Abstract
The emergence of drug-resistant strains in recent years has fueled the epidemic of tuberculosis. This necessitates the development of new chemical scaffolds to curb resistant tuberculosis for effective control of this disease. In this study, we have designed and synthesized two series of benzimidazole derivatives. Their antimycobacterial activities were initially evaluated using Mycobacterium tuberculosis H37RV strains. The most potent analog (6h) was further assessed using various drug-resistant M. tuberculosis strains. This report described the importance of benzimidazoles as new antitmycobacterial agents targeting both the M. tuberculosis H37RV as well as the drug-resistant-tuberculosis strains. The trifluoromethyl group which was essential for antimycobacterial activity was also highlighted.
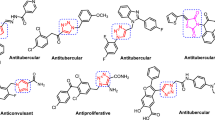
Graphical Abstract
Two series of benzimidazole derivatives and their antimycobacterial activities were evaluated using M. tuberculosis H37RV (MTB-H37RV) strains. Compound 6h was identified as the most potent among all synthesized compounds. The most potent analog was further assessed using various drug-resistant MTB strains. In addition, the trifluoromethyl was identified as an important substitution in giving good antimycobacterial effect. 
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) remains a global health problem and the second leading cause of death from an infectious disease worldwide after AIDS (Dutt and Stead 1999). The introduction of first-line drugs such as streptomycin, p-aminosalicylic acid, and isoniazid for TB treatment some 50 years ago has witnessed a remarkable decline in TB cases. The active TB is currently treated with a four first-line drug regimen comprising mainly isoniazid (INH), rifampicin (RMP), pyrazinamide, and ethambutol for a period of at least 6 months (Snider and Roper 1992; Bass et al. 1994). However, the infectious disease has been undergoing resurgence since the last two decades as Mycobacterium tuberculosis (MTB), the bacteria which cause TB, gain resistance to the above-mentioned drugs.
By definition, multidrug-resistant TB (MDR-TB) strains lose their susceptibility to two of the first-line drugs used in therapy, while extensively drug-resistant TB strains are in addition, resistant to at least one fluoroquinolone and one of the injectable anti-TB drugs, such as kanamycin (Dorman and Chaisson 2007). Treatment of MDR-TB requires longer time and is much more expensive compared to TB. The problem is compounded with the recent isolation of strains resistant to all of the standard first- and second-line drugs used in TB therapy, giving rise to the likely threat of a virtually incurable infection (Velayati et al. 2009).
Recently, there have been reports on utilizing benzimidazole derivatives to counter TB from work done by our group (Yoon et al. 2013, 2015) as well as by others (Pieroni et al. 2011; Kumar et al. 2011; Gong et al. 2014). Benzimidazoles, such as albendazole and thiabendazole were shown to inhibit the activity of MTB, albeit at relatively high concentration (MIC between 60 and 160 µM) (Slayden et al. 2000). Benzimidazoles were previously demonstrated to also inhibit the GTPase activity of MTB FtsZ and septum formation (Margalit et al. 2004; Slayden et al. 2006), further strengthening the notion of using benzimidazoles as antitubercular agent. Moreover, benzimidazoles share structural similarity with indoles, which are found to be present in mycobacterial InhA inhibitors such as Genz-10850 (Kuo et al. 2003). However, these reported inhibitors suffer mostly from its poor cellular activity, which may be due to low membrane permeability or activation of efflux pump although they have very potent in vitro (InhA) inhibitory activity (Kuo et al. 2003).
Therefore, developing a benzimidazole-based compound, which has good membrane permeability as antimycobacterial agent, is an interesting proposition. Our previous studies have underlined the antimycobacterial potential of benzimidazole derivatives in cell-based assay using M. tuberculosis H37RV (MTB-H37RV) strains. In the present work, we continued to expand our library of compounds in the search of new antitubercular agents, by reporting the synthesis and activity study of two new series of benzimidazole derivatives. Key findings such as the importance of trifluoromethyl group were also discussed.
Materials and methods
Chemistry
All chemicals were supplied by Sigma-Aldrich (USA) and Merck Chemicals (Germany). Thin layer chromatography (TLC) using silica gel G were performed in the solvent system chloroform–methanol (9:1). The spots were located under short (254 nm)/long (365 nm) UV light. Elemental analyses were performed on Perkin Elmer 2400 Series II CHN Elemental Analyzer and were within ±0.4% of the calculated values. 1H and 13C NMR were performed on Bruker Avance 300/500 (1H: 300/500 MHz, 13C: 75/125 MHz) spectrometer in CDCl3 using tetramethylsilane as internal standard. Mass spectra were recorded on Varian 320-MS TQ liquid chromatography/mass spectrometry using electrospray ionization (ESI). X-ray structural analysis was carried out using Bruker SMART APEXII charge-coupled device area-detector diffractometer.
