Abstract
2-Chloroquinoline-3-carbaldehyde (2) was synthesized via Vilsmeier–Haack method using acetanilide. Phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1H-benzimidazol-1-yl)acetohydrazide (7a–c) were synthesized using 2-[2-(phenoxy/naphthalen-1-yl/naphthalen-2-yloxy methyl)-1H-benzimidazol-1-yl]acetohydrazide (6a–c). The title compounds 2-chloro-3-{5-[(2-phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1-H-benzimidazol-1-yl)methyl]-1,3,4-oxadiazol-2-yl}quinolone (8a–c) were prepared using chloramine-T. In the second series, (2-chloroquinolin-3-yl)methylidene]-substituted benzohydrazide (11a–i) were prepared by the reaction of 2-chloroquinoline-3-carbaldehyde (2) and an acid hydrazide (10a–i). The synthesized compounds were characterized by IR, NMR, Mass spectrometry, elemental analysis and screened for their antibacterial (serial dilution technique and disc diffusion method) and anticancer activity by NCI 60 cell screen at a single high dose (10−5 M) on various panel/cell lines. The synthesized compounds (8a, 8c, 12a, 12b, 12c and 12h) were acting as a magic bullet against gram-positive strains of Bacillus cereus MTCC1305, and the compounds (12a, 12c and 12h) were also found to be extremely active against Klebsiella pneumonia NCTC7447. In the in vitro screen on tested cancer cell line, the compound (12d) showed 95.70 growth percent (GP) and highly active on SNB-75 (CNS cancer) and UO-31 (renal cancer) (GP = 53.35 and 64.35, respectively), and the compound (8a) showed 96.86 GP and highly active on SNB-75 (CNS cancer GP 51.27).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocyclic compounds with nitrogen are considered as great interest in natural products (Jin, 2003) as they are used frequently in medicinal chemistry. Among them oxadiazoles are considered important as there is furan ring with two methane (–CH=) groups which are replaced by two pyridine types of nitrogen (–N=) atoms. Four types of isomers are observed which depend on the position of nitrogen atoms present in the ring (Somani and Shirodkar, 2009) as described in Fig. 1.
Literature survey reveals that the heterocyclic compounds containing 1,3,4-oxadiazole moiety have been used as a pi-conjugation which are used to prepare large number of donor–acceptor molecules that carry a rich pi-electron aromatic ring. Hence, compounds with 1,3,4-oxadiazole moiety are considered as a good choice for optical material or biologically active chemicals (Dabiri et al., 2006) with various applications like HIV-integrase inhibitor raltegravir (Steigbigel et al., 2008), furamizole as nitrofuran antibacterial (Ogata et al., 1971), antihypertensive agents tiodazosin (Vardan et al., 1983) and nesapidil (Schlecker and Thieme, 1988), anticancer (Sengupta et al., 2008; Jin et al., 2006; Holla et al., 2005), anticonvulsant (Almasirad et al., 2004; Aziz et al., 2009), antimicrobial (Shetgiri and Nayak, 2005; Manjunatha et al., 2010; Shailaja et al., 2010; Mulwad and Chaskar, 2006; Ansari and Lal, 2009), anti-inflammatory analgesic (Bhandari et al., 2008; Dewangan et al., 2010; Amir, 2007; Kumar et al., 2008; Jayashankar et al., 2009), dyes and pigments (Shui et al., 2010), ulcerogenic (Gilani et al., 2010), antitubercular (Ali and Shaharyar, 2007) etc. which are based on 1,3,4-oxadiazole moiety (Fig. 2).
A large number of antimicrobial agents show resistance to clinically significant bacteria which give rise to number of problems like local irritation, difficulty in wound healing process, hypersensitivity reactions, systemic toxicity and the emergence of resistance. So, due to this, the demand of clinical importance of drug resistant microbial pathogens has additional led to urgency in microbiological and antifungal research. Cancer treated as proliferation of cells which occurs in various organs of the body but does not show any certain etiopathology, so the treatment regime becomes bit difficult. Hence, different methods are employed for the treatment of cancer, i.e., immunotherapy, surgery, radiotherapy and chemotherapy. Cytotoxic agents play a very important role in the chemotherapeutics, which reduces the proliferation of malignant cells. The significant side-effects of chemotherapy are diarrhoea, nausea, vomiting, serious infections, hair loss and growth of the tumour cell population (Isikdag et al., 2011). Thus, our main aim was to develop a new molecule for antibacterial and anticancer drugs which can be used as a lead compound for further development.
Materials and methods
Chemistry
The chemical used for experimental work was commercially procured from various chemical units viz. E. Merck India Ltd., CDH and S.D. Fine Chem. and Qualigens. These solvent and reagents were of LR grade and purified before use. The silica gel G (160–120 mesh) used for analytical chromatography (TLC) was obtained from E. Merck India Ltd. Two solvent systems were used, i.e., benzene:acetone (9:1 and 8:2) and toluene:ethyl acetate:formic acid (5:4:1). Ashless Whatman no. 1 filter paper was used for vacuum filtration. Melting points were determined in open glass capillary using melting point apparatus and are uncorrected. The 1H NMR and 13C NMR were recorded on Bruker 300 MHz instrument in DMSO/CDCl3 using tetramethylsilane [(CH3)4Si] as internal standard. The infrared spectra of the compound were recorded in KBr on Perkin-Elmer FTIR spectrometer, and mass spectra were recorded on API 2000 LC/MS/MS system. The iodine chamber and UV lamp were used for visualisation of TLC spots. The commercially available grades of solvents and reagents were found to be of adequate purity. However, the presence of undesirable impurities and others were likely to be used for experimental work was purified/dried.
Synthesis of 2-chloro-quinoline-3-carbaldehyde (2)
2-Chloroquinoline-3-carbaldehyde was synthesized from acetanilide via Vilsmeier–Haack reaction (Srivatava and Singh, 2005). To a solution of acetanilide (0.005 mol; 0.67 g) in dry dimethylformamide (0.015 mol; 1.09 ml) at 0–5 °C with stirring, phosphorous oxychloride (0.06 mol; 5.59 ml) was added drop wise and the mixture stirred at 80–90 °C for 12 h. The mixture was poured into crushed ice and stirred for 5 min, and the resulting solid filtered was washed well with water and dried. The compound was purified by recrystallization from ethyl acetate. It was obtained as yellow solid, yield 65 %; m.p. 146–148 °C; IR (KBr) v max 2871, 1687, 749 cm−1; 1H NMR (DMSO, 300 MHz): δ = 10.25 (s, 1H, CHO), 7.70 (t, 1H, J = 6.2, H-7), 7.25 (d, 1H, J = 6.5 Hz, H-6), 6.89 (d, 1H, J = 6.3 Hz, H-8), 6.78 (t, 1H, J = 7.1 Hz, H-5); 13C NMR (DMSO, 75 MHz): δ = 190 (CH, –CHO), 153 (C, C-2), 145.4 (CH, C-4), 134 (CH, C-8), 129.8 (C, C-3), 129.3 (CH, C-6), 128.5 (C, C-5), 128 (CH, C-9), 127.2 (CH, C-5); EIMS m/z: 191.01 (M +); Anal. Calcd. for C10H6ClNO: C, 62.68; H, 3.16; N, 7.31. Found: C, 62.71; H, 3.18; N, 7.33.
Synthesis of phenoxy/2-naphthalen-1-yl/naphthoxy-methyl-1H-benzimidazole (4a–c)
A mixture of o-phenylenediamine 3 (0.05 mol; 0.54 g) and phenoxyacetic acid/naphthylacetic acid/naphthoxyacetic acid (0.05 mol) was refluxed in 4 N HCl for 4 h on a heating mantle. After completion of reaction, solution was poured onto crushed ice; ammonia solution was added drop wise to neutralize, and the resulting solid was filtered, washed with cold water, dried and recrystallized with ethanol.
2-Phenoxymethyl-benzimidazole (4a)
It was obtained as yellow solid, yield 80 %; m.p. 160–164 °C. IR (KBr) v max 3450, 1540, 1240 cm−1; 1H NMR (DMSO, 300 MHz) δ = 12.31 (s, 1H, NH), 7.70 (d, 1H, J = 9.5 Hz, H-1), 7.47 (d, 1H, J = 9.2 Hz, H-4), 7.29 (t, 2H, J = 14.8 Hz, H-2, 3), 7.19 (s, 2H, H-6, 8), 7.07 (d, 2H, J = 8.7, H-5, 9), 6.85 (t, 1H, J = 12 Hz, H-7), 5.29 (s, 2H, OCH2); 13C NMR (DMSO, 75 MHz): δ = 154.3 (CH2, –CH2O), 142.7 (C, C-2), 138.9 (C, C-4, 9), 130.1 (CH, C-5′), 124.8 (CH, C-3′), 124 (CH, C′-4), 123.1 (CH, C-6, 7), 115 (CH, C-5, 8), 112.3 (CH, C-2′, 6′); EIMS m/z: 224 (M +); Anal. Calcd. for C14H12N2O: C, 74.98; H, 5.39; N, 12.49. Found: C, 75.0; H, 5.35; N, 12.45.
