Abstract
Cathepsins H and L, lysosomal cysteine proteases have been found in elevated levels in tumor invasion, metastasis, inflammation, atherosclerosis, and various other tissue degenerative diseases. In the past decade, work has largely been focused on evaluation of some non-peptidyl inhibitors of cathepsins as these have been considered as viable drug targets for major diseases. Semicarbazones and thiosemicarbazones, carbonyl derivatives are extensively studied for wide variety of biological activities such as anticonvulsant, anticancer, anti-inflammatory, antihypertensive, antimicrobial, and antiparasitic. These derivatives have also shown to possess parasiticidal activity against Plasmodium falciparum, Plasmodium berghei, Trypanosoma cruzi, Trypanosoma brucei rhodesiense and Toxoplasma gondii. With this background, the present work involved the inhibition and kinetic studies of substituted semicarbazones and thiosemicarbazones on cathepsin H and L. A comparative account of structure–activity relationship for inhibition exerted by synthesized semicarbazones and thiosemicarbazones with varied functional moieties on cathepsins H and L is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cathepsins initially thought to be ‘housekeeping’ molecules for cell’s garbage disposals because of their lysosomal localization are also known to play vital role in specific physiological processes, and are also linked to many severe genetic disorders (Wolters and Chapman 2000; Jedeszko and Sloane 2004; Toomes et al. 1999). Cathepsins B, H, and L belonging to Papain super family have been found to be key factors in the pathogenesis of cancer invasion, arthritis, osteoporosis, and microbial infections. Targeting these enzymes is therefore one of the strategies in the development of new chemotherapeutic agents for a number of diseases (Selzer et al. 1999). These enzymes are also involved in pathology of chronic inflammatory diseases of airways and joints such as asthma (Cimerman et al. 2001) and certain forms of arthritis (Maciewicz and Etherington 1988), periodontal disease (Lah et al. 1986), muscular dystrophy (Kamatsu et al. 1986), pancreatitis (Mort and Buttle 1997), and tumor growth and metastasis (Frohlich et al. 2001).
Implications of cathepsins B, H, and L in tumorigenesis (Mohamed and Sloane 2006; Joyce and Hanahan 2004; Lankelma et al. 2010; Henkin 1993; Van der Stappen et al. 1991), recognition of these as prognostic markers in several types of cancer, including breast, with increased primary tumor expression associated with poor outcome (Jain et al. 2010; Lah et al. 2000; Nouh et al. 2011) and correlation of their over expression with advancement of tumor (Chan et al. 1986; Podgorski and Sloane 2003; Ostensen et al. 1983; Kirschke 1977; Waghray et al. 2002; Linnerth et al. 2005; Schweiger et al. 2004; Turk et al. 2002; Liu et al. 2006) focus the development of selective inhibitors of these enzymes.
Semicarbazones and thiosemicarbazones, a class of small molecules that have been extensively studied for wide variety of biological activities, have been evaluated as anticancer (Nutting et al. 2009), antihypertensive (Warren et al. 1977), anti-inflammatory (Swathi and Sarangapani 2014), antimicrobial (Nfor et al. 2011), and anticonvulsant agents (Puthucode et al. 1998). These derivatives have also shown to possess parasiticidal activity against Plasmodium falciparum, Plasmodium berghei, Trypanosoma cruzi, Trypanosoma brucei rhodesiense and Toxoplasma gondii (De-Oliveira et al. 2008; Aguiree et al. 2004; Fujii et al. 2005; Tenorio et al. 2005). Thiosemicarbazones have been demonstrated as potential inhibitors of cathepsin L (Kumar et al. 2010a, b) and are proposed to have potential application in the treatment of chagas disease, sleeping sickness and malaria probably due to their inhibitory potency on parasitic cysteine proteases.
Recent studies by our group have demonstrated the effectiveness of the various non-peptidyl inhibitors (i-ix) of cathepsin B and cathepsin H such as bischalcones based quinazoline-2(1H)-ones, quinazoline-2(1H)-thiones (Raghav and Singh 2014a), acyl hydrazides, triazoles (Raghav and Singh 2014b), hydrazones (Raghav and Singh 2014c), hydroxyl chalcones (Raghav and Garg 2014a) and their cyclized derivatives, formyl and benzoyl pyrazolines (Raghav and Garg 2014b).

