Abstract
An efficient microwave-assisted method of synthesis of some novel 2-arylidene-2H-furo[2,3-f]chromen-3(7H)-ones 6a–j in excellent yields was described and structural assignment of the products was confirmed on the basis of IR, 1H NMR, 13C NMR, MS, and analytical data. The synthesized compounds were screened for antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl and hydrogen peroxide radicals. The compounds 5d, 5h, 6a, 6e, and 6j exhibited better radical scavenging ability. Antimicrobial activity results demonstrated that compounds 5b, 5e, 6b, 6c, and 6e showed promising antimicrobial potency. The in silico molecular docking studies were also carried out for the inhibition of cyclooxygenase-II enzyme. These molecular docking results were well complemented to the antioxidant activity studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free radicals that are reactive oxygen species such as hydroxyl radicals, superoxide radicals, singlet oxygen, hydrogen peroxide radical are constantly formed as a result of normal organ functions or excessive oxidative stress (Yin et al. 2011). Cellular damage arising from these free radicals is one of the fundamental mechanisms underlying a number of human neurodegenerative disorders such as diabetes, cancer, coronary heart diseases, inflammation, and aging (Hangum-balkir and Mckenney 2012; Turkoglu et al. 2007). Antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole, which are commercially available, are currently being used as radical scavengers. However, since suspected actions as promoters of carcinogenesis and other side effects have been reported, their use in food, cosmetic, and pharmaceutical products has been decreasing (Politeo et al. 2007; Tepe et al. 2005; Ku and Mun 2008; Gulcin et al. 2003). Thus, there has been an upsurge of interest in discovery of new and more effective antioxidant agents. On the other hand, infectious diseases caused by microorganisms are one of the main threats to the population of the world. There is an urgent need to search for new antibacterial and antifungal drugs with broad spectrum of activity because of the increasing resistance of microbial pathogens. It is desirable to find drugs with improved potency and wide activity spectrum.
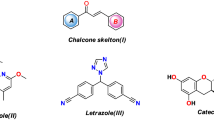
Aurones (2-benzylidenebenzofuran-3(2H)-ones) are a class of flavonoids found in yellow pigments of plants (Pare et al. 1991). Natural and synthetic aurones have been shown to possess a broad spectrum of bioactivity including anticancer (Sim et al. 2011; Cheng et al. 2010), antioxidant (Detsi et al. 2009), antiparasitic (Roussaki et al. 2012; Kerboeuf et al. 2008) antiviral, antiparasitic, antifungal, antidiabetic (Boumendjel 2003), and neuroprotective (Shrestha et al. 2013). 2-Benzylidine-benzofuranone based flavopiridol drug (A) is used as selective CDK1 inhibitor (Schoepfer et al. 2002) and Sulfuretin (B) is a naturally occuring aurone flavonoid known to possess diverse biological activities, such as anti-nociceptive (Kim et al. 2004), antioxidant (Jung et al. 2003), anti-mutagenic (Park et al. 2004), and antidiabetic (Lee et al. 2008; Song et al. 2010) (Fig. 1). 4′-Chloroaurone (C) is a naturally occuring aurone known to possess antioxidant and antibacterial activity (Venkateswarlu et al. 2007) (Fig. 1). In recent years microwave-assisted organic synthesis has gained popularity as an environmental benign technology. Microwave-assisted synthesis leads to significantly reduced reaction times, enhanced conversions and known to be environment friendly (Ashok et al. 2016).
Keeping in view of the therapeutic importance of aurones and furochromenone scaffolds, in order to investigate the combined enhanced effect of these motifs on biological potency, and in continuation of our search on biologically potent molecules, we hereby report the synthesis, characterization, and biological studies of some novel 2-arylidene-2H-furo[2,3-f]chromen-3(7H)-ones under microwave irradiation as well as conventional heating. The new derivatives were screened for antioxidant and antimicrobial activity. The molecular-docking studies of the compounds for better understanding of the drug-receptor interaction were also carried out.
Results and discussion
Chemistry
Traditionally, aurones have been prepared from 2-hydroxychalcones, which were synthesized from 2-hydroxyacetophenones and aromatic benzaldehydes under Claisen–Schmidt conditions, which could undergo oxidative cyclization to furnish aurone ring system. The synthetic route for the 2-arylidene-2H-furo[2,3-f]chromen-3(7H)-ones is illustrated in Scheme 1 and Scheme 2. All these syntheses involve the preliminary preparation of 1-(5-hydroxy-2H-chromen-6-yl)ethanone (3) starting from resacetophenone (1) upon treating with propargyl bromide in presence of anhydrous K2CO3 in dry acetone yielded 1-(2-hydroxy-4-(prop-2-yn-1-yloxy)phenyl)ethanone (2), was further refluxed in N,N-dimethyl aniline at 180 °C for 3 h gave compound (3) (Sreenivas 2011) (Scheme 1). Claisen–Schmidt condensation between 1-(5-hydroxy-2H-chromen-6-yl)ethanone (3) and substituted aromatic aldehydes (4a–j) in presence of powdered KOH under microwave irradiation for 5–7 min (Scheme 2) gave (E)-3-(aryl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-ones (5a–j). These chalcones were then oxidatively cyclized using mercury(II) acetate as the oxidative agent in pyridine under microwave irradiation to furnish the aurone derivatives 6a–j in excellent yields.
Preliminarily, the synthesis of compounds 5a–j and 6a–j was carried out under conventional heating method, but this method suffers from poor yields (40–54%). In order to improve the yields and reduce the reaction time, the synthesis approach was changed to microwave irradiation method. Microwave-assisted synthesis of title compounds 6a–j is advantageous over conventional method in terms of higher yields in shorter reaction times. Comparison of yields of title compounds in conventional and microwave irradiation methods was demonstrated in Table 1. Formation of 2-arylidene-2H-furo[2,3-f]chromen-3(7H)-ones 6a–j was confirmed by recording IR, 1H-NMR, 13C-NMR, mass and elemental analyses. IR (Infra red) spectrum of compound 6c showed absorption band at 1699 cm−1 due to α,β unsaturated C=O group. The 1H-NMR (nuclear magnetic resonance) spectrum of 6c showed a singlet at δ 6.98 corresponds to pyrazole ring proton, a singlet at δ 6.52 due to Hβ of α,β unsaturated C=O and a quartet at δ 5.02 was due to O–CH2 protons. The 13C-NMR spectrum of 6c showed a peak at δ 181.47 corresponds to C=O and δ 66.56 due to O–CH2 carbon. Similarly the mass spectrum (MS) was recorded and reported as [M + H]+ values, exhibited the peak at m/z 310 for the compound 6c.
