △LC24 = 3.406–4.344 log10CFU/mL) against one of the 10 MRSA strains, respectively. The reduced MICs of these agents showed potential use of their combinatory therapy of MRSA infected patients with fewer amounts of dosages and less toxic responses. Our results of the potentiation of antibiotics effects by UA on clinical multi-drug resistant isolates of MRSA indicate that UA can serve as a lead compounds for the future development of new anti-MRSA drugs and anti-MRSA regimens.
References
Araujo AA, de Melo MG, Rabelo TK, Nunes PS, Santos SL, Serafini MR, Santos MR, Quintans-Junior LJ, Gelain DP (2015) Review of the biological properties and toxicity of usnic acid. Nat Prod Res 29:2167–2180
Bruniera FR, Ferreira FM, Saviolli LR, Bacci MR, Feder D, Da LGPM, Sorgini Peterlini MA, Azzalis LA, Campos Junqueira VB, Fonseca FL (2015) The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci 19:694–700
Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ (2008) Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J Antimicrob Chemother 61:365–370
Clinical and Laboratory Standards Institute (2012) Table 2C. Zone diameter and MIC interpretive standards for Staphylococcus spp. performance standards for antimicrobial susceptibility testing. Twenty Second Informational Supplement. CLSI, Wayne, PA Approved Standard M100–S22
Cocchietto M, Skert N, Nimis PL, Sava G (2002) A review on usnic acid, an interesting natural compound. Naturwissenschaften 89:137–146
Cole ST (2014) Who will develop new antibacterial agents? Philos Trans R Soc Lond B Biol Sci 369:20130430
Elo H, Matikainen J, Pelttari E (2007) Potent activity of the lichen antibiotic (+)-usnic acid against clinical isolates of vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Naturwissenschaften 94:465–468
Esposito S, Purrello SM, Bonnet E, Novelli A, Tripodi F, Pascale R, Unal S, Milkovich G (2013) Central venous catheter-related biofilm infections: an up-to-date focus on meticillin-resistant Staphylococcus aureus. J Glob Antimicrob Resist 1:71–78
Felczykowska A, Pastuszak-Skrzypczak A, Pawlik A, Bogucka K, Herman-Antosiewicz A, Guzow-Krzeminska B (2017) Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. BMC Complement Altern Med 17:300
Ferraz-Carvalho RS, Pereira MA, Linhares LA, Lira-Nogueira MC, Cavalcanti IM, Santos-Magalhaes NS, Montenegro LM (2016) Effects of the encapsulation of usnic acid into liposomes and interactions with antituberculous agents against multidrug-resistant tuberculosis clinical isolates. Mem Inst Oswaldo Cruz 111:330–334
Francolini I, Norris P, Piozzi A, Donelli G, Stoodley P (2004) Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob Agents Chemother 48:4360–4365
Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15:639–652
Hu ZQ, Zhao WH, Asano N, Yoda Y, Hara Y, Shimamura T (2002) Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46:558–560
Levine DP (2006) Vancomycin: a history. Clin Infect Dis 42(Suppl):S5–S12
Lodise TP, Lomaestro B, Graves J, Drusano GL (2008) Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336
Lu X, Zhao Q, Tian Y, Xiao S, Jin T, Fan X (2011) A metabonomic characterization of (+)-usnic acid-induced liver injury by gas chromatography-mass spectrometry-based metabolic profiling of the plasma and liver in rat. Int J Toxicol 30:478–491
Maciag-Dorszynska M, Wegrzyn G, Guzow-Krzeminska B (2014) Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol Lett 353:57–62
Mcguinness WA, Malachowa N, Deleo FR (2017) Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med 90:269–281
Moreira CT, Oliveira AL, Comar JF, Peralta RM, Bracht A (2013) Harmful effects of usnic acid on hepatic metabolism. Chem Biol Interact 203:502–511
Nanjing University of Traditional Chinese Medicine (NUTCM) (ed) (2005) Dictionary of Chinese crude drugs. second ed. Shanghai Scientific Technologic Publisher, Shanghai, China, pp 1815–1816
Nithyanand P, Beema Shafreen RM, Muthamil S, Karutha Pandian S (2015) Usnic acid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol Res 179:20–28
Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109:309–318
Sanchez W, Maple JT, Burgart LJ, Kamath PS (2006) Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc 81:541–544
Segatore B, Bellio P, Setacci D, Brisdelli F, Piovano M, Garbarino JA, Nicoletti M, Amicosante G, Perilli M, Celenza G (2012) In vitro interaction of usnic acid in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus clinical isolates determined by FICI and DeltaE model methods. Phytomedicine 19:341–347
Shibata S, Ukita T (1948) Relation between chemical constitutions and antibacterial effects of usnic acid and its derivatives. Jpn J Med 1:152–155
Wagner H, Ulrich-Merzenich G (2009) Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 16:97–110
Walters MS, Eggers P, Albrecht V, Travis T, Lonsway D, Hovan G, Taylor D, Rasheed K, Limbago B, Kallen A (2015) Vancomycin-resistant Staphylococcus aureus - Delaware, 2015. MMWR Morb Mortal Wkly Rep 64:1056
Zuo GY, Li Y, Wang GC, Li ZS, Han J (2014) Synergistic effects of berberines with antibiotics on clinical multi-drug resistant isolates of methicillin-resistant Staphylococcus aureus (MRSA). Med Chem Res 23:2439–2444
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC 81173504) and the supporting fund of Yunnan Province of China (2008PY001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zuo, GY., Fu, RC., Yu, W. et al. Potentiation effects by usnic acid in combination with antibiotics on clinical multi-drug resistant isolates of methicillin-resistant Staphylococcus aureus (MRSA). Med Chem Res 27, 1443–1448 (2018). https://doi.org/10.1007/s00044-018-2161-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2161-5