2-(Naphthalene-1-ylmethyl)-1H-benzimidazole (4b)
It was obtained as white solid, yield 85 %; m.p. 125–126 °C; IR (KBr) v max 3,302, 1,528, 2,867 cm−1; 1H NMR (DMSO, 300 MHz): δ = 12.37 (s, 1H, NH), 8.19 (d, 1H, J = 13.2 Hz, H-5′), 7.92 (d, 2H, J = 9.5 Hz, H-6, 9), 7.84 (d, 1H, J = 7.8 Hz, H-8′), 7.45 (d, 1H, J = 8 Hz, H-3′), 7.32 (d, 2H, J = 8.3 Hz, H-6′, 7′), 7.25 (d, 2H, J = 8.5 Hz, H-7, 8), 7.20 (t, 1H, J = 12.7 Hz, H-2′), 7.06 (d, 1H, J = 6.7 Hz, H-1′), 4.62 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 141.5 (C, C-2), 138.1 (C, C-5, 9), 134.2 (C, C-5′), 133.8 (C, C-10′), 132.5 (C, C-9′), 128.6 (CH, C-6′), 127.2 (CH, C-3′), 126.9 (CH, C-2′, 4′), 125.7 (CH, C-7′, 8′), 124.3 (CH, C-9′), 123.1 (CH, C-5, 6), 115.4 (CH, C-5, 8); EIMS m/z: 258.1 (M +); Anal. Calcd. for C18H14N2: C, 83.69; H, 5.46; N, 10.84. Found: C, 83.67; H, 5.49; N, 10.82.
2-[(Naphthalene-2-yloxy)methyl]-1H-benzimidazole (4c)
It was obtained as creamy-white solid, yield 84 %; m.p. 205–207 °C; IR (KBr) v max 3010, 3297, 1464, 1226 cm−1; 1H NMR (DMSO, 300 MHz): δ = 7.70 (d, 2H, J = 5.4 Hz, H-6, 9), 7.68 (d, 1H, J = 5.6 Hz, H-5′), 7.64 (d, 2H, J = 6.9 Hz, H-3′, 8′), 7.60 (t, 1H, J = 13.7 Hz, H-7′), 7.30 (d, 2H, J = 6 Hz, H-7, 8), 7.47 (t, 1H, J = 15.2 Hz, H-6′), 7.30 (s, 1H, J = 5.8 Hz, H-13′), 7.26 (s, 1H, H′), 5.34 (s, 2H, CH2O); 13C NMR (DMSO, 75 MHz): δ = 155.3 (C, C-2′), 141.7 (C, C-2), 138.2 (C, C-4, 9), 135.2 (C, C-10′), 128.2 (C, C-5′), 127.8 (CH, C-4′), 127 (CH, C-6′), 126.7 (CH, C-8′), 125.7 (CH, C-8′), 123.4 (CH, C-4′), 120.5 (CH, C-6, 7), 119.9 (CH, C-3′), 116.4 (CH, C-5, 8), 104.1 (CH, C-2′), 73.4 (CH2, OCH2); EIMS m/z: 274.1 (M +); Anal Calcd. for C18H14N2O: C, 78.81; H, 5.14; N, 10.21. Found: C, 78.84; H, 5.15; N, 10.25.
Synthesis of ethyl 2-(phenoxymethyl/naphthylmethy/naphthalen-2-yloxy)methyl]-1-yl}acetate (5a–c)
To a suspension of 2-[(phenoxymethyl/naphthylmethy/naphthalen-2-yloxy)methyl]-1H-benzimidazole (0.01 mol) and anhydrous potassium carbonate (2 g) in dry acetone, ethyl chloroacetate (0.01 mol; 1.2 ml) was added drop wise at room temperature for a period of 20–30 min. The reaction mixture was stirred at room temperature for 10–12 h. The inorganic solid was filtered off, and filtrate was concentrated under reduced pressure.
Ethyl [2-(phenoxymethyl)-1H-benzimidazol-1-yl]acetate (5a)
It was obtained as white solid, yield 80 %; m.p. 160–164 °C; IR (KBr) v max 3450, 1540, 1240 cm−1; 1H NMR (DMSO, 300 MHz): δ = 12.31 (s, 1H, NH), 7.59 (d, 2H, J = 12.3 Hz, H-6, 9), 7.30 (d, 2H, J = 9.7 Hz, H-7, 8), 6.97 (t, 1H, J = 11.5 Hz, H-4′), 6.85 (d, 2H, J = 6.7 Hz, H-2′, 6′), 5.29 (s, 2H, OCH2), 4.12 (q, 2H, CH2), 2.30 (t, 3H, J = 12.7 Hz, CH3); 13C NMR (DMSO, 75 MHz): δ = 163.4 (C, C-1′), 142.1 (C, C-2), 138.7 (C, C-4, 9), 123.3 (CH, C-6, 7), 120.1 (CH, C-4′), 115.7 (CH, C-5, 8), 114.6 (CH, C-2′, 6′), 66.7 (CH2, –OCH2), 51.5 (CH2), 14 (CH2, –CH2CH3); EIMS m/z: 310.1 (M +); Anal. Calcd. for C14H12N2O: C, 74.98; H, 5.39; N, 12.49. Found: C, 75.0; H, 5.35; N, 12.45.
Ethyl [2-(naphthalen-1-ylmethyl)-1H-benzimidazol-1-yl]acetate (5b)
It was obtained as white solid, yield 72 %; m.p. 108–112 °C; IR (KBr) v max 3010, 3206, 1436, 1229 cm−1; 1H NMR (DMSO, 300 MHz): δ = 7.79 (d, 2H, J = 7.0 Hz, H-6, 9), 7.71 (d, 1H, J = 6.3 Hz, H-6′), 7.64 (d, 1H, J = 4.0 Hz, H-3′), 7.60 (d, 1H, J = 4.8 Hz, H-7′), 7.55 (d, 2H, J = 7.5 Hz, H-7, 8), 7.50 (t, 1H, J = 9.8 Hz, H-2′), 7.46 (d, 2H, J = 5.8 Hz, H-1′6′), 5.06 (s, 2H, CH2CO), 4.14 (q, 2H, CH2), 2.31 (t, 3H, J = 15.4 Hz, CH3); 13C NMR (DMSO, 75 MHz): δ = 141.7 (C, C-2), 138.5 (C, C-5, 9), 134.1 (C, C-5′), 133.5 (C, C-10′), 132.5 (C, C-9′), 128.9 (CH, C-2′, 4′), 128.6 (CH, C-6′), 127.2 (CH, C-3′), 123.2 (CH, C-7′8′), 120.5 (CH, C-9′), 116.4 (CH, C-5, 8), 50.5 (CH2, –OCH2), 39.4 (CH2), 15.6 (CH2, –CH2CH3); EIMS m/z: 344.1 (M +); Anal. Calcd. for C22H20N2O2: C, 76.72; H, 5.85; N, 8.13. Found: C, 76.70; H, 5.88; N, 8.12; O, 9.29.
Ethyl {2-[(naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl}acetate (5c)
It was obtained as brownish solid, yield 69 %; m.p. 105–109 °C; IR (KBr) v max 2950, 1736, 1657, 1464, 1226 cm−1; 1H NMR (DMSO, 300 MHz): δ = 7.61 (d, 2H, J = 7.8 Hz, H-6, 9), 7.46 (d, 1H, J = 5.5 Hz, H-5′), 7.40 (d, 2H, J = 7.2 Hz, H-3, 8′), 7.30 (t, 1H, J = 13.5 Hz, H-7′), 7.21 (d, 2H, J = 10.8 Hz, H-7, 8), 7.09 (d, 1H, J = 5.6 Hz, H-2′), 7.09 (d, 1H, J = 5.6 Hz, H-2′), 6.98 (t, 1H, J = 11.5 Hz, H-6′), 6.90 (s, 1H, H-10′), 5.21 (s, 2H, CH2O), 4.72 (q, 2H, CH2), 2.35 (t, 3H, J = 14.4 Hz, CH3). 13C NMR (DMSO, 75 MHz): δ = 171 (C, –CO), 158.1 (C, C-1′), 142 (C, C-4, 9), 137.1 (CH, C-6′), 129.3 (CH, C-8′), 124.8 (CH, C-4′), 121.3 (CH, C-6, 7), 115.4 (CH, C-5, 8), 92.45 (CH, C-2′), 62.1 (CH2, OCH2), 50.7 (CH2, –CH2CO), 47.5 (CH2), 13.6 (CH2, –CH2CH3); EIMS m/z 360.1 (M +); Anal. Calcd. for C22H20N2O3: C, 73.32; H, 5.59; N, 7.77. Found: C, 73.29; H, 5.60; N, 7.80.
Synthesis of 2-[2-(phenoxy/naphthalen-1-yl/naphthalen-2-yloxy methyl)-1H-benzimidazol-1-yl]acetohydrazide (6a–c)
To an ethanolic solution of ethyl [2-phenoxymethyl/(naphthalen-1-ylmethyl)/(naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl]acetate (0.01 mol), hydrazine hydrate (98 %) (0.01 mol; 0.49 ml) was added and the mixture was refluxed for 3 h. After completion of the reaction, the mixture was cooled, and the solid so obtained was filtered, washed with cold water and recrystallized from methanol.