Previously, we have synthesized semicarbazones and thiosemicarbazones with different functionalities and evaluated these as inhibitors of cathepsin B (Raghav and Kaur 2014). In this direction to screen their affectivity toward other cathepsins, the present work deals with the inhibition studies of semicarbazones and thiosemicarbazones on cathepsins H and L. Supportive kinetic studies and in silico docking studies are also performed to compare the results.
Experimental
Materials and methods
All the chemicals were of analytical grade. Fast Garnet GBC (o-aminoazotoluene diazonium salt), Leu-βNA and ZPheArg-4mβNA were purchased from Bachem Feinchemikalien AG, Switzerland. The protein sample was concentrated using Amicon stirred cells with YM 10 membrane under nitrogen pressure of 4–5 psi. The source of enzyme was fresh goat liver obtained from local slaughter house.
Melting points were taken in open capillaries and are uncorrected. The progress of the reactions was monitored on silica gel G plates using iodine vapor as visualizing agent. Elisa plate reader was used for measuring absorbance in the visible range. The spectrofuge was used for centrifugation purpose. IR spectra were recorded on Horizon 300 MHz spectrometer. NMR spectra were recorded on Bruker 300 MHz instrument. The chemical shifts are expressed in ppm units from an internal TMS standard. All commercially available reagents were used as-received.
Synthesis
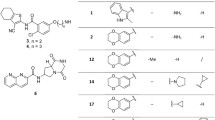
The synthesis and characterization of title compounds semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) by IR, NMR were previously reported (Raghav and kaur 2014) (Fig. 1).
Pharmacology
Purification of cathepsins H and L
All the purification steps were carried out at 4 °C. Cathepsins H and L were extracted and purified from goat liver by the established procedure reported previously (Raghav et al. 2015) including the steps of acetone powder preparation, homogenization, acid autolysis, 30–70% (NH4)2SO4 fractionation, molecular sieve chromatography on sephadex G-100 and ion exchange chromatographies on CM Sephadex C-50 and DEAE A-50 sephadex. The specific activities of the cathepsin H and L were equal to ~24.01 nmol/min/mg and ~16.78 nmol/min/mg respectively.
Enzyme inhibition studies
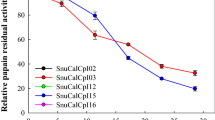
Cathepsin H activity was determined using Leu-βNA substrate at pH 7.0 whereas cathepsin L activity was determined using ZPheArg-4mβNA (Raghav et al. 2015) substrate at pH 6.0 respectively. Effect of synthesized semicarbazones (1a–1j) was observed on the activities of cathepsins H and L at 1 × 10−3 M, 1 × 10−8 M final concentration of each compound, respectively whereas effect of synthesized thiosemicarbazones (2a–2j) was observed on the activities of cathepsins H and L at 1 × 10−3 M, 1 × 10−7 M final concentration of each compound, respectively. First of all, enzyme was equilibrated in buffer of appropriate pH at 37 °C. Then 20 μl of individual compound was added in the reaction mixture separately to effect the final concentration of each compound as quoted before. After an incubation time of 30 min residual enzyme activity was estimated by the usual enzyme assay using the respective substrates. The experiments were performed in triplicate for each concentration and % activity has been calculated with respect to the control, where no compound was added but an equivalent amount of solvent was present (Table 1). Enzyme assays were similarly conducted at lower concentrations of each compound to observe the inhibitory effect of compounds at varying concentrations. The results are presented in Figs 2, 3 for cathepsins H and L respectively.
Enzyme kinetic studies
After establishing the inhibitory action of semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) on cathepsins H and L experiments were designed to evaluate the type of inhibition and to determine the K i value of these compounds on cathepsin H and L. For that, enzyme activity was evaluated at different substrate concentration in presence and absence of a fixed concentration of inhibitor. Line-weaver Burk plots were drawn between 1/[S] and 1/V (Figs 4, 5). The K m value of cathepsins H and L for Leu βNA and ZPheArg-4mβNA was found to be 5.0 × 10−4 M and 0.5 × 10−4 M respectively. The K i values have been summarized in Table 2.