Synthesis of ethyl-4-fluoro-3-nitrobenzoate (1)
4-Fluoro-3-nitrobenzoic acid (5 g, 27 mmol) was refluxed in ethanol (50 mL) and concentrated H2SO4 (2 mL) for 8 h. After completion of reaction (as evident from TLC), the solvent was evaporated under reduced pressure. The aqueous layer was extracted with ethyl acetate (25 mL × 3). The organic layer was dried over Na2SO4 and concentrated under reduced pressure to yield 1 as cream-colored powder (75%).
Synthesis of 4-(2-substituted amino)-3-nitro-ethylbenzoate (2)
Ethyl-4-fluoro-3-nitrobenzoate, 1 (0.5 g, 2.34 mmol), amine [for 5: N-(3-aminopropyl)imidazole; for 6: aniline] (2.58 mmol) and N,N-diisopropylethylamine, DIPEA (0.49 mL, 2.78 mmol) were mixed in dichloromethane (10 mL). The reaction mixture was stirred overnight at room temperature. After completion of reaction (as evident from TLC), the reaction mixture was washed with water (10 mL × 2) followed by 10% Na2CO3 solution (10 mL). The organic layer was dried over Na2SO4 and concentrated under reduced pressure to afford 2 as yellow solid (80–90%).
Synthesis of ethyl-3-amino-4-(2-substituted amino)benzoate (3)
4-(2-Substituted amino)-3-nitro-ethylbenzoate, 2 (1 mmol), ammonium formate (3 mmol) and Pd/C (50 mg) were mixed in ethanol (10 mL). The reaction mixture was refluxed until completion (solution turned colorless). The reaction mixture was then filtered through Celite 545. The filtrate was evaporated under reduced pressure. It was resuspended in ethyl acetate (20 mL) and washed with water (10 mL × 2), dried over Na2SO4 and evaporated to dryness to yield 3 (55–65%).
General procedure for the preparation of sodium bisulfite addcuts of 4-substituted benzaldehyde (4a–h)
Appropriate benzaldehyde (10 mmol) was dissolved in ethanol (20 mL). Sodium metabisulfite (15 mmol) in 5 mL water was added in portion over 5 min. The reaction mixture was stirred at room temperature for 1 h and subsequently stirred at 4 °C overnight. The precipitate formed was filtered and dried to afford sodium bisulfite adducts (55–90%).
General procedure for the preparation of 2-substituted benzimidazole derivatives (5a–h and 6a–h)
Ethyl-3-amino-4-(2-substituted amino)benzoate, 3 (1 mmol) and various sodium bisulfite adducts, 4 (1.5 mmol) were dissolved in DMF (5 mL). The reaction mixture was stirred at 90 °C under N2 atmosphere for 24 h. After completion of reaction (evident by TLC), the reaction mixture was diluted in ethyl acetate (25 mL) and washed with water (10 mL × 3). The organic layer was collected, dried over Na2SO4 and evaporated under reduced pressure to afford compounds the final compounds in 52–92% yields.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-phenyl-1H-benzo[d]imidazole-5-carboxylate (5a)
Yield: 84%; 1H NMR (300 MHz, CDCl3): δ 1.45 (3H, t, J = 7.2 Hz), 2.20–2.30 (2H, m), 3.88 (2H, t, J = 6.6 Hz), 4.31 (2H, t, J = 6.6 Hz), 4.44 (2H, q, J = 7.2 Hz), 6.75 (1H, s), 7.08 (1H, s), 7.30 (1H, d, J = 8.4 Hz), 7.32 (1H, d, J = 8.4 Hz), 7.58 (2H, d, J = 8.4 Hz), 7.59 (1H, s), 7.66 (2H, d, J = 8.4 Hz), 8.08 (1H, dd, J = 1.5 Hz, 8.4 Hz), 8.57 (1H, s). 13C NMR (75 MHz, CDCl3): 14.67, 61.29, 110.14, 122.56, 124.70, 125.41, 126.09, 128.68, 129.22, 129.83, 130.54, 139.05, 143.10, 156.19, and 167.44. Electrospray ionization-mass spectroscopy (ESI-MS): m/z 375.2 [M+H]+. Anal. calcd for C22H22N4O2: C, 70.57%; H, 5.92%; N, 14.96%. Found: C, 70.61%; H, 5.97%; N, 14.93%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-hydroxyphenyl)-1H-benzo[d]imidazole-5-carboxylate (5b)