Biological evaluation
Antioxidant activity
The antioxidant activity of synthesized compounds was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Burits and Bucar 2000; Cuendet et al. 1997) and H2O2 radical scavenging assays (Ruch et al. 1989). Ascorbic acid and BHT were used as standard antioxidants. DPPH radical scavenging assay results (Table 2) revealed that, compounds 5d, 5h, 6e, and 6j exhibited strong scavenging ability with potent IC50 values of 09.43 ± 1.20, 14.25 ± 0.54, 12.46 ± 1.56, and 08.34 ± 0.40 µg/mL, respectively. These values were lower than the standard drugs ascorbic acid (15.54 ± 0.67 µg/mL) and BHT (17.22 ± 0.21 µg/mL). It can be observed that the precursor chalcone compounds 5d and 5h comprised of electron rich isopropyl and dimethoxy substitutions on phenyl ring, respectively, displayed potent free radical scavenging ability. However, up on cyclization, the aurone derivatives 6e and 6j having electronegative dichloro atoms and indolyl nucleus, respectively, exhibited promising antioxidant activity.
It can be envisaged that substituent with lone pair of electrons on the aurone scaffold enhanced the radical scavenging potency. It can be justified by the highest scavenging activity displayed by compound 6j with indolyl substitution. The remaining compounds showed moderate activity.
The same series was screened for H2O2 radical scavenging activity (Table 2). As observed from the results, compound 6j emerged as the better radical scavenger with potent IC50 10.10 ± 0.27 µg/mL. Compounds 5h, 6a, and 6e exhibited good antioxidant activity with IC50 13.46 ± 0.67, 11.72 ± 0.46, and 12.21 ± 0.35 µg/mL, respectively, with more scavenging potency than the standard drugs ascorbic acid (14.65 ± 0.76 µg/mL) and BHT (16.21 ± 0.14 µg/mL). The rest of the compounds displayed moderate antioxidant activity. From the antioxidant screening, it can be concluded that presence of lone pair of electrons on the aurone nucleus played an important role to exhibit radical scavenging ability.
Antimicrobial activity
Antibacterial activity
The synthesized compounds were screened in vitro for their antibacterial activity against Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 29213) as examples of Gram-positive bacteria and Escherichia coli (ATCC 11229), Proteus vulgaris (ATCC 29213) as examples of Gram-negative bacteria. Agar well-diffusion method was used to assay the antibacterial activity against test strains on Mueller–Henton agar plates. Gentamicin was employed as standard antibacterial drug. The results obtained as minimum inhibitory concentration (MIC) in µg/mL and measurements are presented in Table 3. Investigation of the antibacterial efficiency of the synthesized compounds revealed that most of the tested compounds displayed variable inhibitory effects on the growth of the tested gram-positive and gram-negative bacterial strains. It is evident from Table 3, compounds 5b (Ar = 4-bromophenyl) displayed MIC 3.125 µg/mL against gram-positive bacteria and 6.25 µg/mL against gram-negative bacteria, 5e (Ar = 2,4-dichlorophenyl) exhibited MIC 6.25 µg/mL against most of the bacteria, 6b showed MIC 1.56 µg/mL against S.aureus and 3.125 µg/mL against rest of the bacteria, 6c (Ar = 2-chlorophenyl) displayed 3.125 µg/mL against most of the bacteria except E.coli (6.25 µg/mL) and 6e with dichloro substitution exhibited 3.125 µg/mL against all the bacteria. It was envisaged from the analysis of antibacterial activity results that electro-negative atoms such as chloro, bromo on phenyl ring were found more potent as compared to control drug gentamicin (1.56 µg/mL).
Antifungal activity
All the title compounds were evaluated for their in vitro antifungal activity against Aspergillus niger (ATCC 9029) and Candida albicans (ATCC 10231) fungal strains (Table 3). Agar well-diffusion method was used to evaluate the antifungal activity against test strains on PDA plates. Fluconazole was used as standard antifungal drug. Compound 6b emerged as the promising antifungal agent with MIC 6.25 µg/mL. Compounds 5b and 6e were able to induce appreciable promising growth inhibitory activity in the range of MIC 6.25 µg/mL—12.5 µg/mL when compared with standard antifungal agent fluconazole (MIC 3.125 µg/mL). Thus we hypothesized that, compounds with electro-negative groups such as chloro, bromo on phenyl ring were exhibited highest antifungal inhibitory potency.
Molecular docking
In order to gain more insight in to the interactions of the most potent compounds towards antioxidant activity, in silico molecular docking of the title compounds with the cyclooxygenase-II enzyme was carried out. The best poses of the docked compound are shown in the Figs. 2 and 3, and the results are depicted in Table 4. The most potent antioxidant compounds 5d and 6j displayed the better MolDock score values −139.102 and −137.388, respectively. The standard drug ascorbic acid exhibited Moldock score of −86.9613. Most of the compounds showed the hydrogen bond interactions with one or more amino acid residues of the active pocket. The amino acids involved interactions with the compounds Met-215, His-208, Gly-216, Asn-205, Arg-209, Pro-201, Val-217, Val-218, Ala-44, Lys-47, Asn-205, His-60, and Val-218.
The compound 5d showed a hydrogen bond interaction between O atom of keto group with Lys-47 (green dotted lines) (Fig. 2a). Compound 6j formed three hydrogen bonds, two hydrogen bonds between O atoms of aurone ring moiety with Asn-205, Arg-209, and one hydrogen bond between N atom of indole nucleus with Met-215 (green dotted lines) (Fig. 2b). Compounds 5h and 6e exhibited relatively less binding affinity with dock score values of −141.714 and −140.311. Compound 5h formed one hydrogen bond between O atom of keto group with Lys-47 (Fig. 3a). Ascorbic acid showed six hydrogen bonds, O atom of hydroxyl group bonded with Asn-205. The O atom of methylene group formed three hydrogen bonds with His-42, His-60, His-208, and another O atom of methylene group formed two hydrogen bonds with Gly-216, Val-217 (Fig. 3b). These in silico findings are well supported by results of antioxidant activity.
Experimental
Chemistry
All melting points were recorded on Stuart SMP3 melting-point apparatus and are uncorrected. The IR spectra ῦ in cm−1 (KBr) were recorded on Shimadzu FTIR 8400S spectrometer. All the microwave irradiation experiments were performed in a CEM Discover Microwave System and reaction temperatures were monitored by an equipped IR sensor. The 1H NMR and 13C NMR spectra were run on Bruker Avance-400 spectrometer at 400 and 100 MHz, respectively, using tetramethylsilane as an internal reference and DMSO-d6 as solvent. The mass spectra were recorded on Finnigan MAT 1020 mass spectrometer; in m/z. Elemental analyses were recorded on a Karlo Erba 1106 elemental analyzer. All the reactions were monitored on silica gel percolated TLC plates of Merck 60 F254 and spots were visualized with UV light.