Drug modeling studies
Docking studies were performed using iGemdock software. To conduct these, small molecular weight ligands were prepared using marvin sketch and were saved as MDL Mol File. Enzyme structure active site was retrieved from the Protein Data Bank (http://www.rcsb.org/) cav8PCH H_NAG.pdb and cav3BC3L_CSW (Guncar et al. 1998; Chowdhary et al. 2008). The prepared ligands and the binding site was loaded in the iGemdock program and docking was run by setting GA parameters for Standard Docking Accuracy Settings, Docking experiments show a decrease in energy when enzyme and ligands interact. The E total resulting after H-bonding and van der Waals interactions are presented in Tables 3, 4. The docking poses of the most inhibitory compounds 1b, 2g for cathepsin H and 1g, 2h for cathepsin L are shown in Figs 6, 7.
Result and discussion
In drug discovery, one of the strategies is to design structural analogs of potent inhibitors of enzymes involved in physiological disorder. Preferential inhibition of one enzyme over the other can add to the drug potential with reduced toxicity where the otherwise unwanted enzyme inhibition can lead to severe side effects. In the present work we report the inhibition studies of semicarbazones and thiosemicarbazones already established as inhibitors of cathepsins B (Raghav and Kaur 2014) on cathepsins H and L, two other significant and related cysteine proteases. Enzyme kinetics and structure–function relationship has been studied, which is vital to understand the mode of action of drug molecule and is fundamental to the modern design of pharmaceuticals in industries (Sami and Shakoor 2011).
In the past decade, work was focused on the identification and development of cysteine protease inhibitors and their potential use as anti parasitic agents (Du et al. 2002; Greenbaum et al. 2004; Romeiro et al. 2009; Brak et al. 2010). A large work has been accomplished on peptidyl or peptidyl analogs as inhibitors to cysteine proteases (Otto and Schirmeister 1997; Steverding 2011). However, these inhibitors are not considered to be viable drug candidates for treating diseases like cancer, and apoptosis, because of the possibility of immunogenic reactions or gastric instability. Besides peptidyl inhibitors there were also triumph for non-peptidyl inhibitors of cysteine proteases (Dana et al. 2013; Schenker et al. 2008). Therefore our attempt is to find out such non-peptidyl inhibitors of cathepsins B, H, and L that can lead to drug research and development toward these enzymes. The work on cathepsin B has already been published (Raghav and Kaur 2014). In the present work we report the inhibitory effect of semicarbazones and thiosemicarbazones on cathepsins H and L, two other pharmacologically significant lysosomal cysteine proteases.
Effect of synthesized compounds on the activity of cathepsins H and L
The effect of differently substituted semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) on the activity of cathepsins H and L at varying concentrations is shown in Figs 2, 3. From these plots of % residual activities vs. the concentrations of different compounds, it can be observed that at a particular concentration all the synthesized compounds inhibited cathepsin L activity more than cathepsin H.
The inhibition type and K i values
The type of inhibition caused by various compounds was determined through Lineweaver–Burk double reciprocal plot. In order to establish inhibition ability of the under consideration compounds, results were compared with potent inhibitors of cathepsin L, e.g., Leupeptin and cathepsin H e.g. Leu-CH2Cl, respectively. As reported in literature, K
i value for human liver cathepsin H was reported to be 9.2 × 10–6 M (Azaryan and Galoyan 1987) and K
i value for goat brain cathepsin L was reported to be 1.45 × 10–9 M (Kamboj et al. 1993).
For evaluating the type of inhibition caused by different semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) cathepsins H and L activity was measured at varying substrate i.e., Leu βNA and ZPheArg-4mβNA concentration in presence and absence of a fixed concentration of compound. The plots of 1/V and 1/[S] were straight lines intersecting at the Y-axis and shows that value of V max remains constant in all the compounds, whereas the value of K m’ change with each compound. These studies suggested that semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) are competitive inhibitors to cathepsins H and L. Using the Lineweaver–Burk equation of competitive inhibition the Ki values were calculated, which has been presented in Table 2.