Yield: 76%; 1H NMR (300 MHz, CDCl3): δ 1H NMR: 1.45 (3H, t, J = 7.0 Hz), 2.20–2.30 (2H, m), 3.88 (2H, t, J = 6.5 Hz), 4.30 (2H, t, J = 7.5 Hz), 4.43 (2H, q, J = 7.0 Hz), 6.79 (1H, s), 7.07 (1H, s), 7.28 (1H, d, J = 8.5 Hz), 7.39 (1H, s), 7.77 (2H, d, J = 8 Hz), 7.80 (2H, d, J = 8 Hz), 8.09 (1H, dd, J = 1.5 Hz, 8.5 Hz), 8.56 (1H, s). 13C NMR: 14.62, 29.05, 42.01, 43.83, 62.02, 107.83, 109.87, 110.24, 111.06, 123.07, 126.59, 131.18, 137.76, 151.74, 153.40, 157.67, and 167.89. ESI-MS: m/z 391.2 [M+H]+. Anal. calcd for C22H22N4O3: C, 67.68%; H, 5.68%; N, 14.35%. Found: C, 67.66%; H, 5.62%; N, 14.50%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylate (5c)
Yield: 82%; 1H NMR (500 MHz, CDCl3): δ 1H NMR: 1.44 (3H, t, J = 7.0 Hz), 2.10–2.30 (2H, m), 3.88 (2H, t, J = 6.5 Hz), 3.91 (3H, s), 4.27 (2H, t, J = 7.5 Hz), 4.43 (2H, q, J = 7.0 Hz), 6.79 (1H, s), 7.07 (1H, s), 7.28 (1H, d, J = 8.5 Hz), 7.39 (1H, s), 7.77 (2H, d, J = 8 Hz), 7.80 (2H, d, J = 8 Hz), 8.09 (1H, dd, J = 1.5 Hz, 8.5 Hz), 8.55 (1H, s). 13C NMR: 14.62, 28.94, 41.01, 43.93, 59.24, 61.67, 108.15, 109.29, 120.12, 121.80, 130.93, 137.22, 150.04, 151.47, 151.62, and 167.68. ESI-MS: m/z 405.2 [M+H]+. Anal. calcd for C23H24N4O3: C, 68.30%; H, 5.98%; N, 13.85%. Found: C, 68.16%; H, 5.93%; N, 13.95%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-morpholinophenyl)-1H-benzo[d]imidazole-5-carboxylate (5d)
Yield: 77%; 1H NMR (300 MHz, CDCl3): δ 1H NMR: 1.44 (3H, t, J = 7.2 Hz), 2.10–2.30 (2H, m), 3.29 (4H, t, J = 4.5 Hz), 3.88 (2H, t, J = 6.6 Hz), 3.92 (4H, t, J = 4.5 Hz), 4.31 (2H, t, J = 6.6 Hz), 4.43 (2H, q, J = 7.2 Hz), 6.75 (1H, s), 7.08 (1H, s), 7.32 (1H, d, J = 8.4 Hz), 7.58 (2H, d, J = 8.4 Hz), 7.59 (1H, s), 7.66 (2H, d, J = 8.4 Hz), 8.08 (1H, dd, J = 1.5 Hz, 8.4 Hz), 8.56 (1H, s). 13C NMR: 14.39, 30.79, 41.77, 43.89, 60.86, 63.40, 108.90, 112.01, 116.32, 118.53, 121.79, 123.95, 125.01, 129.86, 130.03, 136.97, 138.55, 142.90, 151.54, 155.98, and 167.11. ESI-MS: m/z 460.3 [M+H]+. Anal. calcd for C26H29N5O3: C, 67.95%; H, 6.36%; N, 15.24%. Found: C, 68.12%; H, 6.22%; N, 15.28%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-chlorophenyl)-1H-benzo[d]imidazole-5-carboxylate (5e)
Yield: 75%; 1H NMR (300 MHz, CDCl3): δ 1H NMR: 1.44 (3H, t, J = 6.9 Hz), 2.20–2.40 (2H, m), 4.09 (2H, t, J = 6.6 Hz), 4.33 (2H, t, J = 6.6 Hz), 4.43 (2H, q, J = 6.9 Hz), 6.98 (1H, s), 7.07 (1H, s), 7.50–7.70 (6H, m), 8.08 (1H, dd, J = 1.5 Hz, 8.7 Hz), 8.40 (1H, s). 13C NMR: 14.43, 28.99, 41.00, 43.03, 61.05, 109.96, 123.41, 124.66, 125.92, 126.13, 127.45, 131.54, 137.44, 137.97, 142.72, 151.37, 154.59, and 167.86. ESI-MS: m/z 410.1 [M+H]+. Anal. calcd for C22H21N4O2Cl: C, 64.62%; H, 5.18%; N, 13.70%. Found: C, 64.67%; H, 5.22%; N, 13.80%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-bromophenyl)-1H-benzo[d]imidazole-5-carboxylate (5f)