2-[2-(Phenoxymethyl)-1H-benzimidazol-1-yl]acetohydrazide (6a)
It was obtained as white solid, yield 80 %; m.p. 178–180 °C; IR (KBr): v max 3287, 3034, 1656, 1600, 1242, 1030 cm−1; 1H NMR (DMSO, 300 MHz): δ = 9.43 (s, 1H, CONH), 7.69 (d, 2H, J = 12.6 Hz, H-6, 9), 7.31 (d, 2H, J = 12.6 Hz, H-7, 8), 7.18 (d, 2H, J = 6.0 Hz, H-3, 5′), 7.02 (t, 1H, J = 9.5 Hz, H-4′), 6.92 (d, 2H, J = 6.2 Hz, H-2′, 6′), 5.48 (s, 2H, CH2O), 4.90 (s, 2H, CH2), 2.52 (s, 1H, NH2); 13C NMR (DMSO, 75 MHz): δ = 169.3 (C, CONH), 165 (C, C-1′), 141.3 (C, C-2), 136.7 (C, 4, 9), 130.5 (CH, C-3′, 122 (CH, C-6′, 7′), 120.1 (CH, C-4′), 113.4 (CH, C-5, 8), 112.1 (CH, C-2′, 6′), 67.3 (CH2, –OCH2), 51.9 (CH2, –NCH2). EIMS m/z: 296.1 (M +). Anal. Calcd. for C16H16N4O2: C, 64.85; H, 5.44; N, 18.91. Found: C, 64.86; H, 5.42; N, 18.89.
2-[2-(Naphthalen-1-ylmethyl)-1H-benzimidazol-1-yl]acetohydrazide (6b)
It was obtained as white solid, yield 82 %; m.p. 147–150 °C; IR (KBr) v max 3302, 3043, 1643, 1528, 1233 cm−1; 1HNMR (DMSO, 300 MHz): δ = 9.25 (s, 1H, CONH), 8.15 (d, 2H, J = 11.2 Hz, H-6, 9), 7.58 (d, 1H, J = 9.0 Hz, H-3′), 7.64 (d, 2H, J = 9.5 Hz, H-5′, 8′), 7.35 (d, 2H, J = 7.5 Hz, H-7, 8), 7.30 (d, 2H, J = 8.9 Hz, H-6′, 7′), 7.18 (t, 1H, J = 9.2 Hz, H-2′), 7.13 (d, 1H, J = 5.6 Hz, H-1′), 4.90 (s, 2H, CH2), 2.50 (s, 1H, NH2); 13C NMR (DMSO, 75 MHz): δ = 170.3 (C, CONH), 140.5 (C, C-2), 138.4 (C, C-4, 9), 134.7 (C, C-10′), 133.7 (C, C-9′), 133.5 (CH, C-5′), 128.4 (CH, C-6′, 7′), 126.7 (CH, C-3′), 126.2 (CH, C-2′, 4), 123.9 (CH, C-7′, 8′), 113.7 (CH, C-5, 8), 53.1 (CH2, –NCH2), 32.1 (CH2); EIMS m/z: 330.4 (M +); Anal. Calcd. for C22H18N4O: C, 72.71; H, 5.49; N, 16.96. Found: C, 72.71; H, 5.50; N, 16.94.
2-{2-[(Naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl}acetohydrazide (6c)
It was obtained as white solid, yield 82 %; m.p. 208–210 °C; IR (KBr) v max 3292, 3056, 1668, 1466, 1254 cm−1. 1H NMR (DMSO, 300 MHz): δ = 9.05 (s, 1H, CONH), 7.70 (d, 2H, J = 5.7 Hz, H-6, 9), 7.65 (d, 1H, J = 5.5 Hz, H-5′), 7.58 (d, 2H, J = 6.5 Hz, H-3′, 8′), 7.52 (t, 1H, J = 11.6 Hz, H-7′), 7.32 (d, 2H, J = 6 Hz, H-7, 8), 7.17 (t, 1H, J = 12.2 Hz, H-6′), 7.03 (s, 1H, J = 4.7 Hz, H-2′), 6.92 (s, 1H, H-10′), 5.35 (s, 1H, CH2O), 4.89 (s, 2H, CH2), 2.53 (s, 1H, NH2); 13C NMR (DMSO, 75 MHz): δ = 170.3 (C, CONH), 158.8 (C, C-2′), 142.6 (C, C-2), 138.9 (C, C-4, 9), 133.5 (C, C-10′), 130.7 (C, C-5′), 130.3 (CH, C-4′), 127.6 (CH, C-6′), 127.1 (CH, C-9′), 126.2 (CH, C-8′), 122.6 (CH, C-7), 120.3 (CH, C-6, 7), 117.8 (CH, C-3′), 109.4 (CH, C-5, 8), 103.8 (CH, C-1′), 66.2 (CH2, CH2O), 51.9 (CH2, NCH2). EIMS m/z: 346.4 (M +); Anal. Calcd. for C22H18N4O2: C, 69.35; H, 5.24; N, 16.17. Found: C, 69.30; H, 5.21; N, 16.21.
Synthesis of phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1H-benzimidazol-1-yl)acetohydrazide (7a–c)
A mixture of (2-phenoxy/naphthalen-1-yl/naphthalen-2-yloxy methyl)-benzoimidazol-1-yl) acetohydrazide (0.005 mol) and 2-chloroquinoline-3-carbaldehyde (0.005 mol; 0.95 g) in ethanol was refluxed for 5 h. After completion of the reaction, the reaction mixture was concentrated, cooled and poured in ice-cold water, the precipitate so formed was filtered, dried and recrystallized to give the desired compound.
(2-Phenoxymethyl-benzoimidazol-1-yl)-acetic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (7a)
It was obtained as yellowish solid, yield 72 %; m.p. 202–206 °C; IR (KBr) v max 3310, 3107, 1654, 1535, 1081, 723 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.94 (s, 1H, CONH), 8.43 (s, 1H, N=CH), 8.26 (s, 1H, H-4′′), 8.12 (d, 1H, J = 8.6 Hz, H-9″), 7.97 (d, 2H, J = 6.9 Hz, H-6, H-9), 7.78 (t, 1H, J = 13.6 Hz, H-8″), 7.66 (t, 1H, J = 8.8 Hz, H-7″), 7.48 (t, 1H, J = 11.6 Hz, H-4′), 7.20–7.34 (m, 3H, H-6″, H-7, H-8), 7.06 (d, 2H, J = 8.7 Hz, H-3′, H-5′), 6.96 (d, 2H, J = 8.8 Hz, H-2′, H-6′), 5.66 (s, 2H, CH2O), 5.11 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 173.1 (C, CONH), 163.7 (C, C-1′), 154.8 (CH, N=CH), 142.7 (C, C-2), 138.2 (C, C-4, C-9), 130.4 (CH, C-8″), 129.5 (CH, C-3′,C-5′), 128.3 (CH, C-9″), 126.9 (CH, C-7″), 126.7 (C, C-5″), 124.2 (C, C-3″), 123.3 (CH, C-6, C-7), 121.8 (CH, C-4′), 115.1 (CH, C-5, C-8), 110.1 (CH, C-2′, C-6′), 66 (CH2, CH2O), 52.2 (CH2, NCH2); EIMS m/z: 469.1 (M +); Anal. Calcd. for C26H20ClN5O2: C, 66.45; H, 4.29; N, 14.90. Found C, 66.39; H, 4.39; N, 14.87.
(2-Naphthalen-1-ylmethyl-benzoimidazol-1-yl)-acetic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (7b)
It was obtained as yellowish solid, yield 72 %; m.p. 232–236 °C; IR (KBr) v max 3317, 3107, 1654, 1531, 1078, 733 cm−1; 1H NMR (DMSO, 300 MHz): δ = 12.32 (s, 1H, CONH), 8.33 (s, 1H, N=CH), 8.20 (s, 1H, H-4″), 8.15 (d, 1H, J = 8.1 Hz, H-9″), 8.09 (d, 1H, J = 10.2 Hz, H-5′), 7.92 (d, 2H, J = 9.5 Hz, H-6, H-9), 7.63 (t, 1H, J = 8.8 Hz, H-7″), 7.41 (d, 1H, J = 6.3 Hz, H-3′), 7.32 (d, 2H, J = 10.3 Hz, H-6′, H-7′), 7.20–7.34 (m, 3H, H-6″, H-7, H-8), 7.13 (t, 1H, J = 11.7 Hz, H-2′), 6.91 (d, 1 H, J = 6.7 Hz, H-1′), 5.16 (s, 2H, CH2), 5.13 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 173 (C, CONH), 156.1 (C, C–Cl), 153.3 (CH, N=CH), 152.3 (C, C-10″), 142.7 (C, C-2), 137.9 (C, C-4, C-9), 137.7 (CH, C-4″), 134 (C, C-10′), 133.1 (C, C-5′), 130.2 (CH, C-8″), 128.3 (C, C-9″), 127.9 (CH, C-6′, C-9′), 126.7 (CH, C-6″), 126.4 (CH, C-2′, C-3′), 126.1 (CH, C-7″), 125.7 (C, C-5″), 124.1 (CH, C-7, C-8), 122.7 (C, C-3″), 121.7 (CH, C-6, C-7), 112.1 (CH, C-5, C-8), 52.1 (CH2, N-CH2), 28.1 (CH2); EIMS m/z: 503.1 (M +); Anal. Calcd. for C30H22ClN5O: C, 71.49; H, 4.40; N, 13.90. Found: C, 69.3; H, 4.27; N, 13.49.