Lineweaver–Burk plots of different semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) for cathepsins H and L are shown in Figs 4, 5.
Structure–activity relationship
Out of various synthesized semicarbazones (1a–1j) and thiosemicarbazones (2a–2j), m-chlorobenzaldehyde semicarbazone, (1b) and o-nitrobenzaldehyde thiosemicarbazone, (2g) with K i values of 2.7 × 10−5 M and 0.9 × 10−5 M showed maximum inhibition on cathepsin H, whereas in case of cathepsin L, o-nitrobenzaldehyde semicarbazone, (1g) and m-nitrobenzaldehyde thiosemicarbazone, (2h) with K i values of 0.5 × 10−10 M and 0.5 × 10−9 M showed maximum inhibition. Followed by these results it was concluded that the synthesized compounds showed more inhibition on activity of cathepsin L than on cathepsins H. By comparing the results obtained from the previous study (Raghav and kaur 2014) it is concluded that the semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) were less inhibitory to the activity of cathepsin B than cathepsin L but more inhibitory than cathepsin H. The inhibitory trend obtained on these three lysosomal cathepsins, the synthesized compounds semicarbazones (1a–1j) and thiosemicarbazones (2a–2j) showed maximum inhibition towards cathepsin L followed by cathepsin B and then cathepsin H. Also by comparing the synthesized compounds on the activity of individual cathepsin it was accomplished that thiosemicarbazones (2a–2j) showed maximum inhibition than semicarbazones (1a–1j) towards cathepsin B and H whereas in case of cathepsin L thiosemicarbazones (2a–2j) showed less inhibition than semicarbazones (1a–1j). These results can lead to the development of selective inhibitors of cathepsins B, H, and L.
The structure–activity relationship study revealed that the maximum inhibitory compounds for cathepsins B, H, and L either possessed chlorine or nitro moiety. The effect of nitro substituent can be explained on the basis of the electronic effect induced by the substituent. Nitro being strongly electron withdrawing may affect the nucleophilic center in the molecule rendering it more susceptible to the attack of sulfhydryl group of target enzymes. The electronic effect of chloro can also be explained similarly. The chloro group can have an added advantage being more lipophilic in nature. A proposed mechanism of inhibition of cathepsin B by semicarbazones and thiosemicarbazones is also reported (Raghav and kaur 2014).
Semicarbazones containing peptidyl inhibitor have been previously reported as potential inhibitors of cathepsins B (Barrett 1986). The inhibitory capacity of these molecules has been attributed to the specific peptidyl binding of inhibitory compound with the enzyme binding sites. In the non-peptidyl semicarbazones and thiosemicarbazones the subsite P2 is occupied by the aromatic ring orienting the –CONH2, –CSNH2 group toward the P1 site and competes with the binding of substrate with the enzyme (Fig. 8). Hence, these have evaluated as competitive inhibitors (cf enzyme kinetic studies (Figs 4 and 5) and docking (Figs 6 and 7), respectively).
Docking studies
The docking approach was used to study the interaction of compounds with the active site of cathepsin B, H, and L to observe binding poses of individual compounds. Individual binding poses of each compound was assessed and their interactions in the active site of the enzyme were analyzed. The empirical scoring function of iGemDOCK is the estimated sum total of van der Waals, H-bonding and electrostatic energy. Figure 6 show the binding of most inhibitory compound 1b and 2g in the active site of cathepsin H. It is clearly observed that Ser-69 and Glu-73 residues present at the catalytic site of the enzyme are involved in the binding of compounds. In addition, Gln-78 and Asn-112 amino acids residues are also involved in the stabilization of compounds in binding site.