General procedure for the synthesis of compounds (5a–j)
Conventional method
To a stirred solution of 1-(5-hydroxy-2H-chromen-6-yl)-ethanone (3) (0.1 g, 0.52 mmol), KOH (1.04 mmol) in ethanol (20 mL), was added substituted aromatic aldehydes (4a–j) (0.52 mmol) and refluxed for 10–24 h. Progress of the reaction was monitored by TLC (EtOAc:hexane 1:5 v/v). After completion of the reaction, reaction mixture was poured into ice cold water and neutralized with 10% dil. HCl solution. The solid was filtered and recrystallized from ethanol to get the pure compound.
Microwave irradiation method
To a mixture of 1-(5-hydroxy-2H-chromen-6-yl)-ethanone (3) (0.1 g, 0.52 mmol) and substituted aromatic aldehydes (4a–j) (0.52 mmol) in ethanol (5 mL), powdered KOH (1.04 mmol) was added and subjected to microwave irradiation at 180 W for 4–7 min. Progress of the reaction was monitored by TLC (EtOAc:hexane 1:5 v/v). After completion of the reaction, reaction mixture was poured into ice cold water and neutralized with 10% dil. HCl solution. The solid was filtered and recrystallized from ethanol to get the pure compound.
(E)-1-(5-hydroxy-2H-chromen-6-yl)-3-phenylprop-2-en-1-one (5a)
Pale yellow solid; yield (91%); m.p.: 102–103 °C; IR (KBr) (cm−1): 3056 (OH), 2927 (H–C=), 1636 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.61 (s, 1H, OH), 7.88 (d, 1H, J = 15.48 Hz, Hβ), 7.71 (d, 1H, J = 8.87 Hz, Ar–H), 7.64 (m, 2H, Ar–H), 7.55 (d, 1H, J = 15.48 Hz, Hα), 7.42 (t, 3H, Ar–H), 6.82 (d, 1H, J = 10.19 Hz, Ar–CH = C), 6.37 (d, 1H, J = 8.87 Hz, Ar–H), 5.72 (m, 1H, Ar–C = CH), 4.91 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 192.1 (C=O), 160.2 (C8a), 159.7 (C5), 144.3 (Cβ), 134.3 (C1′), 132.2 (C4′), 130.7 (C7), 129.0, 128.8 (C2′, C3′), 120.7 (C3), 120.5 (C4), 116.8 (Cα), 113.9 (C6), 109.4 (C4a), 107.5 (C8), 65.8 (OCH2); LC-MS (m/z): 279 (M + H)+. Elemental analysis (C18H14O3): calcd. C, 77.68; H, 5.07. found: C, 77.90; H, 4.83%
(E)-3-(4-bromophenyl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-one (5b)
Yellow solid; yield (88%); m.p.: 170–172 °C; IR (KBr) (cm−1): 3058 (OH), 2934 (H–C=), 1635 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.74 (Broad-s, 1H, OH), 8.20 (d, 1H, J = 8.92 Hz, Ar–H), 8.07 (d, 1H, J = 15.40 Hz, Hβ), 7.88 (d, 2H, J = 8.55 Hz, Ar–H), 7.79 (d, 1H, J = 15.40 Hz, Hα), 7.67 (d, 2H, J = 8.43 Hz, Ar–H), 6.69 (d, 1H, J = 10.14 Hz, Ar–CH=C), 6.42 (d, 1H, J = 8.80 Hz, Ar–H), 5.86 (m, 1H, Ar–C=CH), 4.92 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 190.1 (C=O); 160.2 (C8a), 157.0 (C5), 138.8 (Cβ), 132.9 (C1′), 132.3 (C4′), 131.9 (C7), 131.4, 129.0 (C2′, C3′), 127.7 (C3), 122.1 (C4), 117.5 (Cα), 115.4 (C6), 110.6 (C4a), 107.9 (C8), 66.0 (OCH2); LC-MS (m/z): 357 (M + H)+. Elemental analysis (C18H13BrO3): calcd. C, 60.52; H, 3.67. found: C, 60.31; H, 3.98%.
(E)-3-(2-chlorophenyl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-one (5c)
Pale yellow solid; yield (89%); m.p. 118–120 °C; IR (KBr) (cm−1): 3062 (OH), 2938 (H–C=), 1636 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.62 (s, 1H, OH), 8.26 (dd, 1H, Ar–H), 8.21 (d, 1H, J = 8.92 Hz, Ar–H), 8.14 (d, 1H, J = 15.40 Hz, Hβ), 8.05 (d, 1H, J = 15.40 Hz, Hα), 7.58 (m, 1H, Ar–H), 7.48 (m, 2H, Ar–H), 6.69 (d, 1H, J = 10.14 Hz, Ar–CH=C), 6.45 (d, 1H, J = 8.92 Hz, Ar–H), 5.87 (m, 1H, Ar–C=CH), 4.93 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 191.6 (C=O), 160.4 (C8a), 159.7 (C5), 138.6 (Cβ), 134.4 (C1′), 132.4, 132.0 (C4′, C5′), 131.9 (C6′), 129.9 (C2′), 128.6 (C7), 127.5 (C3′), 123.4 (C3), 120.6 (C4), 116.7 (Cα), 113.9 (C6), 109.4 (C4a), 107.6 (C8), 65.8 (OCH2); LC-MS (m/z): 313 (M + H)+. Elemental analysis (C18H13ClO3): calcd. C, 69.13; H, 4.19. found: C, 69.33; H, 4.41%.
(E)-1-(5-hydroxy-2H-chromen-6-yl)-3-(4-isopropylphenyl)prop-2-en-1-one (5d)
Yellow solid; yield (94%); m.p.: 72–74 °C; IR (KBr) (cm−1): 3053 (OH), 2959 (H–C=), 1636 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.86 (s, 1H, OH), 8.19 (d, 1H, J = 9.06 Hz, Ar–H), 7.94 (s, 1H, J = 15.48 Hz, Hβ), 7.82 (t, 3H, Ar–H), 7.34 (d, 2H, J = 8.12 Hz, Ar–H), 6.70 (d, 1H, J = 10.19 Hz, Ar–CH=C), 6.44 (d, 1H, J = 8.87 Hz, Ar–H), 5.86 (m, 1H, Ar–C=CH), 4.92 (q, 2H, OCH2), 2.94 (m, 1H, CH), 1.22 (d, 6H, J = 6.98 Hz, 2 × CH3); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 191.8 (C=O), 159.9 (C8a), 159.4 (C5), 151.4 (Cβ), 144.2 (C1′), 131.9 (C4′), 131.8 (C7), 128.9, 126.5 (C2′, C3′), 120.3 (C3), 119.4 (C4), 116.5 (Cα), 113.7 (C6), 109.1 (C4a), 107.2 (C8), 65.5 (OCH2), 33.0 (CH), 23.2 (2 C, 2 × CH3); LC-MS (m/z): 321 (M + H)+. Elemental analysis (C21H20O3): calcd. C, 78.73; H, 6.29. found: C, 78.52; H, 6.50%.