Yield: 85%; 1H NMR (300 MHz, CDCl3): δ 1H NMR: 1.45 (3H, t, J = 7.2 Hz), 2.20–2.40 (2H, m), 3.92 (2H, t, J = 6.6 Hz), 4.27 (2H, t, J = 6.6 Hz), 4.43 (2H, q, J = 7.2 Hz), 6.79 (1H, s), 7.12 (1H, s), 7.28 (1H, d, J = 8.4 Hz), 7.38 (1H, s), 7.52 (2H, d, J = 8.4 Hz), 7.68 (2H, d, J = 8.4 Hz), 8.09 (1H, dd, J = 1.5, 8.4 Hz), 8.56 (1H, s). 13C NMR: 14.55, 29.01, 41.24, 43.94, 61.03, 108.10, 122.65, 124.78, 125.40, 126.19, 128.77, 129.21, 129.82, 130.22, 130.56, 137.05, 139.11, 143.13, 150.12, 156.25, and 167.18. ESI-MS: m/z 453.2 [M+H]+. Anal. calcd for C22H21N4O2Br: C, 58.29%; H, 4.67%; N, 12.36%. Found: C, 58.22%; H, 4.77%; N, 12.40%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-(trifluoromethoxy)phenyl)-1H-benzo[d]imidazole-5-carboxylate (5g)
Yield: 92%; 1H NMR (500 MHz, CDCl3): δ 1H NMR: 1.43 (3H, t, J = 7.0 Hz), 2.10–2.30 (2H, m), 3.90 (2H, t, J = 6.5 Hz), 4.27 (2H, t, J = 7.5 Hz), 4.43 (2H, q, J = 7.0 Hz), 6.79 (1H, s), 7.07 (1H, s), 7.28 (1H, d, J = 8.5 Hz), 7.39 (1H, s), 7.77 (2H, d, J = 8 Hz), 7.80 (2H, d, J = 8 Hz), 8.09 (1H, dd, J = 1.5, 8.5 Hz), 8.56 (1H, s). 13C NMR: 14.40, 29.15, 40.92, 43.73, 61.74, 110.15, 121.18, 122.80, 124.99, 127.88, 129.26, 130.76, 131.59, 137.85, 139.06, 143.04, 150.78, 151.44, 154.83, and 166.98. ESI-MS: m/z 459.2 [M+H]+. Anal. calcd for C23H21N4O3F3: C, 60.26%; H, 4.62%; N, 12.43%. Found: C, 60.32%; H, 4.62%; N, 12.34%.
Ethyl 1-(3-(1H-imidazol-1-yl)propyl)-2-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazole-5-carboxylate (5h)
Yield: 90%; 1H NMR (500 MHz, CDCl3): δ 1H NMR: 1.43 (3H, t, J = 7.0 Hz), 2.20–2.30 (2H, m), 3.92 (2H, t, J = 6.5 Hz), 4.27 (2H, t, J = 7.5 Hz), 4.43 (2H, q, 7.0 Hz), 6.76 (1H, s), 7.09 (1H, s), 7.28 (1H, d, J = 8.5 Hz), 7.39 (1H, s), 7.77 (2H, d, J = 8 Hz), 7.80 (2H, d, J = 8 Hz), 8.09 (1H, dd, J = 1.5, 8.5 Hz), 8.56 (1H, s). 13C NMR: 14.42, 30.89, 41.93, 43.81, 61.12, 109.30, 118.29, 122.78, 125.24, 125.85, 126.10, 126.21, 129.54, 130.43, 132.49, 133.32, 137.01, 138.37, 142.67, 153.33, and 166.79. ESI-MS: m/z 444.2 [M+H]+. Anal. calcd for C23H21N4O2F3: C, 62.44%; H, 4.78%; N, 12.88%. Found: C, 62.44%; H, 4.78%; N, 12.89%.
Ethyl 1,2-diphenyl-1H-benzo[d]imidazole-5-carboxylate (6a)
Yield: 77%; 1H NMR (300 MHz, CDCl3): δ 1.43 (3H, t, J = 7.2 Hz), 4.42 (2H, q, J = 7.2 Hz), 7.20–7.60 (11H, m), 7.99 (1H, dd, J = 1.5, 8.4 Hz), 8.60 (1H, s). 13C NMR (75 MHz, CDCl3): 14.51, 61.42, 110.34, 111.80, 111.99, 112.56, 113.14, 113.91 115.67, 118.82, 119.05, 130.24, 131.00, 136.56, 140.28, 142.44, 152.16, and 167.88. ESI-MS: m/z 343.1 [M+H]+. Anal. calcd for C22H18N2O2: C, 77.17%; H, 5.30%; N, 8.18%. Found: C, 77.06%; H, 5.35%; N, 8.32%.
Ethyl 2-(4-hydroxyphenyl)-1-phenyl-1H-benzo[d]imidazole-5-carboxylate (6b)
Yield: 77%; 1H NMR (500 MHz, CDCl3): δ 1.43 (3H, t, J = 7.1 Hz), 4.42 (2H, q, J = 7.1 Hz), 7.19 (2H, d, J = 9 Hz), 7.28 (1H, d, J = 9 Hz), 7.35–7.60 (5H, m), 7.47 (2H, d, J = 9 Hz), 7.99 (1H, dd, J = 1.5, 9 Hz), 8.56 (1H, s). 13C NMR (125 MHz, CDCl3): 14.26, 61.05, 118.95, 120.87, 122.54, 124.79, 126.95, 127.26, 128.08, 129.17, 130.34, 132.96, 136.15, 140.28, 143.03, 158.69, and 167.00. ESI-MS: m/z 359.1 [M+H]+. Anal. calcd for C22H18N2O3: C, 73.73%; H, 5.06%; N, 7.82%. Found: C, 73.56%; H, 5.16%; N, 7.88%.