The binding energies of title compounds in the amino acyl binding site of cathepsin H (cav8PCHH_NAG) is presented in Table 3. Experimental results obtained can be correlated with the ligand–binding interactions. It is observed that for 1b and 2g the binding energies computed come out to be −71.75 and −89.06. In each series, these most inhibitory compounds show a decrease in binding energy toward higher side. The binding energies show effective interaction between the enzyme binding site and inhibitory compounds may be responsible for these inhibition patterns. These results are somewhat different than the results of in vitro studies, which clearly indicate that thiosemicarbazones are more effective inhibitors than semicarbazones (Table 3). Figure 6c shows the docking results of substrate Leu-βNA with the cathepsin H active site. The amino acids Ser-69, Glu-78, and Asn-122 which interact with the most inhibitory compounds 1b (Fig. 6a) and 2g (Fig. 6b) can be observed interacting with the substrate Leu-βNA. The results are in correlation with the enzyme kinetic studies, where the compounds have been evaluated as competitive inhibitors.
Figure 7 shows the binding of most inhibitory compounds 1g and 2h in the active site of cathepsin L. The results of the docking studies support the in vitro experimental studies conducted on goat liver cathepsin L, which shows that semicarbazones are better inhibitor than thiosemicarbazones and this is different in contrast with the results obtained in case of cathepsin H. The binding energies of title compounds in the amino acyl binding site of cathepsin L (cav3BC3L_CSW) is presented in Table 4. The binding energies of 1g and 2h were found to be −98.99 and −87.43, respectively.
Figure 7c shows the docking results of substrate Z-Phe-Arg-4mβNA with the cathepsin L active site. It can be observed that the results are in correlation with the enzyme kinetic studies, where the compounds have been evaluated as competitive inhibitors as the amino acids Asp-162, Gly-164, and His-163 interact with the most inhibitory compounds 1g (Fig. 7a) and 2h (Fig. 7b) can be observed interacting with the substrate Z-Phe-Arg-4mβNA.
Conclusion
The present work concluded that the synthesized title compounds have been evaluated as better inhibitors for cathepsin L than cathepsin H and previously reported cathepsin H. One more aspect of the present work is concluded that cathepsin L inhibited semicarbazones more than that of thiosemicarbazones, whereas in case of cathepsin H thiosemicarbazones show more inhibition than semicarbazones. Best inhibitor for cathepsin H has been evaluated as m-chlorobenzaldehyde semicarbazone, (1b) and o-nitrobenzaldehyde thiosemicarbazone, (2g) with K i values of 0.27 × 10−4 M and 0.09 × 10−4 M, for cathepsin L o-nitrobenzaldehyde semicarbazone, (1g) and m-nitrobenzaldehyde thiosemicarbazone, (2h) showed maximum inhibition with K i values of 0.005 × 10−8 M and 0.05 × 10−8 M.
References
Aguiree G, Boiani L, Cerecetto H, Fernandez M, Gonzalez M, Denicola A, Otero L, Gambino D, Rigol C, Olea-Azar C, Faundez M (2004) In-vitro activity and mechanism of action against the protozoan parasite trypanosome cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg Med Chem 12:4885–4893
Azaryan A, Galoyan A (1987) Human and bovine brain cathepsin L and cathepsin H: Purification, physic-chemical properties and specificity. Neurochem Res 12:207–213
Brak K, Kerr ID, Barrett KT, Fuchi N, Debnath M, Ang K, Engel JC, McKerrow JH, Doyle PS, Brinen LS, Ellman JA (2010) Non-peptidic tetrafluoro phenoxymethyl ketone cruzain inhibitors as promising new leads for chagas disease. Chemother J Med Chem 53:1763–1773