(E)-3-(2,4-dichlorophenyl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-one (5e)
Yellow solid; yield (86%); m.p.: 128–130 °C; IR (KBr) (cm−1): 3068 (OH), 2946 (H–C=), 1633 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.57 (s, 1H, OH), 8.28 (d, 1H, J = 8.49 Hz, Ar–H), 8.20 (d, 1H, J = 8.87 Hz, Ar–H), 8.05 (s, 2H, Ar–H), 7.75 (s, 1H, Ar–H), 7.75 (d, 1H, J = 8.49 Hz, Ar–H), 6.68 (d, 1H, J = 10.00 Hz, Ar–CH=C), 6.44 (d, 1H, J = 8.68 Hz, Ar–H), 5.87 (m, 1H, Ar–C=CH), 4.92 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 191.5 (C=O), 160.6 (C8a), 159.7 (C5), 137.4 (Cβ), 135.8, 135.2 (2C, C1′, C2′), 132.5 (Ar–C), 131.0 (C7), 129.9, 129.5, 127.9 (3C, Ar–C), 124.1 (C3), 120.8 (C4), 116.7 (Cα), 114.0 (C6), 109.4 (C4a), 107.8 (C8), 65.9 (OCH2); LC-MS (m/z): 347 (M + H)+. Elemental analysis (C18H12Cl2O3): calcd. C, 62.27; H, 3.48. found: C, 62.49; H, 3.66%.
(E)-1-(5-hydroxy-2H-chromen-6-yl)-3-(p-tolyl)prop-2-en-1-one (5f)
Wine red solid; yield (93%); m.p.: 116–118 °C; IR (KBr) (cm−1): 3072 (OH), 2955 (H–C=), 1636 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.86 (s, 1H, OH), 8.18 (d, 1H, J = 8.87 Hz, Ar–H), 7.93 (s, 1H, J = 15.48 Hz, Hβ), 7.80 (m, 3H, Ar–H), 7.27 (d, 2H, J = 7.93 Hz, Ar–H), 6.68 (d, 1H, J = 10.19 Hz, Ar–CH=C), 6.42 (d, 1H, J = 8.87 Hz, Ar-H), 5.85 (m, 1H, Ar–C=CH), 4.90 (q, 2H, OCH2), 2.34 (s, 3H, Ar–CH3); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 192.2 (C=O), 160.2 (C8a), 159.7 (C5), 144.5 (Cβ), 141.0 (C4′), 132.2 (C1′), 131.7 (C7), 129.5, 129.1 (C2′, C3′), 120.6 (C3), 119.6 (C4), 116.8 (Cα), 114.0 (C6), 109.5 (C4a), 107.6 (C8), 65.8 (OCH2), 21.0 (Ar–CH3); LC-MS (m/z): 293 (M + H)+. Elemental analysis (C19H16O3): calcd. C, 78.06; H, 5.52. found: C, 78.24; H, 5.31%.
(E)-1-(5-hydroxy-2H-chromen-6-yl)-3-(4-methoxyphenyl)prop-2-en-1-one (5g)
Orange solid; yield (91%); m.p.: 112–114 °C; IR (KBr) (cm−1): 3063 (OH), 2943 (H–C=), 1635 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.98 (s, 1H, OH), 8.16 (d, 1H, J = 8.87 Hz, Ar–H), 7.85 (m, 4H, Ar–H), 7.02 (d, 2H, J = 8.87 Hz, Ar–H), 6.70 (d, 1H, J = 10.19 Hz, Ar–CH=C), 6.42 (d, 1H, J = 8.68 Hz, Ar–H), 5.85 (m, 1H, Ar–C=CH), 4.90 (q, 2H, OCH2), 3.83 (s, 3H, OCH3); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 192.0 (C=O), 161.5 (C8a), 160.0 (C5), 159.7 (C4′), 144.4 (Cβ), 131.9 (C1′), 131.0 (Ar–C), 127.0 (C7), 120.3 (C3), 117.9 (C4), 116.9 (Cα), 114.3 (Ar–C), 114.0 (C6), 109.4 (C4a), 107.4 (C8), 65.7 (OCH2), 55.2 (OCH3); LC-MS (m/z): 309 (M + H)+. Elemental analysis (C19H16O4): calcd. C, 74.01; H, 5.23. found: C, 74.20; H, 5.01%.
(E)-3-(3,4-dimethoxyphenyl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-one (5h)
Orange solid; yield (91%); m.p.: 88–90 °C; IR (KBr) (cm−1): 3060 (OH), 2939 (H–C=), 1632 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.85 (s, 1H, OH), 8.05 (d, 1H, J = 8.87 Hz, Ar–H), 7.83 (d, 1H, J = 15.49 Hz, Hβ), 7.18 (m, 3H, Ar–H), 6.84 (d, 1H, J = 8.72 Hz, Ar–H), 6.71 (d, 1H, J = 10.19 Hz, Ar–CH=C), 6.45 (d, 1H, J = 8.87 Hz, Ar–H), 5.85 (m, 1H, Ar–C=CH), 4.91 (q, 2H, OCH2), 3.86, 3.82 (s, 6H, (OCH3)2); 13C-NMR (DMSO-d6) δ p.p.m.: 192.1 (C=O), 160.0 (C8a), 159.7 (C5), 151.5, 148.9 (C3′, C4′), 144.9 (Cβ), 132.1 (C1′), 132.0 (C7), 127.2 (C3), 120.5 (C4), 118.0 (Ar–C), 116.9 (Cα), 114.0 (C6), 110.8, 110.7 (Ar–C), 109.4 (C4a), 107.3 (C8), 65.7 (OCH2), 55.7, 55.5 (2C,OCH3); LC-MS (m/z): 339 (M + H)+. Elemental analysis (C20H18O5): calcd. C, 70.99; H, 5.36. found: C, 70.78; H, 5.57%.
(E)-1-(5-hydroxy-2H-chromen-6-yl)-3-(thiophen-2-yl)prop-2-en-1-one (5i)
Yellow solid; yield (87%); m.p.: 101–102 °C; IR (KBr) (cm−1): 3075 (OH), 2965 (H–C=), 1639 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 13.64 (s, 1H, OH), 8.00 (d, 1H, J = 15.10 Hz, Hβ), 7.68 (d, 1H, J = 8.87 Hz, Ar–H), 7.38 (m, 3H, Ar–H), 7.10 (m, 1H, Ar–H), 6.83 (d, 1H, J = 10.19 Hz, Ar–CH=C), 6.37 (d, 1H, J = 8.87 Hz, Ar–H), 5.73 (m, 1H, Ar–C=CH), 4.92 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 192.1 (C=O), 160.2 (C8a), 159.6 (C5), 141.0 (C1′), 139.2 (Cβ), 132.8 (C4′), 130.1 (C7), 129.9, 129.3 (2C, Ar–C), 128.0 (Cα), 124.1 (C3), 120.8 (C4), 113.6 (C6), 109.6 (C4a), 107.5 (C8), 65.2 (OCH2); LC-MS (m/z): 285 (M + H)+. Elemental analysis (C16H12O3S): calcd. C, 67.59; H, 4.25. found: C, 67.78; H, 4.56%.