[2-(Naphthalen-2-yloxymethyl)-benzoimidazol-1-yl]-acetic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (7c)
It was obtained as brownish solid, yield 72 %; m.p. 272–276 °C; IR (KBr) v max 3307, 2967, 1667, 1555, 1079, 744 cm−1; 1H NMR (DMSO, 300 MHz): δ = 12.12 (s, 1H, CONH), 8.43 (s, 1H, N=CH), 8.16 (s, 1H, H-4″), 8.08 (d, 1H, J = 10.1 Hz, H-9″), 7.78 (d, 1H, J = 7.6 Hz, H-5′), 7.62 (t, 1H, J = 7.9 Hz, H-7″), 7.54 (d, 2H, J = 5.4 Hz, H-3′, H-8′), 7.43 (t, 1H, J = 10.7 Hz, H-7′), 7.35 (d, 2H, J = 6.7 Hz, H-7, H-8), 7.18–7.31 (m, 3H, H-6″, H-7, H-8), 7.17 (t, 1H, J = 15.6 Hz, H-6′), 6.98 (d, 1H, J = 5.5 Hz, H-2′), 6.82 (s, 1H, H-10′), 5.71 (s, 2H, OCH2), 5.40 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 173 (C, CONH), 156.9 (C, C-2″), 154.9 (N=CH), 151.1 (C, C-10″), 142.1 (C, C-2), 137.8 (C, C-4, 9), 135.3 (C, C-5′, C-10′), 129.1 (CH, C-8″), 128.2 (CH, C-9″), 127.3 (CH, C-7″), 126.4 (CH, C-6″), 125.7 (C, C-5″), 124.9 (C, C-3″), 123.7 (CH, C-7′, C-8′), 121.9 (CH, C-6′, C-9′), 120.8 (CH, C-6, C-7), 119.7 (CH, C-3′), 112.3 (CH, C-5, C-8), 103.7 (CH, C-1′), 65.7 (CH2, CH2O), 52.2 (CH2, NCH2); EIMS m/z: 519.1 (M +); Anal. Calcd. for C30H22ClN5O2: C, 69.3; H, 4.26; N, 13.47. Found: C, 69.3; H, 4.27; N, 13.49.
Procedure for the synthesis of 2-chloro-3-{5-[(2-phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1-H-benzimidazol-1-yl)methyl]-1,3,4-oxadiazol-2-yl}quinoline (8a-c)
To an ethanolic solution of 2-chloroquinolin-3-yl)methylidene]-2-(2-phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1H-benzimidazol-1-yl)acetohydrazide (0.005 mol), chloramine-T (0.01 mol; 2.27 g) was added. The solution was refluxed for 4 h, sodium chloride which separated out during the course of reaction was filtered off. Excess ethanol was completely removed from the filtrate by distillation under reduced pressure, leaving behind a solid mass which was crystallized from ethanol to give the desired compound.
2-Chloro-3-[5-(2-phenoxymethyl-benzoimidazol-1-ylmethyl)-[1,3,4]oxadiazol-2-yl]-quinoline (8a)
It was obtained as yellowish solid, yield 65 %; m.p. 238–240 °C; IR (KBr) v max 2962, 1598, 1231, 1034, 743 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.08 (s, 1H, H-4″), 8.02 (d, 1H, J = 6.6 Hz, H-9″), 7.94 (d, 2H, J = 4.8 Hz, H-6, H-9), 7.88 (t, 1H, J = 13.6 Hz, H-8″), 7.53 (t, 1H, J = 7.8 Hz, H-7″), 7.55 (t, 1H, J = 10.4 Hz, H-4′), 7.18–7.31 (m, 3H, H-6″, H-7, H-8), 7.09 (d, 2H, J = 8.2 Hz, H-3′, H-5), 6.91 (d, 2H, J = 8.4 Hz, H-2′, H-6′), 5.64 (s, 2H, CH2O), 5.43 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 166.7 (C, C-5oxa), 166.2 (C, C-2oxa), 161 (C, C-1′), 155.7 (C, C-2″), 143.2 (C, C-2), 138.2 (C, C-4, C-9), 130.1 (CH, C-8″), 128.9 (CH, C-3′, C-5′), 127.8 (CH, C-9″), 127.1 (CH, C-7″), 125.6 (C, C-5″), 124.2 (C, C-3″), 122.7 (CH, C-6, C-7), 121.9 (CH, C-4′), 113.4 (CH, C-5, C-8), 112.1 (CH, C-2′, C-6′), 76.5 (CH2, CH2O), 46.2 (CH2, NCH2); EIMS m/z: 467.1 (M +); Anal. Calcd. for C26H18ClN5O2: C, 66.74; H, 3.88; N, 14.97. Found: C, 66.70; H, 3.82; N, 14.99.
2-Chloro-3-[5-(2-naphthalen-1-ylmethyl-benzoimidazol-1-ylmethyl)-[1,3,4]oxadiazol-2-yl]-quinoline (8b)
It was obtained as yellowish-white solid, yield 65 %; m.p. 197–200 °C; IR (KBr) v max 2952, 1588, 1234, 1033, 740 cm−1. 1H NMR (DMSO, 300 MHz): δ = 8.04 (s, 1H, H-4″), 8.01 (d, 2H, J = 6 Hz, H-8″, H-9″), 7.96 (d, 1H, J = 9.2 Hz, H-5′), 7.74 (d, 3H, J = 7.8 Hz, H-8′, H-6, H-9), 7.61 (t, 1H, J = 8.6 Hz, H-7″), 7.39 (d, 1H, J = 4.8 Hz, H-3′), 7.20–7.34 (m, 5H, H-6″, H-7, H-8, H-6′, H-7′), 7.13 (t, 1H, J = 11.2 Hz, H-2′), 6.91 (d, 1 H, J = 6.7 Hz, H-1′), 5.54 (s, 2H, CH2), 5.31 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 166.8 (C, C-5oxa), 166.3 (C, C-2oxa), 147.7 (C, C-10″), 139.9 (C, C-2), 135.6 (C, C-4, C-9), 134 (C, C-5′, C-10′), 130.1 (CH, C-4″, C-8″), 128.6 (C, C-9″), 127.9 (CH, C-6′, C-9′), 127.1 (CH, C-6″), 126.5 (CH, C-2′, C-3′), 126.3 (CH, C-7″), 125.1 (C, C-5″), 124.4 (CH, C-7′, C-8′), 123.1 (C, C-3″), 115.3 (CH, C-6, C-7), 112.3 (CH, C-5, C-8), 45.6 (CH2, N-CH2), 28.4 (CH2); EIMS m/z: 501.13 (M +); Anal. Calcd. for C30H20ClN5O: C, 71.78; H, 4.02; N, 13.95. Found: C, 71.82; H, 4.07; N, 13.94.
2-Chloro-3-{5-[2-(naphthalen-2-yloxymethyl)-benzoimidazol-1-ylmethyl]-[1,3,4]oxadiazol-2-yl}-]-quinoline (8c)
It was obtained as white solid, yield 65 %; m.p. 151–156 °C; IR (KBr) v max 2952, 1588, 1238, 1023, 729 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.54 (s, 1H, H-4″), 8.13 (d, 2H, J = 8.1 Hz, H-5′, H-9″), 7.94 (t, 1H, J = 18.0 Hz, H-7″), 7.56–7.85 (m, 5H, H-7, H-8, H-3′, H-7′, H-8′), 7.20–7.42 (m, 3H, H-6″, H-7, H-8), 6.97 (t, 1H, J = 21 Hz, H-6′), 6.81 (d, 1H, J = 7.2 Hz, H-2′), 6.13 (s, 1H, H-10′), 5.74 (s, 2H, CH2), 5.53 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 166.6 (C, C-5oxa), 166.1 (C, C-2oxa), 161 (C, C-2′), 155.9 (C, C-2″), 150.3 (C, C-10″), 140.5 (C, C-2), 135.8 (C, C-4, C-9), 130 (C, 5′, C-10′), 129.1 (CH, C-8), 128.4 (CH, C-8″), 126.7 (C, C-5″), 126.3 (C, C-3″), 124.9 (CH, C-7′, C-8′), 121.7 (CH, C-6′, C-9′), 120.1 (CH, C-6, 7), 116.7 (CH, C-3′), 112.8 (CH, C-5, C-8), 104.7 (CH, C-1′), 67.2 (CH2, CH2O), 46.2 (C’H2, NCH2); EIMS m/z: 517.3 (M +); Anal. Calcd. for C30H20ClN5O2: C, 69.56; H, 3.89; N, 13.52. Found: C, 69.61; H, 3.91; N, 13.53.
General procedure for the preparation of acid hydrazides (10a–i)
The appropriate aromatic acids 9a–i (0.01 mol) were dissolved in absolute ethanol (10 ml). Hydrazine hydrate (0.02 mol, 1 ml) and few drops of conc. sulphuric acid were added. The reaction mixture was refluxed for 6 h. The resulting solid obtained was filtered, dried and crystallized from methanol. The completion of reaction was monitored by thin-layer chromatography and infrared spectrophotometer (Jha et al., 2010). (10a: naphthoxy acetic acid hydrazide; 10b: phenylacetic acid hydrazide; 10c: p-nitrobenzoic acid hydrazide; 10d: o-chlorobenzoic acid hydrazide; 10e: p-chlorobenzoic acid hydrazide; 10f: nicotinic acid hydrazide; 10g: phenoxyacetic acid hydrazide; 10h: 3,5-dinitrobenzoic acid hydrazide; 10i: salicylic acid hydrazide.)