Barrett A (1986) In proteinase inhibitors. In: Barrett A, Salvesen G, (eds) Proteinase Inhibitors. Elsevier, Amsterdam, pp 3–22
Chan SJ, Segundo BS, McCormick MB, Steiner DF (1986) Nuclotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci 83:7721–7725
Chowdhary SF, Joseph L, Kumar S, Tulsidas SR, Bhat S, Ziomek E, Menard R, Sivaraman J, Purisima EO (2008) Exploring inhibitor binding at the S’ subsites of cathepsin L. J Med Chem 51:1361–1368
Cimerman N, Bruguljan PM, Krasovec M, Suskovic S, Kos J (2001) Serum concentration and circadian profiles of cathepsins B, H and L, and their inhibitors, stefins A and B, in asthma. Clin Chim Acta 310:113–122
Dana D, Davalos AR, De S, Rathod P, Gamage RK, Huestis J, Afzal N, Zavlanov Y, Paroly SS, Rotenberg SA, Subramaniam G, Mark KJ, Chang EJ, Kumar S (2013) Development of cell-active non-peptidyl inhibitors of cysteine cathepsin. Bioorg Med Chem 21:2975–2987
De-Oliveira RB, De Sauza-Fagundes EM, Soares RP, Andrade AA, Kretti AU, Zani CL (2008) Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur J Med Chem 43:1983–1988
Du X, Guo C, Hansell E, Doyle PS, Caffrey CR, Holler TP, McKerrow JH, Cohen FE (2002) Synthesis and structure–activity relationship study of potent trypanocidal thiosemicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J Med Chem 45:2695–2707
Frohlich E, Schlagenhauff B, Mohrle M, Weber E, Klessen C, Rassner G (2001) Activity expression and transcription of the cathepsins B, D, H and L in cutaneous malignant melanoma. Cancer 91:972–982
Fujii N, Mallari JP, Hansell JE, Mackey HZ, Doyle P, Zhou YM, Gut J, Rosenthal PJ, McKerrow JH, Guy RK (2005) Discovery of potent thiosemicarbazone inhibitors of rhodesain and cruzain. Bioorg Med Chem Lett 15:121–123
Guncar G, Podobnik M, Pungercar J, Strukelj B, Turk V, Turk D (1998) Crystal structure of porcine cathepsin H determined at 2.1 Å resolution: location of the mini-chain C-terminal carboxyl group defines cathepsin H aminopeptidase function. Structure 6:51–61
Greenbaum DC, Mackey Z, Hansell E, Doyle P, Gut J, Caffrey CR, Lehrman J, Rosenthal PJ, McKerrow JH, Chibale K (2004) Synthesis and structure–activity relationships of parasiticidal thiosemicarbazone cysteine protease inhibitors against Plasmodium falciparum, Trypanosoma brucei and Trypanosoma cruzi. J Med Chem 47:3212–3219
Henkin J (1993) Proteases and metastasis. Ann Rep Med Chem 28:151–160
Jain M, Bakhshi S, Shukla AA, Chauhan SS (2010) Cathepsins B and L in peripheral blood mononuclear cells of pediatric acute myeloid leukemia: potential poor prognostic markers. Ann Hematol 89:1223–1232
Jedeszko C, Sloane BF (2004) Cysteine cathepsins in human cancer. Biol Chem 385:1017–1027
Joyce JA, Hanahan D (2004) Mutiple roles for cysteine cathepsins in cancer. Cell Cycle 3:1516–1619
Kamatsu K, Tsukuda K, Hosoya J, Satoh S (1986) Elevation of cathepsin B and Cathepsin L activities in fore limb and hind limb muscles of dystrophic mice. Exp Neurol 93:642–646
Kamboj RC, Pal S, Raghav N, Singh H (1993) Selective colorimetric assay for cathepsin L using Z-Phe-Arg-4-ethoxy-β-naphthylamide. Biochimie 75:873–878
Kirschke H (1977) Cathepsin H: an endoaminopeptidase. Acta Biol Med Ger 36:1547–1548
Kumar GDK, Chavarria GE, Charlton-Sevcik AK, Arispe WM, MacDonough MT, Strecker TE, Chen SE, Siim BG, Chaplin DJ, Trawick ML, Pinney KG (2010a) Design, synthesis and biological evaluation of potent thiosemicarbazone based cathepsin L inhibitors. Bioorg Med Chem Lett 20:1415–1419