(E)-1-(5-hydroxy-2H-chromen-6-yl)-3-(1H-indol-3-yl)prop-2-en-1-one (5j)
Red solid; yield (89%); m.p.: 178–180 °C; IR (KBr) (cm−1): 3075 (OH), 3049 (NH), 2965 (H–C=), 1631 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 11.58 (s, 1H, OH), 8.10 (d, 1H, J = 15.10 Hz, Hβ), 7.91 (m, 1H, Ar–H), 7.78 (m, 3H, Ar–H), 7.49 (d, 1H, J = 15.10 Hz, Hα), 7.42 (m, 1H, Ar–H), 7.18 (m, 2H, Ar–H), 6.70 (d, 1H, J = 10.19 Hz, Ar–CH=C), 6.32 (d, 1H, J = 8.68 Hz, Ar–H), 5.69 (m, 1H, Ar–C=CH), 4.83 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 192.3 (C=O), 160.7 (C8a), 160.2 (C5), 138.4 (Cβ), 137.2, 130.5 (2C, Ar–C), 130.3 (C7), 128.4 (Cα), 125.3 (C3), 123.6, 121.9 (2C, Ar–C), 120.7 (C4), 118.9, 118.5, 115.7 (3C, Ar–C), 114.7 (C6), 111.9 (C4a), 107.4 (C8), 103.9 (Ar–C), 66.1 (OCH2); LC-MS (m/z): 318 (M + H)+. Elemental analysis (C20H15NO3): calcd. C, 75.70; H, 4.76; N, 4.41. found: C, 75.39; H, 4.97; N, 4.61%.
General procedure for the synthesis of compounds (6a–j)
Conventional method
To a well stirred solution of (E)-3-(aryl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-ones (5a–j) (0.5 mmol) in pyridine (20 mL), Hg(OAc)2 (0.5 mmol) was added and the reaction mixture was refluxed for 4–5 h. After completion of the reaction, the cooled mixture was poured into ice cold water and acidified with HCl aqueous solution (30%, v/v). The precipitate was extracted with CH2Cl2 and dried over Mg2SO4. After CH2Cl2 was evaporated, the residue was recrystallized from ethanol to give compounds 6a–j.
Microwave irradiation method
A mixture of (E)-3-(aryl)-1-(5-hydroxy-2H-chromen-6-yl)prop-2-en-1-ones (5a–j) (0.5 mmol) and Hg(OAc)2 (0.5 mmol) in pyridine (5 mL) was subjected to microwave irradiation at 180 W for 3–5 min. After completion of the reaction, the cooled mixture was poured into ice cold water and acidified with HCl aqueous solution (30%, v/v). The precipitate was extracted with CH2Cl2 and dried over Mg2SO4. After CH2Cl2 was evaporated, the residue was recrystallized from ethanol to give compounds 6a–j.
(Z)-2-benzylidene-2H-furo[2,3-f]chromen-3(7H)-one (6a)
Pale yellow solid; yield (81%); m.p.: 138–140 °C; IR (KBr) (cm−1): 2960 (H–C=), 1687 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 7.88 (d, 2H, J = 8.30 Hz, Ar–H), 7.55 (d, 1H, J = 8.49 Hz, Ar–H), 7.43 (m, 3H, Ar–H), 6.81 (m, 2H, Hβ, Ar–CH=C), 6.61 (d, 1H, J = 8.49 Hz, Ar–H), 5.88 (m, 1H, Ar–C=CH), 5.02 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.4 (C=O), 161.4 (C5a), 160.9 (C2), 147.1 (Cα), 131.8 (C1′), 131.2 (2C, Ar–C), 129.7 (Ar–C), 128.9 (2C, Ar–C), 124.8 (C4), 123.2 (C8), 115.3 (C9), 114.4 (C3), 112.5 (Cβ), 111.2 (C5), 106.7 (C9a), 66.3 (OCH2); LC-MS (m/z): 277 (M + H)+. Elemental analysis (C18H12O3): calcd. C, 78.25; H, 4.38; found: C, 78.48; H, 4.16%.
(Z)-2-(4-bromobenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6b)
Pale yellow solid; yield (78%); m.p.: 237–240 °C; IR (KBr) (cm−1): 2959 (H–C=), 1688 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 7.92 (d, 2H, J = 8.53 Hz, Ar–H), 7.70 (d, 2H, J = 8.53 Hz, Ar–H), 7.56 (d, 1H, J = 8.28 Hz, Ar–H), 6.87 (m, 2H, Ar–CH=C, Hβ), 6.70 (d, 1H, J = 8.28 Hz, Ar–H), 6.07 (m, 1H, Ar–C=CH), 5.04 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.9 (C=O), 161.8 (C5a), 161.1 (C2), 147.7 (Cα), 131.9 (C1′), 131.1 (2C, Ar–C), 128.8 (Ar–C), 129.3 (2C, Ar–C), 124.9 (C4), 123.6 (C8), 116.0 (C9), 115.2 (C3), 114.1 (Cβ), 111.7 (C5), 106.9 (C9a), 66.3 (OCH2); LC-MS (m/z): 355 (M + H)+. Elemental analysis (C18H11BrO3): calcd. C, 60.87; H, 3.12. found: C, 60.66; H, 3.30%.
(Z)-2-(2-chlorobenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6c)
Pale yellow solid; yield (80%); m.p.: 176–179 °C; IR (KBr) (cm−1): 2959 (H–C=), 1699 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 8.29 (dd, 1H, Ar–H), 7.50 (m, 4H, Ar–H), 6.98 (s, 1H, Hβ), 6.78 (dd, 1H, Ar–CH=C), 6.68 (d, 1H, J = 8.28 Hz, Ar–H), 6.03 (m, 1H, Ar–C=CH), 5.02 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.4 (C=O), 161.6 (C5a), 161.3 (C2), 148.1 (Cα), 134.3, 131.9, 131.2, 129.9, 129.3, 128.0 (6C, Ar–C), 125.2 (C4), 123.5 (C8), 115.3 (C9), 114.1 (Cβ), 112.8 (C3), 106.8 (C5), 105.1 (C9a), 66.5 (OCH2); LC-MS (m/z): 310 (M + H)+. Elemental analysis (C18H11ClO3): calcd. C, 69.58; H, 3.57. found: C, 69.76; H, 3.37%.