Procedure for the synthesis of (2-chloroquinolin-3-yl)methylidene]-substituted benzohydrazide (11a–i)
A mixture of substituted hydrazide 8a–i (0.003 mol) and 2-chloroquinoline-3-carbaldehyde 2 (0.003 mol; 0.57 g) in ethanol was refluxed for 5 h. After completion of the reaction, the reaction mixture was concentrated, cooled and poured in ice-cold water; the precipitate so formed was filtered, dried and recrystallized to give the desired compound.
(Naphthalen-2-yloxy)-acetic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11a)
It was obtained as yellowish-white solid, yield 67 %; m.p. 236–239 °C; IR (KBr) v max 3311, 2921, 1605, 1693, 1605, 1500, 741 cm−1; 1H NMR (DMSO, 300 MHz): δ = 9.03 (s, 1H, CONH), 8.46 (s, 1H, N=CH), 7.95 (d, 1H, J = 9.3 Hz, H-9′), 8.28 (s, 1H, H-4′), 8.10 (d, 1H, J = 6 Hz, H-6′), 7.73–7.82 (m, 4H, H-4, H-6, H-9, H-8′), 7.68 (s, 1H, H-1′), 7.17–7.34 (m, 4H, H-3, H-7, H-8, H-7′), 5.38 (s, 2H, CH2O); 13C NMR (DMSO, 75 MHz): δ = 173.3 (C, CONH), 157 (C, C-2′), 155.7 (C, C-2), 153.3 (C, C-10′), 139 (CH, C-4′), 135.4 (CH, C-5, 10), 130.5 (CH, C-7′), 129.5 (CH, C-4), 128.2 (CH, C-6, C-8, C-9), 127.7 (CH, C-6′, C-9′), 127.1 (CH, C-7′), 126 (C, C-5′), 123.3 (C, C-3′), 121 (CH, C-7), 117.9 (CH, C-3), 107.4 (CH, C-1), 78.3 (CH2, OCH2); EIMS m/z: 389.0 (M +); Anal. Calcd. for C22H16ClN3O2: C 67.78; H, 4.14; N, 10.78. Found: C, 67.75; H, 4.17; N, 10.81.
Phenyl-acetic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11b)
It was obtained as yellowish solid, yield 65 %; m.p. 212–216 °C; IR (KBr) v max 3276, 2931, 1665, 1602, 1503, 734 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.54 (s, 1H, CONH), 8.24 (s, 1H, N=CH), 8.01 (d, 1H, J = 10.6 Hz, H-4′), 7.93 (d, 1H, J = 8.7 Hz, H-9′), 7.69 (t, 1H, J = 7.8 Hz, H-8′), 7.51 (t, 1H, J = 9.6 Hz, H-7′), 7.18 (d, 1H, H-6′), 7.10 (t, 2H, J = 12.3 Hz, H-3, H-5), 7.01–7.06 (m, 3H, H-2, H-4, H-6), 4.55 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 173.5 (C, CONH), 156 (C, C-2′), 153.2 (CH2), 150 (C, C-10′), 139.7(CH, C-4′), 136.2 (C, C-1), 132.5 (CH, C-8′), 129.5(CH, C-2, C-6), 129 (CH, C-3, C-5), 128.3 (CH, C-6′, C-9′), 127 (CH, C-4′), 124.1 (C, C-5′), 123.2 (CH, C-7′), 120.1 (C, C-3′), 40.1 (CH2); EIMS m/z: 323.08 (M +); Anal. Calcd. for C17H11Cl2N3O: C, 59.32; H, 3.22; N, 12.21. Found: C, 59.30; H, 3.25; N, 12.24.
4-Nitro-benzoic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11c)
It was obtained as yellow solid, yield 72 %; m.p. 250–255 °C; IR (KBr) v max 3305, 2940, 1692, 1535, 1460, 756 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.54 (s, 1H, CONH), 8.20 (s, 1H, N=CH), 8.03 (d, 1H, J = 9.7 Hz, H-4′), 7.96 (t, 2H, J = 12.3 Hz, H-3, H-5), 7.88 (d, 1H, J = 8.7 Hz, H-9′), 7.71 (t, 1H, J = 7.8 Hz, H-8′), 7.51 (t, 1H, J = 9.6 Hz, H-7′), 7.26 (d, 2H, J = 5.6 Hz, H-2, H-6), 7.18 (d, 1H, H-6′); 13C NMR (DMSO, 75 MHz): δ = 170.5 (C, CONH), 158.5 (C, C-2′), 153.3 (CH2), 151 (C, C-4), 148.8 (C, C-10′), 140 (C, C-1), 135.3 (CH, C-4′), 130 (CH, C-8′), 128.5 (CH, C-2, C-6), 127 (C, C-9′), 126.4 (CH, C-6′), 126 (CH, C-7′), 125.4 (C, C-5′), 122.5 (C, C-3′), 120.1 (CH, C-3, C-5); EIMS m/z: 354.5 (M +); Anal. Calcd. for C17H11ClN4O3: C, 57.65; H, 3.13; N, 15.79. Found: C, 57.63; H, 3.11; N, 15.83.
3,5-Dimethoxy-benzoic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11d)
It was obtained as yellow solid, yield 63 %; m.p. 288–292 °C; IR (KBr) v max 3193, 2950, 1672, 1562, 1501, 794 cm−1; 1H NMR (DMSO, 300 MHz): δ = 9.02 (s, 1H, CONH), 8.56 (s, 1H, N=CH), 7.94 (d, 1H, J = 10.2 Hz, H-9′), 7.66 (t, 1H, J = 7.1 Hz, H-8), 7.52 (t, 1H, J = 8.4 Hz, H-7′), 7.41 (d, 1H, H-6′), 7.31–7.35 (m, 3H, H-2, H-4, H-6), 3.78 (s, 3H, 5-OCH3), 3.67 (s, 3H, 3-OCH3); 13C NMR (DMSO, 75 MHz): δ = 170.2 (C, CONH), 164 (C, C-3, C-5), 157.1 (CH, CH=N), 153.2 (C, C-10′), 136 (CH, C-4′), 132.5 (C, C-1), 130 (C, C-8′), 128.5 (C, C-9′), 127.5 (CH, C-6′), 126.6 (CH, C-7′), 125.2 (C, C-5′), 122.3 (C, C-3′), 104.6 (CH, C-2, C-6), 102 (CH, C-5), 56 (OCH3); EIMS m/z: 369.08 (M +); Anal. Calcd. for C19H16ClN3O3: C, 61.71; H, 4.36; N, 11.36. Found: C, 61.75; H, 4.37; N, 11.39.
4-Chloro-benzoic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11e)
It was obtained as yellow solid, yield 72 %; m.p. 198–202 °C; IR (KBr) v max 3312, 2945, 1675, 1534, 1464, 736 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.62 (s, 1H, CONH), 8.09 (s, 1H, N=CH), 7.91 (d, 1H, J = 8.7 Hz, H-4′), 7.82 (t, 2H, J = 11.2 Hz, H-3, H-5), 7.67 (d, 1H, J = 9.4 Hz, H-9′), 7.56 (t, 1H, J = 10.6 Hz, H-8′), 7.43 (t, 1H, J = 9.6 Hz, H-7′), 7.33 (d, 1H, H-6′), 7.20 (d, 2H, J = 7.6 Hz, H-2, H-6); 13C NMR (DMSO, 75 MHz): δ = 170 (C, CONH), 158.2 (C, C-2′), 154.1 (CH, N=CH), 151 (C, C-10′), 138.1 (CH, C-4′), 137 (C, C-4), 132.2 (C, C-1), 130.5 (C, C-8′), 129.6 (CH, C-3, C-5), 128.5 (CH, C-2, C-6), 127.9 (C, C-9′), 127.8 (CH, C-6′), 125.6 (CH, C-7′), 125.3 (C, C-5′), 122.3 (C, C-3′); EIMS m/z: 343.02 (M +); Anal. Calcd. for C17H11Cl2N3O: C, 59.32; H, 3.22; N, 12.21. Found: C, 59.35; H, 3.26; N, 12.23.
Nicotinic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11f)
It was obtained as brownish-yellow solid, yield 62 %; m.p. 224–228 °C; IR (KBr) v max 3192, 2927, 1633, 1502, 1454, 726 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.92 (s, 1H, CONH), 8.65 (s, 1H, N=CH), 8.41 (d, 1H, J = 9.7 Hz, H-4′), 7.78–7.84 (m, 4H, pyridyl), 7.63 (d, 1H, J = 9.7 Hz, H-9′), 7.50 (t, 1H, J = 8.9 Hz, H-8′), 7.41 (t, 1H, J = 12.2 Hz, H-7′), 7.38 (d, 1H, H-6′); 13C NMR (DMSO, 75 MHz): δ = 171.4 (C, CONH), 157.2 (C, C-2′), 154 (CH, N=CH), 152.3 (C, C-10′), 149.2 (CH, C-4), 138.3 (CH, C-4′), 136.6 (CH, C-6), 130.6 (CH, C-8′), 129 (CH, C-2), 127.9 (CH, C-9′), 127 (CH, C-6′), 126.5 (CH, C-7′), 125.2 (C, C-5′), 124.7 (CH, C-5), 123.3 (C, C-3′); EIMS m/z: 310.06 (M +); Anal. Calcd. for C16H11ClN4O: C, 61.84; H, 3.57; N, 18.03. Found: C, 61.80; H, 3.52; N, 18.07.