Kumar GDK, Chavarria GE, Charlton-Sevcik AK, Yoo GK, Song J, Strecker TE, Siim BG, Chaplin DJ, Trawick ML, Pinney KG (2010b) Functionalized benzophenone, thiophene, pyridine and fluorine thiosemicarbazone derivatives as inhibitors of cathepsin L. Bioorg Med Chem Lett 20:6610–6615
Lah T, Skaleric U, Babnik J, Turk V (1986) Detection of cathepsin L-like proteinase and cathepsin D in gingival fluid. J Peridontal Res 21:504–509
Lah TT, Kalman E, Najjar D, Gorodetsky E, Brennan P, Somers R, Daskal I (2000) cells producing cathepsins D, B, and L in human breast carcinoma and their association with prognosis. Hum Pathol 31:149–160
Lankelma JM, Voorend DM, Barwari T, Koestsveld J, Vander Spek AH, De Porto AP, Van Rooijen G, Van Noorden CJ (2010) Cathepsin L, target in cancer treatment? Life Sci 86:225–233
Linnerth NM, Sirbovan K, Moorehead RA (2005) Use of a transgenic mouse model to identify markers of human lung tumors. Int J Cancer 114:977–982
Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, Xu WH, Fu H, Dolganov GM, Hu C, Libby P, Shi GP (2006) Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis and vascular cells. Atherosclerosis 184:302–311
Maciewicz RA, Etherington DJ (1988) Enzyme immunoassay for cathepsins B and L in synovial fluids from patients with arthritis. Biochem Soc Trans 16:812–813
Mohamed MM, Sloane BF (2006) Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 6:764–775
Mort JS, Buttle DJ (1997) Cathepsin B. Int J Biochem Cell Biol 29:715–720
Nfor EN, Esemu SN, Ayimele GA, Eno EA, Iniama GE, Offiong OE (2011) Synthesis, stereochemistry and antimicrobial activity of copper(ii) and nickel(ii) complexes of 4-phenylsemicarbazones. Bull Chem Soc Ethiop 25:361–370
Nouh MA, Mohamed MM, EI-Shinawi M, Shaalan MA, Cavallo-Medved D, Khaled HM, Sloane BF (2011) Cathepsin B: a potential prognostic marker for inflammatory breast cancer. J Transl Med 9:1–8. doi:10.1186/1479-5876-9-1
Nutting CM, Van Herpen CML, Miah AB, Bhide SA, Machiels JP, Buter J, Kelly C, de Raucourt D, Harrington KJ (2009) Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann Oncol 20:1275–1279
Ostensen M, Morland B, Husby G (1983) Stimulation of murine macrophage cathepsin B by serum from patients with rheumatoid arthritis: an indicator of disease activity. Clin Exp Immunol 51:103–109
Otto HH, Schirmeister T (1997) Cysteine proteases and their inhibitors. Chem Rev 97:133–171
Podgorski I, Sloane BF (2003) Cathepsin B and its role(s) in cancer progression. Biochem Soc Symp 70:263–276
Puthucode RN, Pugazhenthi U, Quali JW, Stables JP, Dimmock JR (1998) Anticonvulsant activity of various aryl, arylidene and aryloxyaryl semicarbazones. Eur J Med Chem 33:595–607
Raghav N, Garg S (2014a) SAR studies of o-hydroxychalcones and their cyclised analogs and study them as novel inhibitors of cathepsin b and cathepsin H. Eur J Pharm Sci 60:55–63
Raghav N, Garg S (2014b) N-formylpyrazolines and N-benzoylpyrazolines as novel inhibitors of mammalian cathepsin B and cathepsin H. Bioorg Chem 57:43–50
Raghav N, Kaur R (2014) Synthesis and evaluation of some semicarbazone and thiosemicarbazone based cathepsin B inhibitors. Med Chem Res 23:4669–4679
Raghav N, Singh M (2014a) Design synthesis and docking studies of bischalcones based quinazoline-2(1H)-ones and quinazoline-2(1H)-thiones derivatives as novel inhibitors of cathepsin B and cathepsin H. Eur J Pharm Sci 54:28–39
Raghav N, Singh M (2014b) Acyl hydrazide and triazoles as novel inhibitors of mammalian cathepsin B and cathepsin H. Eur J Med Chem 77:231–242