Ethyl 2-(4-methoxyphenyl)-1-phenyl-1H-benzo[d]imida-zole-5-carboxylate (6c)
Yield: 80%; 1H NMR (500 MHz, CDCl3): δ 1.44 (3H, t, J = 7.1 Hz), 3.83 (3H, s), 4.43 (2H, q, J = 7.1 Hz), 6.85 (2H, d, J = 9 Hz), 7.24 (1H, d, J = 9 Hz), 7.35 (2H, d, J = 9 Hz), 7.50–7.97 (5H, m), 7.98 (1H, dd, J = 1.5 Hz, 9 Hz), 8.59 (1H, s). 13C NMR (125 MHz, CDCl3): 14.30, 59.05, 61.25, 116.07, 119.88, 121.31, 122.79, 123.79, 124.00, 129.18, 129.28, 130.31, 133.05, 138.77, 139.41, 143.08, 159.32, and 168.19. ESI-MS: m/z 373.1 [M+H]+. Anal. calcd for C23H20N2O3: C, 74.18%; H, 5.41%; N, 7.52%. Found: C, 74.09%; H, 5.35%; N, 7.64%.
Ethyl 2-(4-morpholinophenyl)-1-phenyl-1H-benzo[d]imidazole-5-carboxylate (6d)
Yield: 84%; 1H NMR (500 MHz, CDCl3): δ 1.44 (3H, t, J = 7.1 Hz), 3.22 (4H, t, J = 5.0 Hz), 3.85 (4H, t, J = 5.0 Hz), 4.44 (2H, q, J = 7.1 Hz), 6.80 (2H, d, J = 9 Hz), 7.20 (1H, d, J = 9 Hz), 7.37 (2H, d, J = 9 Hz), 7.50–7.60 (5H, m), 7.99 (1H, dd, J = 1.5 Hz, 9 Hz), 8.60 (1H, s). 13C NMR (125 MHz, CDCl3): 14.40, 47.97, 52.13, 60.90, 66.67, 109.84, 114.19, 121.43, 124.16, 124.58, 125.49, 127.46, 128.95, 130.07, 130.64, 136.80, 140.23, 143.92, 151.95, and 167.07. ESI-MS: m/z 428.2 [M+H]+. Anal. calcd for C26H25N3O3: C, 73.05%; H, 5.89%; N, 9.83%. Found: C, 73.06%; H, 5.86%; N, 9.80%.
Ethyl 2-(4-chlorophenyl)-1-phenyl-1H-benzo[d]imidazole-5-carboxylate (6e)
Yield: 81%; 1H NMR (500 MHz, CDCl3): δ 1.43 (3H, t, J = 7.1 Hz), 4.42 (2H, q, J = 7.1 Hz), 7.25 (1H, d, J = 9 Hz), 7.28 (2H, d, J = 9 Hz), 7.40–7.60 (5H, m), 7.54 (2H, d, J = 9 Hz), 8.00 (1H, dd, J = 1.5, 9 Hz), 8.52 (1H, s). 13C NMR (125 MHz, CDCl3): 14.40, 61.64, 110.28, 111.80, 116.26, 122.70, 122.97, 124.65, 125.67, 127.91, 129.20, 130.35, 131.18, 137.50, 140.76, 142.71, 152.68, and 167.48. ESI-MS: m/z 377.1 [M+H]+. Anal. calcd for C22H17N2O2Cl: C, 70.12%; H, 4.55%; N, 7.43%. Found: C, 70.10%; H, 4.52%; N, 7.54%.
Ethyl 2-(4-bromophenyl)-1-phenyl-1H-benzo[d]imidazole-5-carboxylate (6f)
Yield: 79%; 1H NMR (500 MHz, CDCl3): δ 1.44 (3H, t, J = 7.1 Hz), 4.44 (2H, q, J = 7.1 Hz), 7.24 (1H, d, J = 9 Hz), 7.32 (2H, d, J = 9 Hz), 7.40–7.60 (5H, m), 7.58 (2H, d, J = 9 Hz), 8.00 (1H, dd, J = 1.5, 9 Hz), 8.60 (1H, s). 13C NMR (125 MHz, CDCl3): 14.39, 61.65, 110.30, 111.82, 116.37, 122.75, 123.89, 124.65, 125.67, 127.91, 129.20, 130.32, 131.08, 137.50, 140.76, 142.71, 152.69, and 167.50. ESI-MS: m/z 421.1 [M+H]+. Anal. calcd for C22H17N2O2Br: C, 62.72%; H, 4.07%; N, 6.65%. Found: C, 62.76%; H, 4.02%; N, 6.73%.