Phenoxyacetic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11g)
It was obtained as yellowish-brown solid, yield 67 %; m.p. 26–219 °C; IR (KBr) v max 3301, 2929, 1694, 1605, 1500, 740 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.72 (s, 1H, CONH), 8.45 (s, 1H, N=CH), 7.62 (d, 1H, J = 10.3 Hz, H-9′), 7.53 (t, 1H, J = 9.4 Hz, H-8′), 7.40 (t, 1H, J = 11.2 Hz, H-7′), 7.34 (d, 1H, J = 6.3, H-6′), 7.32 (t, 1H, J = 7.2, H-4), 7.06 (d, 2H, J = 10.2, H-3, H-5), 6.90 (d, 2H, J = 9.2 Hz, H-2, H-6), 5.01 (s, 1H, OCH2); 13C NMR (DMSO, 75 MHz): δ = 172.8 (C, CONH), 163.4 (C, C-1), 159 (C, C-2′), 154 (CH, N=CH), 150.3 (C, C-10′), 138.3(CH, C-4′), 130.3 (CH, C-8′), 128.5 (CH, C-4), 127.7 (CH, C-9′), 126 (CH, C-6′), 125.1 (CH, C-7′), 123.5 (C, C-5′), 122.6 (C, C-3′), 117.4 (CH, C-4), 110.3 (CH, C-2, 6), 78.6 (CH2, OCH2); EIMS m/z: 339.07 (M +); Anal. Calcd. for C18H14ClN3O2: C, 63.63; H, 4.15; N, 12.37. Found: C, 63.60; H, 4.19; N, 12.31.
3,5-Dinitro-benzoic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11h)
It was obtained as brownish-yellow solid, yield 78 %; m.p. 70–72 °C; IR (KBr) v max 3305, 2924, 1670, 1504, 1461, 793 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.87 (s, 1H, CONH), 8.59 (s, 1H, N=CH), 8.22–8.35 (m, 3H, H-2,H-4, H-6), 7.76 (d, 1H, J = 10.5 Hz, H-9′), 7.58 (t, 1H, J = 10.5 Hz, H-8′), 7.43 (t, 1H, J = 10.4 Hz, H-7′), 7.26 (d, 1H, J = 6.9 Hz, H-6′); 13C NMR (DMSO, 75 MHz): δ = 171.4 (C, CONH), 157.6 (C, C-2′), 154.2 (CH, N=CH), 151.6 (C, C-10′), 150 (C, C-3, C-5), 138.2 (CH, C-4′), 134.7 (C, C-1), 130.5 (CH, C-8′), 128.7 (CH, C-2, C-6), 128 (CH, C-9′), 127.8 (CH, C-6′), 126.4 (CH, C-7′), 126 (CH, C-5′), 125.5 (C, C-3′), 123 (CH, C-4); MS m/z: 399.0 (M +); Anal. Calcd for C17H10ClN5O5: C, 51.08; H, 2.52; N, 17.52. Found: C, 51.05; H, 2.48; N, 17.48.
2-Hydroxy-benzoic acid (2-chloro-quinolin-3-ylmethylene)-hydrazide (11i)
It was obtained as yellow solid, yield 60 %; m.p. 171–173 °C; IR (KBr) v max 3307, 2965, 1664, 1500, 1473, 733 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.81 (s, 1H, CONH), 8.50 (s, 1H, N=CH), 7.57 (d, 1H, J = 10.5 Hz, H-9′), 7.46 (t, 1H, J = 10.7 Hz, H-8′), 7.38 (t, 1H, J = 9.2 Hz, H-7′), 7.29 (d, 1H, J = 7.9, H-6′), 6.67–7.23 (m, 4H, H-2, H-3, H-5, H-6); 13C NMR (DMSO, 75 MHz): δ = 171 (C, CONH), 156 (C, C-2, C-2′), 154.2 (CH, N = CH), 151 (C, C-10′), 139 (CH, C-4′), 134.3 (CH, C-4), 130.7 (CH, C-8′), 129 (CH, C-6), 128.8 (CH, C-9′), 127.5 (CH, C-6′), 126.3 (CH, C-7′), 125.2 (CH, C-5′), 123.3 (C, C-3′), 121.4 (CH, C-5), 119.2 (C, C-1), 117.7 (CH, C-3); EIMS m/z: 325.06 (M +); Anal. Calcd. for C17H12ClN3O2: C, 62.68; H, 3.71; N, 12.90. Found: C, 62.62; H, 3.67; N, 12.94.
Procedure for the synthesis of 2-chloro-3-(5-substituted-phenyl-1,3,4-oxadiazol-2-yl)quinoline (12a–i)
To an ethanolic solution of (2-chloroquinolin-3-yl)methylidene]-substituted benzohydrazide 11a–i (0.002 mol; 0.78 g), chloramine-T (0.004 mol; 0.90 g) was added. The solution was refluxed for 4 h, sodium chloride which separated out during the course of reaction was filtered off. Excess ethanol was completely removed from the filtrate by distillation under reduced pressure, leaving behind a solid mass which was crystallized from ethanol to give the desired compound.
2-Chloro-3-{5-[(naphthalen-2-yloxy)methyl]-1,3,4-oxadiazol-2-yl}quinoline (12a)
It was obtained as brownish solid, yield 76 %; m.p. 147–150 °C; IR (KBr) v max 2916, 1597, 1297, 1045, 749 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.49 (s, 1H, H-9′), 8.23 (s, 1H, H-4′), 8.02 (d, 1H, J = 6 Hz, H-6′), 7.67–7.80 (m, 4H, H-4, H-6, H-9, H-8′), 7.63 (s, 1H, H-1), 7.12–7.37 (m, 4H, H-3, H-7, H-8, H-7′), 4.90 (s, 2H, CH2O); 13C NMR (DMSO, 75 MHz): δ = 166.7 (C, C-5oxa), 166.5 (C, C-2oxa), 157.1 (C, C-2), 154.2 (C, C-2′), 146.7 (C, C-10′), 136.6 (CH, C-4′), 135 (C, C-10), 131.9 (C, C-3′), 130.6 (CH, C-8′), 129.8 (CH and C, C-4, C-5), 127.5 (CH, C-9′), 126.9 (CH, C-6, C-9), 126.5 (CH, C-6′), 126 (CH, C-7′), 125.1 (C, C-5′), 123 (CH, C-7, C-8), 115.5 (CH, C-3), 102.5 (CH, C-1), 70.2 (CH2); EIMS m/z: 387 (M +); Anal. Calcd. for C22H14ClN3O2: C, 68.13; H, 3.64; N, 10.83. Found: C, 68.10; H, 3.65; N, 10.86.
3-(5-Benzyl-1,3,4-oxadiazol-2-yl)-2-chloroquinoline (12b)
It was obtained as yellowish-white solid, yield 73 %; m.p. 178–180 °C; IR (KBr) v max 2945, 1587, 1299, 1047, 728 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.05 (d, 1H, J = 10.9 Hz, H-4′), 7.90 (d, 1H, J = 9.7 Hz, H-9′), 7.67 (t, 1H, J = 9.7 Hz, H-8′), 7.45 (t, 1H, J = 13.7 Hz, H-7), 7.28 (d, 1H, H-6′), 7.19 (t, 2H, J = 10.2 Hz, H-3, H-5), 7.08–7.16 (m, 3H, H-2, H-4, H-6), 4.56 (s, 2H, CH2); 13C NMR (DMSO, 75 MHz): δ = 166.4 (C, C-5oxa), 166 (C, C-2oxa), 155.4 (C, C-2′), 146.4 (C, C-10′), 139.5 (C, C-1), 135 (CH, C-4′), 131.9 (C, C-3′), 130.7 (CH, C-8′), 129.5 (CH, C-2, C-6), 128 (CH, C-3, C-5), 127.8 (CH, C-9′), 127 (CH, C-6′), 126.5 (CH, C-7′), 126.1 (C, C-5′), 125.3 (CH, C-4), 35.8 (CH2); EIMS m/z: 321.6 (M +); Anal. Calcd. for C18H12ClN3O: C, 67.19; H, 3.76; N, 13.06. Found: C, 67.22; H, 3.81; N, 13.05.
2-Chloro-3-[5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl]quinoline (12c)
It was obtained as white solid, yield 66 %; m.p. 79–83 °C; IR (KBr) v max 2916, 1597, 1299, 1043, 748 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.04 (d, 1H, J = 9.0 Hz, H-4′), 7.99 (t, 2H, J = 6.3 Hz, H-3, H-5), 7.88 (d, 1H, J = 8.7 Hz, H-9′), 7.63 (t, 1H, J = 8.8 Hz, H-8′), 7.52 (t, 1H, J = 9.4 Hz, H-7′), 7.28 (d, 2H, J = 14.6 Hz, H-2, H-6), 7.20 (d, 1H, J = 6.6 Hz, H-6′); 13C NMR (DMSO, 75 MHz): δ = 166.9 (C, C-5oxa), 166.5 (C, C-2oxa), 156 (C, C-2′), 147.5 (C, C-10′), 145.8 (C, C-4), 141 (C, C-1), 135.2 (CH, C-4′), 132.7 (C, C-3′), 131.7 (CH, C-8′), 129.3 (CH, C-9′), 128.7 (CH, C-2, C-6), 127.9 (CH, C-6′), 127 (CH, C-7′), 126.5 (C, C-5), 123.2 (CH, C-3, C-5); EIMS m/z: 352.02 (M +); Anal. Calcd. for C17H9ClN4O3: C, 57.89; H, 2.57; N, 15.88. Found: C, 57.92; H, 2.55; N, 15.90.