Raghav N, Singh M (2014c) SAR studies of differently functionalized chalcones based hydrazones and their cyclized derivatives as inhibitors of mammalian cathepsin B and cathepsin H. Bioorg Med Chem 22:4233–45
Raghav N, Singh M, Garg S, Kaur R, Jangra S, Ravish I (2015) Ion exchangers: a useful tool for separation and simultaneous purification of lysosomal cysteine proteinases, cathepsins B, H and L. Int J Pharm Sci Res 6:2944–2949
Romeiro NC, Aguirre G, Hernández P, González M, Cerecetto H, Aldana I, Pérez-Silanes S, Monge A, Barreiro EJ, Lima LM (2009) Synthesis,trypanocidal activity and docking studies of novel quinoxaline-Nacylhydrazones, designed as cruzain inhibitors candidates. Bioorg Med Chem 17:641–652
Sami AJ, Shakoor AR (2011) Cellulase activity inhibition and growth retardation of associated bacterial strains of Aulacophora foviecollis by two glycosylated flavonoids isolated from Mangifera indica leaves. J Med Plants Res 5:184–190
Schenker P, Alfarano P, kolb P, Caflisch A, Baici A (2008) A double-headed cathepsin B inhibitor devoid of warhead. Protein Sci 17:2145–2155
Schweiger A, Christensen IJ, Nielsen HJ, Sorensen S, Brunner N, Kos J (2004) Serum cathepsin H as a potential prognostic marker in patients with colorectal cancer. Int J Biol Markers 19:289–294
Selzer PM, Pingel S, Hsieh I, Ugele B, Chan VJ, Engel JC, Bogyo M, Russell DG, Sakanari JA, Mckerrow JH (1999) Cysteine protease inhibitors as chemotherapy: lessons from a parasite target. Proc Natl Acad Sci 96:11015–11022
Steverding D (2011) The Cathepsin B-selective inhibitors CA-074 and CA-074Me inactivate Cathepsin L under reducing conditions. Open Enzyme Inhib J 4:11–16
Swathi K, Sarangapani M (2014) Synthesis and anti-inflammatory activity of a novel series of isatin hydrazone & isatin thiosemicarbazone derivatives. World J Pharm Pharm Sci 3(2014):2070–2078
Tenorio RP, Carvalho CS, Pessanha CS, de Lima JG, de Faria AR, Alves AJ, de Melo EJT, Goes AJS (2005) Synthesis of thiosemicarbazone and 4-thiazolidinone derivatives and their in vitro anti-toxoplasma gondii activity. Bioorg Med Chem Lett 15:2575–2578
Toomes C, James J, Wood AJ, Wu CL, Mccormlck D, Lench N, Hewitt C, Moynlnam L, Roberts E, Woods CG, Markham A, Wong M, Widmer R, Ghaffar KA, Pemberton M, Husseln IR, Temtamy SA, Davles R, Read AP, Sloan P, Dixon MJ, Thakker NS (1999) Loss of function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet 23:421–424
Turk V, Turk B, Guncar G, Turk D, Kos J (2002) Lysosomal cathepsins: structure, role in antigen processing and presentation and cancer. Adv Enzyme Regul 42(2002):285–303
Van der Stappen WJJos, Paraskeva C, Williams AC, Hague A, Maciewicz RA (1991) Relationship between the secretion of cysteine proteinases and their inhibitors and malignant potential. Biochem Soc Trans 19:362S
Waghray A, Keppler D, Sloane BF, Schuger L, Chen YQ (2002) Analysis of a truncated form of cathepsin H in human prostate tumor cells. J Biol Chem 277:11533–11538
Warren JD, Woodward DL, Hargreaves RT (1977) 4-Substituted semicarbazones of mono and dichlorobenzaldehydes as antihypertensive agents. J Med Chem Res 20:1520–1521
Wolters PJ, Chapman HA (2000) Importance of lysosomal cysteine proteases in lung disease. Respir Res 1:170–177
Acknowledgement
Among the authors, Ravinder Kaur acknowledges Kurukshetra University, Kurukshetra for providing URS and other lab facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Raghav, N., Kaur, R. A comparative account of sar studies of semicarbazones and thiosemicarbazones on cathepsins H and L. Med Chem Res 26, 1723–1734 (2017). https://doi.org/10.1007/s00044-017-1826-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1826-9