Ethyl 1-phenyl-2-(4-(trifluoromethoxy)phenyl)-1H-benzo[d]imidazole-5-carboxylate (6g)
Yield: 81%; 1H NMR (500 MHz, CDCl3): δ 1.43 (3H, t, J = 7.1 Hz), 4.43 (2H, q, J = 7.1 Hz), 7.20 (1H, d, J = 9 Hz), 7.28 (2H, d, J = 9 Hz), 7.40–7.60 (5H, m), 7.55 (2H, d, J = 9 Hz), 7.99 (1H, dd, J = 1.5, 9 Hz), 8.54 (1H, s). 13C NMR (125 MHz, CDCl3): 14.34, 61.56, 111.03, 111.79, 116.30, 121.80, 123.06, 123.99, 124.66, 126.86, 127.25, 129.24, 131.51, 131.82, 136.50, 140.79, 142.70, 154.05, and 167.50. ESI-MS: m/z 427.1 [M+H]+. Anal. calcd for C23H17N2O3F3: C, 64.79%; H, 4.02%; N, 6.57%. Found: C, 64.69%; H, 4.11%; N, 6.54%.
Ethyl 2-(4-(trifluoromethyl)phenyl)-1-phenyl-1H-benzo[d]imidazole-5-carboxylate (6h)
Yield: 89%; 1H NMR (500 MHz, CDCl3): δ 1.44 (3H, t, J = 7.1 Hz), 4.44 (2H, q, J = 7.1 Hz), 7.27 (1H, d, J = 9 Hz), 7.33–7.60 (5H, m), 7.58 (2H, d, J = 9 Hz), 7.70 (2H, d, J = 9 Hz), 8.03 (1H, dd, J = 1.5, 9 Hz), 8.63 (1H, s). 13C NMR (125 MHz, CDCl3): 14.38, 61.03, 110.32, 122.45, 122.67, 124.84, 125.33, 125.36, 125.41, 125.97, 127.29, 130.30, 132.89, 136.15, 140.28, 142.43, 152.26, and 166.89. ESI-MS: m/z 411.1 [M+H]+. Anal. calcd for C24H23N3O2: C, 67.31%; H, 4.18%; N, 6.83%. Found: C, 67.30%; H, 4.12%; N, 6.88%.
Biological evaluation
Antimycobacterial activity against MTB-H37RV strains
All the synthesized compounds 5a–5h and 6a–6h were tested for their antimycobacterial activity in vitro against MTB-H37RV using a modified high throughput screen assay adapted from Collins and Franzblau (1997). The end-point detection was assessed by the Promega reagent BacTiter-GloTM microbial cell viability (BTG) assay and was compared with microdilution AlamarBlue broth assay. Compounds screened in dose response were tested in ten two-fold dilutions from 100 to 0.195 µM. Three standard drugs (INH, cycloserine, and pyrimethamine) were used as references for the assay. Data were analyzed using the IDBS Activity Base software and the dose response result was analyzed using a four parameter logistic fit to the data (Excel Fit equation 205) with the maximum and minimum locked at 100 and 0.
Multidrug-resistant M. tuberculosis MIC testing
The broth micro dilution assay format, following guidelines established by the Clinical and Laboratory Standards Institute, is routinely utilized for MIC testing. Briefly, testing was conducted using 96-well, U-bottom micro plates with an assay volume of 0.2 mL/well. First, the test media was added to each well. Test compounds, dissolved in appropriate solvent and diluted in test media, were then added to appropriate wells at twice the intended starting concentration and serially diluted two-fold across the plate. The assay plates were subsequently incubated at 37 °C for 7 days. Following incubation, the plates were read visually and individual wells scored for inhibition. In addition, an absorbance reading was taken via a plate reader and the results recorded. Testing was conducted in duplicate. The MIC was reported as the lowest concentration of drug that inhibits growth of the organism. The strains that were used in this study were isoniazid-resistant (SRI 1367), rifampin-resistant (SRI 1369) and ofloxacin-resistant (SRI 4000) MTB.
Cytotoxicity assay
Vero cells were treated with 50 µM of interested compounds and allowed to adhere for 72 h. Then, the proliferative activity was determined by MTT assay (CellTiter 96 Non-radioactive cell proliferation assay; Promega, Madison, WI) to monitor the number of viable cells according to the manufacturer’s instructions. Briefly, the dye solution was added at 15 μL/well. After 1 h of incubation at 37 °C in a humidified 5% CO2 atmosphere, 100 µL of the stop solution was added into each well. Absorbance was recorded at 570 nm. All experiments were done in triplicate, and the proliferation rate was calculated as the ratio of absorbance under each experimental condition to that of the control.