2-Chloro-3-[5-(3,5-dimethoxyphenyl)-1,3,4-oxadiazol-2-yl]quinoline (12d)
It was obtained as brownish solid, yield 66 %; m.p. 171–175 °C; IR (KBr) v max 2926, 1527, 1280, 1092, 714 cm−1; 1H NMR (DMSO, 300 MHz): δ = 7.71 (d, 1H, J = 12 Hz, H-9′), 7.60 (t, 2H, J = 8.7 Hz, H-7′, H-8′), 7.46 (d, 1H, J = 7.1 Hz, H-6′), 7.26–7.36 (m, 3H, H-2, H-4, H-6), 3.43 (s, 3H, 5-OCH3), 3.33 (s, 3H, 3-OCH3); 13C NMR (DMSO, 75 MHz): δ = 167.2 (C, C-5oxa), 167 (C, C-2oxa), 163 (C, C-3, C-5), 155 (C, C-2′), 147 (C, C-10′), 141.3 (C, C-1), 135.6 (CH, C-4′), 132.4 (C, C-3′), 131 (CH, C-8′), 128.9 (CH, C-9′), 128.4 (CH, C-6′), 128 (CH, C-7′), 126.5 (C, C-5′), 104 (CH, C-2, 6), 100.7 (CH, C-4), 56 (C, 2-OCH3); EIMS m/z: 367 (M +); Anal. Calcd. for C19H14ClN3O3: C, 62.05; H, 3.84; N, 11.43. Found: C, 62.09; H, 3.80; N, 11.40.
2-Chloro-3-[5-(4-chlorophenyl)-1,3,4-oxadiazol-2-yl]quinoline (12e)
It was obtained as yellowish-white solid, yield 63 %; m.p. 217–221 °C; IR (KBr) v max 2906, 1531, 1289, 1108, 721 cm−1; 1H NMR (DMSO, 300 MHz): δ = 7.89 (d, 1H, J = 9.4 Hz, H-4′), 7.76 (t, 2H, J = 11.7 Hz, H-3, 5), 7.59 (d, 1H, J = 10.7 Hz, H-9′), 7.49 (t, 1H, J = 12.3 Hz, H-8′), 7.38 (t, 1H, J = 8.9 Hz, H-7′), 7.29 (d, 1H, J = 9.5 Hz, H-6′), 7.17 (d, 2H, J = 7.9 Hz, H-2, H-6); 13C NMR (DMSO, 75 MHz): δ = 165.7 (C, C-5oxa), 165.4 (C, C-2oxa), 155 (C, C-2′), 147.8 (C, C-10′), 134.6 (CH, C-4′), 133.5 (C, C-1), 132.5 (C, C-4), 131.7 (C, C-3′), 130.6 (CH, C-8′), 129.8 (CH, C-3, C-5), 129 (CH, C-9′), 128.6 (CH, C-2, C-6), 127 (CH, C-6′), 126.9 (CH, C-7′), 126.3 (C, C-5′); EIMS m/z: 341 (M +); Anal.Calcd. for C17H9Cl2N3O: C, 59.67; H, 2.65; N, 12.28. Found: C, 59.73; H, 2.69; N, 12.31.
2-Chloro-3-[5-(pyridin-3-yl)-1,3,4-oxadiazol-2-yl]quinoline (12f)
It was obtained as white solid, yield 58 %; m.p. 135–136 °C; IR (KBr) v max 3046, 1526, 1299, 1096, 702 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.29 (d, 1H, J = 10.2 Hz, H-4′), 7.72–7.85 (m, 4H, Hpyr), 7.60 (d, 1H, J = 9.4 Hz, H-9′), 7.48 (t, 1H, J = 8.7 Hz, H-8′), 7.38 (t, 1H, J = 12 Hz, H-7′), 7.32 (d, 1H, J = 7.5 Hz, H-6′); 13C NMR (DMSO, 75 MHz): δ = 166.7 (C, C-5oxa), 166.2 (C, C-2oxa), 154.5 (C, C-2′), 149.3 (CH, C-2pyr), 148.1 (CH, C-4pyr), 146.6 (C, C-10′), 136.2 (CH, C-4′), 134.2 (CH, C-6pyr), 133.2 (C, C-1pyr), 132.2 (C, C-3′), 131 (CH, C-8′), 129.6 (CH, C-9′), 128.5 (CH, C-6′), 127.1 (CH, C-7′), 126.2 (C, C-5′), 123 (CH, C-5pyr); EIMS m/z: 308.5 (M +); Anal. Calcd. for C16H9ClN4O: C, 62.25; H, 2.94; N, 18.15. Found: C, 62.21; H, 2.98; N, 18.14.
2-Chloro-3-[5-(phenoxymethyl)-1,3,4-oxadiazol-2-yl]quinolone (12g)
It was obtained as yellowish-white solid, yield 66 %; m.p. 167–170 °C; IR (KBr) v max 2934, 1577, 1297, 1039, 739 cm−1; 1H NMR (DMSO, 300 MHz): δ = 7.58 (d, 1H, J = 8.7 Hz, H-9′), 7.53 (t, 1H, J = 9.2 Hz, H-8′), 7.47 (t, 1H, J = 10.8 Hz, H-7′), 7.42 (d, 1H, J = 7.1, H-6′), 7.37 (t, 1H, J = 11.2, H-4), 7.12 (d, 2H, J = 13.2, H-3, H-5), 6.79 (d, 2H, J = 9.8 Hz, H-2, H-6), 5.94 (s, 1H, OCH2); 13C NMR (DMSO, 75 MHz): δ = 167.7 (C, C-5oxa), 167 (C, C-2oxa), 161.6 (C, C-1), 157 (C, C-2′), 147 (C, C-10′), 137.8 (CH, C-4′), 132.4 (C, C-3′), 131.1 (CH, C-8′), 130 (CH, C-9′), 129.2 (CH, C-3, 5), 128 (CH, C-6′), 126.4 (CH, C-7′), 124.5 (C, C-5′), 120.1 (CH, C-4), 114 (CH, C-2, 6), 75.1 (OCH2); EIMS m/z: 337.6 (M +); Anal. Calcd. for C18H12ClN3O2: C, 64.01; H, 3.58; N, 12.44. Found: C, 64.05; H, 3.61; N, 12.47.
2-Chloro-3-[5-(3,5-dinitrophenyl)-1,3,4-oxadiazol-2-yl]quinoline (12h)
It was obtained as yellow solid, yield 76 %; m.p. 134–135 °C; IR (KBr) v max 3047, 1597, 1298, 1095, 703 cm−1; 1H NMR (DMSO, 300 MHz): δ = 8.22–8.38 (m, 3H, H-2, H-4, H-6), 7.84 (d, 1H, J = 10.2 Hz, H-9′), 7.61 (t, 1H, J = 12.2 Hz, H-8′), 7.43 (t, 1H, J = 10.5 Hz, H-7′), 7.27 (d, 1H, J = 6.6, H-6′); 13C NMR (DMSO, 75 MHz): δ = 166.5 (C, C-5oxa), 166.1 (C, C-2oxa), 156 (C, C-2′), 150.4 (C, C-3, C-5), 147 (C, C-10′), 137.5 (C, C-1), 133 (CH, C-4′), 130.7 (CH, C-8′), 129.4 (CH, C-9′), 128.6 (CH, C-2, C-6), 127.7 (CH, C-6′), 127 (CH, C-7′), 126.1 (C, C-5′), 115.6 (CH, C-4); EIMS m/z: 397.1 (M+); Anal. Calcd. for C17H8ClN5O5: C, 51.34; H, 2.03; N, 17.61. Found: C, 51.37; H, 2.06; N, 17.64.
2-[5-(2-Chloroquinolin-3-yl)-1,3,4-oxadiazol-2-yl]phenol (12i)
It was obtained as yellowish-white solid, yield 63 %; m.p. 141–143 °C; IR (KBr) vmax 3365, 2987, 1595, 1299, 1098, 723 cm−1; 1H NMR (DMSO, 300 MHz): δ = 10.21 (s, OH, 1H), 7.51 (d, 1H, J = 7 Hz, H-9′), 7.41 (t, 1H, J = 10.3 Hz, H-8′), 7.32 (d, 1H, J = 7.0 Hz, H-7′), 7.12 (t, 1H, J = 8.4, H-6′), 6.57–6.97 (m, 4H, H-2, H-3, H-5, H-6); 13C NMR (DMSO, 75 MHz): δ = 166.8 (C, C-5oxa), 166.3 (C, C-2oxa), 157 (C, C-2′), 147 (C, C-10′), 136.9 (CH, C-4′), 132.2 (C, C-3′), 131 (CH, C-8′), 130 (CH, C-9′), 129.5 (CH, C-4, C-6), 127.9 (CH, C-6′), 127.2 (CH, C-7′), 126.1 (C, C-5′), 122.7 (C, C-1), 119.5 (CH, C-5), 115 (CH, C-3); EIMS m/z: 323.4 (M +); Anal. Calcd. for C17H10ClN3O2: C, 63.07; H, 3.11; N, 12.98. Found: C, 63.11; H, 3.13; N, 12.95.