(Z)-2-(4-isopropylbenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6d)
Pale yellow solid; yield (83%); m.p.: 196–198 °C; IR (KBr) (cm−1): 2961 (H–C=), 1696 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 7.90 (d, 2H, J = 8.03 Hz, Ar–H), 7.53 (d, 1H, J = 8.28 Hz, Ar–H), 7.38 (d, 2H, J = 8.03 Hz, Ar–H), 6.83 (m, 2H, Ar–CH=C, Hβ), 6.67 (d, 1H, J = 8.28 Hz, Ar–H), 6.06 (m, 1H, Ar–C = CH), 5.03 (s, 2H, OCH2), 2.94 (m, 1H, CH), 1.23 (d, 6H, J = 6.77 Hz, 2 × CH3); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.4 (C=O), 161.3 (C5a), 160.8 (C2), 150.6 (C4′), 146.8 (Cα), 131.4 (2C, Ar–C), 129.4 (C1′), 127.0 (2C, Ar–C), 124.8 (C4), 123.2 (C8), 115.3 (C9), 114.6 (C3), 112.4 (Cβ), 111.5 (C5), 106.7 (C9a), 66.3 (OCH2), 33.3 (CH), 23.5 (2C, 2×CH3); LC-MS (m/z): 319 (M + H)+. Elemental analysis C21H18O3: calcd. C, 79.22; H, 5.70. found: C, 79.43; H, 5.44%.
(Z)-2-(2,4-dichlorobenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6e)
Pale yellow solid; yield (75%); m.p.: 253–256 °C; IR (KBr) (cm−1): 2957 (H–C=), 1697 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 8.34 (d, 1H, J = 8.53 Hz, Ar–H), 7.82 (d, 1H, Ar–H), 7.60 (m, 2H, Ar–H), 6.95 (s, 1H, Hβ), 6.85 (d, 1H, J = 10.29 Hz, Ar–CH=C), 6.72 (d, 1H, J = 8.53 Hz, Ar–H), 6.08 (m, 1H, Ar–C=CH), 5.06 (s, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.6 (C=O), 161.7 (C5a), 161.4 (C2), 148.2 (Cα), 134.4 (C2′), 132.0, 131.3, 130.0, 129.4, 128.1 (5C, Ar–C), 125.3 (C4), 123.6 (C8), 115.3 (C9), 114.2 (C3), 112.9 (Cβ), 106.9 (C9a), 105.2 (C5), 66.6 (OCH2); LC-MS (m/z): 345 (M + H)+. Elemental analysis (C18H10Cl2O3): calcd. C, 62.63; H, 2.92. found: C, 62.81; H, 2.71%.
(Z)-2-(4-methylbenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6f)
Pale yellow solid; yield (83%); m.p.: 194–196 °C; IR (KBr) (cm−1): 2961 (H–C=), 1697 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 7.88 (d, 2H, J = 8.19 Hz, Ar–H), 7.55 (d, 1H, J = 8.31 Hz, Ar–H), 7.33 (d, 2H, J = 8.06 Hz, Ar–H), 7.27 (d, 1H, J = 8.06 Hz, Ar–H), 6.84 (s, 1H, Hβ), 6.70 (dd, 1H, Ar–CH=C), 6.08 (m, 1H, Ar–C=CH), 5.04 (q, 2H, OCH2), 2.37 (s, 3H, Ar–CH3); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.4 (C=O), 161.3 (C5a), 160.8 (C2), 146.6 (Cα), 139.9 (C4′), 131.2 (2C, Ar–C), 129.6 (2C, Ar–C), 129.0 (Ar–C), 124.8 (C4), 123.3 (C8), 115.4 (C9), 114.6 (C3), 112.4 (Cβ), 111.5 (C5), 106.7 (C9a), 66.3 (OCH2), 21.0 (Ar–CH3); LC-MS (m/z): 291 (M + H)+. Elemental analysis (C19H14O3): calcd. C, 78.61; H, 4.86. found: C, 78.82; H, 4.62%.
(Z)-2-(4-methoxybenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6g)
Pale yellow solid; yield (82%); m.p.: 120–122 °C; IR (KBr) (cm−1): 2960 (H–C=), 1691 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 7.86 (d, 2H, J = 7.36 Hz, Ar–H), 7.49 (d, 1H, J = 7.93 Hz, Ar–H), 7.01 (d, 2H, J = 7.55 Hz, Ar–H), 6.80 (m, 2H, Ar–CH=C, Hβ), 6.62 (d, 1H, J = 7.93 Hz, Ar–H), 5.96 (d, 1H, J = 9.06 Hz, Ar–C=CH), 5.04 (s, 2H, OCH2), 3.87 (s, 3H, OCH3); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.2 (C=O), 161.1 (C5a), 160.6 (C4′), 160.5 (C2), 145.9 (Cα), 133.2 (2C, Ar–H), 124.7 (C4), 124.6 (C8), 124.3 (C1′), 123.2 (C9), 115.4 (C3), 114.5 (2C, Ar–C), 113.8 (Cβ), 112.3 (C5), 106.7 (C9a), 66.3 (OCH2), 55.2 (OCH3); LC-MS (m/z): 307 (M + H)+. Elemental analysis (C19H14O4): calcd. C, 74.50; H, 4.61. found: C, 74.72; H, 4.39%.
(Z)-2-(3,4-dimethoxybenzylidene)-2H-furo[2,3-f]chromen-3(7H)-one (6h)
Pale yellow solid; yield (81%); m.p.: 96–98 °C; IR (KBr) (cm−1): 2951 (H–C=), 1687 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 8.18 (d, 1H, J = 9.00 Hz, Ar–H), 7.81 (m, 2H, Ar–H), 7.55 (s, 1H, Hβ), 7.01 (d, 1H, J = 8.39 Hz, Ar–H), 6.68 (d, 1H, J = 10.07 Hz, Ar–CH=C), 6.41 (d, 1H, J = 8.85 Hz, Ar–H), 5.84 (m, 1H, Ar–C=CH), 4.90 (s, 2H, OCH2), 3.86, 3.81 (s, 6H, (CH3)2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.1 (C=O), 160.9 (C5a), 160.8, 160.7 (C3′, C4′), 160.3 (C2), 144.7 (Cα), 127.4 (C4), 126.2 (C8), 124.3, 124.0 (2C, Ar–C), 124.0 (C9), 114.5 (C3), 113.8 (Cβ), 112.5 (Ar–C), 112.3 (C5), 111.5 (Ar–C), 105.4 (C9a), 65.9 (OCH2), 55.1 (2C, 2×OCH3); LC-MS (m/z): 337 (M + H)+. Elemental analysis (C20H16O5): calcd. C, 71.42; H, 4.79. found: C, 71.65; H, 4.60%.