Results and discussion
Synthesis and characterization of compounds
The synthetic scheme is a four-step pathway (Fig. 1) leading to the formation of a variety of benzimidazole derivatives as described previously (Yoon et al. 2015). Esterification of 4-fluoro-3-nitro benzoic acid in ethanol, with concentrated sulfuric acid as catalyst furnished the ethyl ester 1 (75%). The ethylbenzoate 1 was then treated with amine [for R 1 = 5: N-(3-aminopropyl)imidazole; for R 1 = 6: aniline] and DIPEA in dry dichloromethane at room temperature to afford amino compound 2. It was subsequently reduced to intermediate 3 (60%) using ammonium formate and 10% Pd/C. Intermediate 3 was then refluxed with the bisulfite adduct of various aromatic aldehydes (4a–h) in DMF overnight to afford benzimidazole derivatives 5a–h and 6a–h in moderate to good yields (52–93%). Column chromatography purification for the final compounds were done in solvent system chloroform–methanol (9:1) using Silica Gel 60 (0.063–0.200 mm). The structures of the newly synthesized compounds were then characterized using NMR, CHN, mass spectrometry, and X-ray analysis where possible (Fig. 2) (Yoon et al. 2012).
Biological activity
The synthesized compounds 5a–h and 6a–h were tested for their antimycobacterial activity against MTB-H37Rv in a screening assay adapted using the Promega reagent BTG. The BTG assay is a quantitative ATP assay for bacteria using luciferase production as an end-point detection point. Data was analyzed using the IDBS Activity Base software and the dose response result was analyzed using a four parameter logistic fit to the data (Excel Fit equation 205) with the maximum and minimum locked at 100 and 0. From these curves, IC90 and IC50 values were calculated. As references, three standard drugs, namely cycloserine, isoniazid, and pyrimethamine were also evaluated in the assays.
Of the 16 benzimidazole analogs that have been synthesized, five compounds showed MIC of ≤100 µM against MTB-H37Rv strains (Table 1). Consistent with our previous findings, the most potent compounds from both series contained a trifluoromethyl group (5h and 6h), suggesting the potential of this substituent in giving good antimycobacterial effect against MTB-H37Rv. Although the reason behind this has not been fully elucidated, the ability of both 5h and 6h to permeate into the bacterial cell wall could be one possible reason for their better inhibitory activity. With a steric parameter (Es) of −2.4, trifluoromethyl group (–CF3) is relatively highly lipophilic (Elliott 1995; Welch and Eswarakrishnan 1991) and permeates cell membrane easily, which may give the trifluoromethylated compounds superior activity compared to the rest of the tested compound. Thus, combining the benzimidazole backbone with the –CF3 group may have synergistic advantages.
The most potent analog, ethyl 2-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazole-5-carboxylate (6h) showed IC50 at 8.96 µM (Fig. 3a), IC90 at 12.46 µM, and MIC at 12.50 µM using BTG method. By comparison, it gave IC50 of 11.27 µM (Fig. 3b), IC90 of 12.65 µM and MIC of 50.00 µM using the Alamar Blue method. It was found to be more active than the standard drugs cycloserine and pyrimethamine, but was less potent compared to the first-line drug isoniazid.
Dose response curves of compound 6h against Mycobacterium tuberculosis H37RV strains (MTB-H37RV) using a BacTiter-Glo (BTG) and b Alamar Blue method. Red lines indicated the half maximal inhibitory concentration (IC50); outliers (in blue boxes) were eliminated from curve fitting (color figure online)
However, as many TB cases now are multidrug-resistant, it is important to have new drugs in the pipeline that can be effective against drug-resistant MTB as well. As the benzimidazole scaffold is still considered novel in TB drug discovery, the most potent compound found in the screening assay (6h) was subsequently tested in several drug-resistant MTB strains. Interestingly, 6h was demonstrated to possess antimycobacterial activities against several drug-resistant MTBs such as isoniazid-resistance MTB (INH-R MTB and strain SRI 1367), rifampin-resistance MTB (RMP-R MTB and strain SRI 1369) and ofloxacin-resistance MTB (OFX-R MTB and strain SRI 4000) with MIC of 15.24, 30.50, and 60.00 µM, respectively (Table 2). INH and RMP are two of the most important antituberculosis drugs in the market today and among drug-resistant MTB isolates, resistance to INH was the most commonly observed type along with RMP (Da Silva and Palomino 2011; Somoskovi et al. 2001)
Sensing the potential of these compounds, we next set out to confirm the tolerable toxicity of the synthesized benzimidazoles in mammalian cells. Cytotoxicity test (IC50) was performed using VERO cells. After 72 h of exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega Cell Titer 96 non-radioactive cell proliferation method according to manufacturer’s protocol. No significant cytotoxicity was observed for all of the compounds. This bodes well as we aim to utilize benzimidazole as the skeleton of new antitubercular agents in light of their selectivity and low toxicity.
Conclusion
In conclusion, we have described the synthesis and MTB-H37Rv inhibitory activities of 16 benzimidazole derivatives. In line with our previous work, the present work clearly demonstrated that the trifluoromethyl group is essential for potent MTB-H37Rv inhibitory activity. Although currently none of the benzimiadzole-based compounds are as potent as INH, compound such as 6h warrant further investigations as the benzimidazoles still have much potential to be modified to be useful as second-line drug to counter some of the MDR-TB cases. Moreover, they were found to be non-cytotoxic toward mammalian cells. Further work on trifluoromethyl-substituted benzimidazoles are ongoing in our laboratory.