(Z)-2-(thiophen-2-ylmethylene)-2H-furo[2,3-f]chromen-3(7H)-one (6i)
Pale yellow solid; yield (82%); m.p.: 142–144 °C; IR (KBr) (cm−1): 2953 (H–C=), 1686 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 8.26 (d, 1H, J = 7.78 Hz, Ar–H), 7.55 (dd, 1H, Ar–H), 7.50 (d, 1H, J = 8.28 Hz, Ar–H), 7.43 (m, 1H, Ar–H), 6.95 (s, 1H, Hβ), 6.75 (d, 1H, J = 10.03 Hz, Ar–CH=C), 6.35 (d, 1H, J = 8.28 Hz, Ar–H), 6.00 (m, 1H, Ar–C=CH), 4.99 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 183.9 (C=O), 154.9 (C5a), 150.5 (C2), 147.9 (Cα), 136.7, 131.5, 131.1, 128.6 (4 C, Ar–C), 127.3 (C4), 123.2 (C8), 121.6 (C9), 121.0 (C3), 117.8 (Cβ), 113.5 (C5), 107.3 (C9a), 65.9 (OCH2); LC-MS (m/z): 283 (M + H)+. Elemental analysis (C16H10O3S): calcd. C, 68.07; H, 3.57. found: C, 68.29; H, 3.36%.
(Z)-2-((1H-indol-3-yl)methylene)-2H-furo[2,3-f]chromen-3(7H)-one (6j)
Red solid; yield (79%); m.p.: 253–256 °C; IR (KBr) (cm−1): 2965 (H–C=), 1692 (α,β-unsaturated C=O); 1H-NMR (400 MHz, DMSO-d6) δ p.p.m.: 9.87 {s, 1H, NH), 7.97 (d, 2H, J = 8.69 Hz, Ar–H), 7.52 (d, 1H, J = 8.39 Hz, Ar–H), 7.07 (m, 2H, Ar–H), 7.02 (m, 1H, Ar–H), 6.87 (d, 1H, J = 10.80 Hz, Ar–CH=C), 6.83 (s, 1H, Hβ), 6.67 (d, 1H, J = 8.54 Hz, Ar–H), 6.05 (m, 1H, Ar–C=CH), 5.03 (q, 2H, OCH2); 13C-NMR (100 MHz, DMSO-d6) δ p.p.m.: 181.8 (C=O), 156.2 (C5a), 152.4 (C2), 145.3 (Cα), 137.1, 129.1 (2 C, Ar–C), 128.3 (C4), 125.8 (C8), 122.8, 121.1 (2 C, Ar–C), 121.0 (C9), 120.1 (C3), 119.5 (Cβ), 119.1, 118.4, 115.0 (3 C, Ar–C), 114.1 (C5), 109.1 (C9a), 104.1 (Ar–C), 66.1 (OCH2); LC-MS (m/z): 316 (M + H)+. Elemental analysis (C20H13NO3): calcd. C, 76.18; H, 4.16; N, 4.44. found: C, 76.37; H, 4.37; N, 4.20%.
Biological assay
DPPH radical scavenging activity
The hydrogen atom or electron donation ability of the compounds was measured from the bleaching of the purple colored methanol solution of DPPH. The spectrophotometric assay uses the stable radical DPPH as a reagent. 1 mL of various concentrations of the test compounds (5, 10, 25, 50, and 100 µg/mL) in methanol was added to 4 mL of 0.004% (w/v) methanol solution of DPPH. After a 30 min incubation period at room temperature, the absorbance was read against blank at 517 nm. The percent of inhibition of free radical production from DPPH was calculated by the following equation.
where A control is the absorbance of the control reaction (containing all reagents except the test compound) and A sample is the absorbance of the test compound. Tests were carried at in triplicate.
Hydrogen peroxide (H2O2) scavenging activity
The H2O2 scavenging ability of the compounds was determined according to the method of Ruch et al., A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). 5, 10, 25, 50, and 100 µg/mL concentrations of the test compounds in 3.4 mL phosphate buffer were added to H2O2 solution (0.6 mL, 40 mM). The absorbance value of the reaction mixture was recorded at 230 nm. The percent of scavenging of H2O2 was calculated using Eq. (1).
Antimicrobial activity
The in vitro antimicrobial studies were carried out by agar well-diffusion method against test organisms (Chung et al. 1990; Azoro 2002). Nutrient broth (NB) plates were swabbed with 24 h old broth culture (100 mL) of test bacteria. Using the sterile cork borer, wells (6 mm) were made into each petriplate. The different concentrations of test samples dissolved in DMSO were added into the wells by using sterile pipettes. Simultaneously, the standard antibiotics, gentamicin for antibacterial activity, fluconazole for antifungal activity were tested against the pathogens. The plates were incubated at 37 °C for 24 h for bacteria and at 28 °C for 48 h for fungi. After appropriate incubation, the diameter of zone of inhibition of each well was measured. Duplicates were maintained and the average values were calculated for eventual antibacterial activity. Broth dilution test was used to determine MIC of the above mentioned samples (Janovska et al. 2003; Bishnu et al. 2009). Freshly prepared NB was used as diluents. The 24 h old culture of the test bacteria B. subtilis, S. aureus, E. coli, and P. vulgaris and the test fungi A. niger and C. albicans were diluted 100 folds in NB (100 mL bacterial cultures in10 mL NB). Increasing concentrations of the test samples were added to the test tubes containing the bacterial and fungal cultures. All the tubes were incubated at 37 °C for 24 h for bacteria and at 28 °C for 48 h for fungi. The tubes were examined for visible turbidity and using NB as control. The lowest concentration that inhibited visible growth of the tested organisms was recorded as MIC.
Molecular docking
Molecular docking was performed on crystal structure of tyrosinase from B. megaterium (PDB code: 3NM8) and imported into MVD.4.0 (Kalgutkar et al. 1998). All water molecules and co-factors were deleted. Then they were exported as mol2 files and docked by using MVD. We used the template docking available in MVD and evaluated MolDock score, Rerank score, and protein–ligand interaction score from MolDock and MolDock [GRID] options. Ascorbic acid was selected as reference compound for the template. It was used the default settings, including a grid resolution of 0.30 Å, the MolDock optimizer as a search algorithm, and the number of runs was set to 10. A population size of 50, maximum iteration of 2000, scaling factor of 0.50, crossover rate of 0.90 was set. The maximum number of poses to generate was increased to 10 from a default value of 5.
Conclusions
In summary, we synthesized a new series of compounds 5a–j and 6a–j under conventional and microwave irradiation methods. In microwave irradiation method, reactions were completed in short reaction time, mild reaction conditions, high yields and convenient operation. All the titled compounds have been screened for antioxidant activity using DPPH and H2O2 radical scavenging assays. The compounds 5d, 5h, 6e, and 6j were most potent with DPPH radical and the compounds 5h, 6a, 6e, and 6j were exhibited better radical scavenging ability with H2O2 radical. Antimicrobial activity screening revealed that compounds 5b, 5e, 6b, 6c, and 6e displayed better microbial inhibition. The binding mode of the synthesized compounds with protein active site was predicted using molecular docking. Compounds 5d, 5h, and 6j showed promising dock score values with more number of hydrogen bonding interactions. These in silico findings are well supported by results of antioxidant activity.
Supplementary materials
Spectral data of compounds 5a–j and 6a–j; 1H NMR, 13C NMR and mass spectra of representative synthesized derivatives are provided in supplementary materials.
References
Ashok D, Rangu K, Rao VH, Gundu S, Srilata B, Vijjulatha M (2016) Microwave-assisted synthesis, molecular docking and antimicrobial activity of novel 2-(3-aryl,1-phenyl-1H-pyrazol-4-yl)-8H-pyrano[2,3-f]chromen-4-ones. Med Chem Res 25:501–514
Azoro C (2002) Antibacterial activity of crude aqueous of Azadrichta indica on Salmonella typhi. World J Biotechnol 3:347–351
Bishnu J, Sunil L, Anuja S (2009) Antibacterial property of different medicinal plants: Ocimum sanctum, Cinnamomum zeylanicum, Xanthoxylum armatum and Origanum majorana. J Sci Eng Technol 5:143–150
Boumendjel A (2003) Aurones: a subclass of flavones with promising biological potential. Curr Med Chem 10:2621–2630
Burits M, Bucar F (2000) Antioxidant activity of Nigella sativa essential oil. Phytother Res 14:323–328
Cheng H, Zhang L, Liu Y, Chen S, Cheng H, Lu X, Zheng Z, Zhou GC (2010) Design, synthesis and discovery of 5-hydroxyaurone derivatives as growth inhibitors against HUVEC and some cancer cell lines. Eur J Med Chem 45:5950–5957
Chung KT, Thomasson WR, Wu-Yuan CD (1990) Growth inhibition of selected food-borne bacteria, particularly Listeria monocytogenes, by plant extracts. J Appl Bacteriol 69:498–503
Cuendet M, Hostettmann K, Potterat O, Dyatmiko W (1997) Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta 80:1144–1152
Detsi A, Majdalani M, Kontogiorgis CA, Hadjipavlou-Litina D, Kefalas P (2009) Natural and synthetic 2′-hydroxy-chalcones and aurones. Bioorg Med Chem 17:8073–8085
Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu OI (2003) Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. subsp. pallsiana (Lamb.) Holmboe. J Ethnopharmacol 86:51–58
Hangum-balkir Y, Mckenney ML (2012) Determination of antioxidant activities of berries and Resveratrol. Green Chem Lett Rev 5:147–153
Janovska D, Kubikova K, Kokoska L (2003) Screening for antimicrobial activity of some medicinal plants species of traditional Chinese medicine. J Food Sci 21:107–110
Jung MJ, Chung HY, Kang SS, Choi JH, Bae KS, Choi JS (2003) Antioxidant activity from the stem bark of Albizzia julibrissin. Arch Pharm Res 6:458–462
Kalgutkar AS, Crews BC, Rowlinson SW, Garner C, Seibert K, Marnett LJ (1998) Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science 280:1268–1270
Kerboeuf D, Riou M, Guégnard F (2008) Flavonoids and related compounds in parasitic disease control. Mini Rev Med Chem 8:116–28
Kim IT, Park YM, Shin KM, Ha JH, Choi JW, Jung HJ, Park HJ, Lee KT (2004) Anti-inflammatory and anti-nociceptive effects of the extract from Kalopanax pictus, Pueraria thunbergiana and Rhus verniciflua. J Ethnopharmacol 94:165–173
Ku CS, Mun SP (2008) Characterization of seed oils from fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioresour Technol 99:2852–2856
Lee EH, Song DG, Lee JY, Pan CH, Um BH, Jung SH (2008) Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts. Biol Pharm Bull 31:1626–1630
Pare PW, Dmitrieva N, Mabry TJ (1991) Phytoalexin aurone induced in Cephalocereus senilis liquid suspension culture. Phytochemistry 30:1133–1135
Park KY, Jung GO, Lee KT, Choi JW, Choi MY, Kim GT, Jung HJ, Park HJ (2004) Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J Ethnopharmacol 90:73–79
Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101:379–385
Roussaki M, Lima SC, Kypreou AM, Kefalas P, Cordeiro da Silva A, Detsi A (2012) Aurones: a promising heterocyclic scaffold for the development of potent antileishmanial agents. Int J Med Chem 196921, doi:10.1155/2012/196921
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008
Schoepfer J, Fretz H, Chaudhuri B, Muller L, Seeber E, Meijer L, Lozach O, Vangrevelinghe E, Furet P (2002) Structure-based design and synthesis of 2-benzylidene-benzofuran-3-ones as flavopiridol mimics. J Med Chem 45:1741–1747
Shrestha S, Natarajan S, Park JH, Lee DY, Cho JG, Kim GS, Jeon YJ, Yeon SW, Yang DC, Baek NI (2013) Potential neuroprotective flavonoid-based inhibitors of CDK5/p25 from Rhus parviflora. Bioorg Med Chem Lett 23:5150–5154
Sim HM, Loh KY, Yeo WK, Lee CY, Go ML (2011) Aurones as modulators of ABCG2 and ABCB1. Chem Med Chem 6:713–724
Song MY, Jeong GS, Kwon KB, Ka SO, Jang HY, Park JW, Kim YC, Park BH (2010) Sulfuretin protects against cytokine-induced beta-cell damage and prevents streptozotocin-induced diabetes. Exp Mol Med 42:628–638
Sreenivas P (2011) Synthesis and antibacterial activity of some new spiro[pyrano[2,3-f]chromene-2,10-cycloalkan]-4-ones and 10-alkylspiro[pyrano[2,3-f]chromene-2,40-piperidin]-4-ones. Indian J Chem Sect B 50B:1484–1490
Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M (2005) Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem 90:333–340
Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K (2007) Antioxidant and antimicrobial activities of laetiporus sulphurus (bull.) Murrill. Food Chem 101:267–273
Venkateswarlu S, Panchagnula GK, Gottumukkala AL, Subbaraju GV (2007) Synthesis, structural revision, and biological activities of 4′-chloroaurone, a metabolite of marine brown alga Spatoglossum variabile. Tetrahedron 63:6909–6914
Yin H, Xu L, Porter NA (2011) Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 111:5944–5972
Acknowledgements
The authors are grateful to Department of Chemistry, Osmania University for providing laboratory facilities, CFRD, and spectral analysis. One of the authors, K.R. is thankful to UGC-New Delhi, India, for granting Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ashok, D., Rangu, K., Gundu, S. et al. Microwave-assisted synthesis, molecular docking, and biological evaluation of 2-arylidene-2H-furo[2,3-f]chromen-3(7H)-ones as antioxidant and antimicrobial agents. Med Chem Res 26, 1735–1746 (2017). https://doi.org/10.1007/s00044-017-1834-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1834-9