References
Bass JB, Farer LS, Hopewell PC, O’Brien R, Jacobs RF, Ruben F, Dixie E, Snider J, Thornton G (1994) Treatment of tuberculosis and tuberculosis infection in adults and children. Am J Respir Crit Care Med 149:1359–1374
Collins L, Franzblau SG (1997) Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009
Da Silva PEA, Palomino JC (2011) Molecular basis and mechanisms of drug-resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother 66:1417–1430
Dorman SE, Chaisson RE (2007) From magic bullets back to the magic mountain: the rise of extensively drug-resistant tuberculosis. Nat Med 3:295–298
Dutt AK, Stead WW (1999) Epidemiology and host factors. In: Schlossberg D (ed) Tuberculosis and nontuberculous mycobacterial infections, 4th edn.. WB Saunders Company, Philadelphia, PA, pp 3–16
Elliott AJ (1995) Chemistry of organic fluorine compounds II: A critical review. ACS monograph 187. American Chemical Society, Washington DC, WA, pp 1119–1125
Gong Y, Somersan Karakaya S, Guo X, Zheng P, Gold B, Ma Y, Little D, Roberts J, Warrier T, Jiang X, Pingle M, Nathan CF, Liu G (2014) Benzimidazole-based compounds kill Mycobacterium tuberculosis. Eur J Med Chem 75:336–353
Kumar K, Awasti D, Lee SY, Zanardi I, Ruzsicska B, Knudson S, Tonga PJ, Slayden RA, Ojima I (2011) Novel trisubstituted benzimidazoles, targeting Mtb FtsZ as a new class of antitubercular agents. J Med Chem 54:374–381
Kuo MR, Morbidoni HR, Alland D, Sneddon SF, Gourlie BB, Staveski MM, Leonard M, Gregory JS, Janjigian AD, Yee C, Musser JM, Kreiswirth B, Iwamoto H, Perozzo R, Jacobs Jr WR, Sacchettini JC, Fidock DA (2003) Targeting tuberculosis and malaria through inhibition of Enoyl reductase: compound activity and structural data. J Biol Chem 278:20851–20859
Margalit DN, Romberg L, Mets RB, Hebert AM, Mitchison TJ, Kirschner MW, Ray Chaudhuri D (2004) Targeting cell division: small-molecule inhibitors of FtsZ GTPase perturb cytokinetic ring assembly and induce bacterial lethality. Proc Natl Acad Sci USA 101:11821–11826
Pieroni M, Tipparaju SK, Lun S, Song Y, Sturm AW, Bishai WR, Kozikowski AP (2011) Pyrido[1,2-a]benzimidazole-based agents active against tuberculosis (TB), multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB. ChemMedChem 6:334–342
Slayden RA, Knudson DL, Belisle JT (2006) Identification of cell cycle regulators in mycobacterium tuberculosis by inhibition of septum formation and global transcriptional analysis. Microbiology 152:1789–1797
Slayden RA, Lee RE, Barry 3rd CE (2000) Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol Microbiol 38:514–525
Snider Jr DE, Roper WL (1992) The new tuberculosis. N Engl J Med 326:703–705
Somoskovi A, Parsons LM, Salfinger M (2001) The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir Res 2:164–168
Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE (2009) Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant or totally drug-resistant strains in Iran. Chest 36:420–425
Welch JT, Eswarakrishnan S (1991) Fluorine in bioorganic chemistry. Wiley-Interscience, New York, NY
Yoon YK, Ali MA, Choon TS, Arshad S, Razak IA (2012) Ethyl 1-phenyl-2-[4-(trifluoromethyl)-phenyl]-1H-benzimidazole-5-carboxylate. Acta Crystallogr E 68:o1864–o1865
Yoon YK, Ali MA, Choon TS, Ismail R, Wei AC, Kumar RS, Osman H, Beevi F (2013) Antituberculosis: synthesis and antimycobacterial activity of novel benzimidazole derivatives. Biomed Res Int 2013:926309
Yoon YK, Ali MA, Wei AC, Choon TS, Ismail R (2015) Synthesis and evaluation of antimycobacterial activity of new benzimidazole aminoesters. Eur J Med Chem 93:614–624
Acknowledgements
The authors wish to express their gratitude and appreciation to Pharmacogenetics and Novel Therapeutics Research Cluster, Institute for Research in Molecular Medicine, Universiti Sains Malaysia for supporting this work. This work was funded through Research Grant No.RUC (1001/PSK/8620012). This work was also supported by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases (contract no. HHSN272201100012I).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yeong, K.Y., Ang, C.W., Ali, M.A. et al. Antituberculosis agents bearing the 1,2-disubstituted benzimidazole scaffold. Med Chem Res 26, 770–778 (2017). https://doi.org/10.1007/s00044-017-1784-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1784-2