Antibacterial activity
Determination of minimum inhibitory concentration by serial dilution technique
The stock solutions of synthesized compounds were reconstituted with a minimum amount of dimethyl sulfoxide (DMSO). This solvent did not possess any antimicrobial activity of its own. Calculated volume of this stock solution were dispensed in a series of McCartney bottles previously containing calculated volume of sterile cooled molten nutrient agar media (40–45 °C) to prepare final volume of 30 ml each with dilutions of 5, 12.5, 25, 50, 100, 200 and 400 µg/ml. Then these molten media containing varying concentration of compounds were poured aseptically in pre sterilized petridishes (70 mm) to give sterile nutrient agar plates with varying dilution of the compounds. These plates were then kept in the refrigerator at 4 °C for 24 h to ensure uniform diffusion of compounds. Then these plates were dried at 37 °C before spot inoculations. One loopful culture (loop diameter: 6 mm) of an overnight grown bacterial strains suspension (105 CFU/ml) was added in each quadrant as marked by checkerboard technique. The spotted plates were incubated at 37 °C for 24 h in an incubator, and MIC values were obtained (Asamenew et al., 2011; Mazumder et al., 2004).
Determination of zones of inhibition of disc diffusion method
A 200 µg/ml solution of both synthesized compounds and ciprofloxacin (solvent: DMSO) were prepared in sterilized McCartney bottles. Sterile molten media plates were prepared and incubated at 25 °C for 24 h to check for the presence of any sort of contamination. Then each sterilized nutrient agar plates were flooded with liquid culture of bacterial strains and dried for 30 min at 25 °C. The sterile Whatman filter paper disc (4-mm diameter) was soaked in solution of synthesized compounds and placed in appropriate position of the plates marked as quadrant at the back of petridishes. All the flooded plates with corresponding paper discs soaked with solution of synthesized compounds were incubated at 25 °C for 24 h, and diameter of zone of inhibition was measured in mm. Similar procedure was adopted for ciprofloxacin, and corresponding zone diameters were measured and compared accordingly.
Anticancer activity
Treatment of tumour cell lines
All compounds were submitted to the National Cancer Institute, and three compounds were selected for anticancer screening on NCI 60 cell lines initially at a single high dose (10−5 M) on leukaemia (CCRF-CEM, HL-60 (TB), K-562, MOLT-4, RPMI-8226, SR), non-small cell lung cancer (A549/ATCC, HOP-62, HOP-92, NCI-H226, NCI-H23, NCI-H322M, NCI-H460, NCI-H522), colon cancer (COLO 205, HCC-2998, HCT-116, HCT-15, HT29, KM12, SW-620), CNS cancer (SF-268,SF-295,SF-539,SNB-19, SNB-75), melanoma (LOX IMVI, MALME-3M, M14, MDA-MB-435, SK-MEL-2, SK-MEL-28, SK-MEL-5, UACC-257, UACC-62), ovarian cancer (IGROV1, OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8 NCI/ADR-RES 99.24, SK-OV-3), renal cancer (786-0, A498, ACHN, CAKI-1, SN12C, TK-10, UO-31), prostate cancer (PC-3, DU-145) and breast cancer (MCF7, MDA-MB-231/ATCC, HS 578T, BT-549, T-47D, MDA-MB-468) cell lines, nearly 60 in number. The One-dose data were reported as a mean graph of the percent growth of treated cells. The number reported for the one-dose assay is growth relative to the no-drug control, and relative to the time zero number of cells. The anticancer screening was carried out as per the NCI US protocol reported elsewhere (http://dtp.nci.nih.gov; Monks et al., 1991; Boyd and Paull, 1995; Shoemaker, 2006). Using the seven absorbance measurements [time zero (Tz), control growth (C), and test growth in the presence of drug at the five concentration levels (Ti)], the percentage growth was calculated at each of the drug concentrations levels.
Percentage growth inhibition is calculated as
All values are statistically analysed and are expressed as mean with a range of growth for various cell lines.
Results and discussion
Synthesis
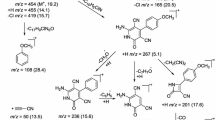
2-Chloroquinoline-3-carbaldehyde (2) was prepared from acetanilide via Vilsmeier–Haack approach. In Scheme 1, the 2-[2-(phenoxy/naphthalen-1-yl/naphthalen-2-yloxy methyl)-1H-benzimidazol-1-yl]acetohydrazide (6a–c) were prepared using o-phenylenediamine and the corresponding acid by a series of step (Salahuddin et al., 2014a). The phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1H-benzimidazol-1-yl)acetohydrazide (7a–c) were prepared using 2-chloroquinoline-3-carbaldehyde (2) and 2-[2-(phenoxy/naphthalen-1-yl/naphthalen-2-yloxymethyl)-1H-benzimidazol-yl]acetohydrazide (6a–c) in the presence of ethyl alcohol. The syntheses of 2-chloro-3-{5-[(2-phenoxy/naphthalene-1-yl/naphthalen-2-yloxy methyl-1-H-benzimidazol-1-yl)methyl]-1,3,4-oxadiazol-2-yl}quinolone (8a–c) were prepared using chloramine-T. In Scheme 2, the different hydrazide (10a–i) were synthesized using different aromatic acids (Jha et al., 2010). The (2-chloroquinoline-3-yl)methylidene]-substituted benzohydrazide (11a–i) were synthesized by reacting with 2-chloroquinoline-3-carbaldehyde (2) and the different hydrazide (10a–i). In the final step, 2-chloro-3-(5-substituted-phenyl-1,3,4-oxadiazol-2-yl)quinoline (12a–i) were synthesized with chloramine-T as the titled compounds. In general, the IR spectra of the compounds 7a, 7b and 7c showed absorption peaks of C=O at 1654, 1654 and 1667 and NH peaks at 3310, 3317 and 3307, respectively. 1H NMR spectra of the compounds 7a, 7b and 7c show singlet between δ5 and δ6 of CH2 and CH2O respectively. The Schiff bases (7a–c) explained the presence of –CONH and –N=CH from the presence of two singlet between δ8 and δ9. In IR spectra of the compounds 8a–c, disappearance of C=O and NH peaks indicates the formation of desired compounds. 1H NMR spectra of the compounds 8a–c also show two singlet between δ5 and δ6 of CH2 and CH2O respectively. In Scheme 2, compounds 11a–i show two absorption peaks of C=O and –NH in IR spectra, and these two peaks were absent in the compounds 12a–i confirm the formation of oxadiazole. The 13C NMR spectra of 1,3,4-oxadiazole (8a–c and 12a–i) shows two peaks between 165 and 168.
Antibacterial activity
It was observed that the maximum number of synthesized compounds (8a, 8c, 12a, 12b, 12c and 12h) was acting as magic bullet against various gram-positive strains of Bacillus cereus MTCC1305 as they were inhibited at very low concentration of the compounds. The compound 12c was as potent as pure ciprofloxacin against B. cereus MTCC1305. The synthesized compounds (12a, 12c and 12h) were also found to be extremely active against Klebsiella pneumonia NCTC7447, and hence they may be regarded as potent-promising agent for controlling pneumonia. It was also noted that 8c and 12f were very instrumental in inhibiting most of the pathogenic multidrug resistant of various gram-negative species including E. coli 35B, Vibrio cholera 765 and Proteus vulgaris AP169. Compounds 8b and 12b were having a MIC of 50 µg/ml against Acetobacter aceti AP586 and Mongonella morganii ATCC 25830 when tested. Shigella sonnei E08869, Pseudomonas putida MTCC 2252 and Shigella dysenteriae 9 found to show significant degree of resistance against majority of synthesized compounds; however, both the strains of S. dysenteriae 9 and S. sonnei E08869 were effectively controlled by the synthesized compound (12f) at a concentration as low as 50 µg/ml (Table 1). Thus, we observe from our study that these compounds have a fairly broad spectrum of antibacterial efficacy and hence may be regarded as a potent candidate to control the global thread of drug resistant in future.
The disc diffusion study reveals that the diameter of zone of inhibition was in coherence with the study of MIC conducted on various synthesized compounds against the selected microorganisms. It was observed that compound with lower MIC showed a great zone of inhibition against all the test microorganisms. Compounds (8a, 8c, 12a, 12b, 12c and 12h) was found to be most active against gram-positive bacteria particularly B. cereus MTCC1305 showing zone of inhibition 9.5–11 mm. Compounds 12a, 12c and 12h were found to be most active against K. pneumonia NCTC7447 and it shows zone of inhibition MIC of 12.5 µg/ml and maximum zone of inhibition 10.5, 10 and 11 mm, respectively (Table 2).
Anticancer activity (Salahuddin et al., 2014b)
The synthesized compounds displayed moderate to low activity in the in vitro screen on all tested cancer cell lines and are given in Table 3. The compounds 8a and 12d were found to be the most active compound of the series and much better growth interaction as compared to 12f against SNB-75. The compound 12d showed 95.70 growth percent (GP) and highly active on SNB-75 (CNS cancer), UO-31 and CAKI-1 (renal cancer) (GP = 53.35, 64.35, and 77.71, respectively), and the compound 8a showed 96.86 GP and highly active on SNB-75 (CNS cancer), UO-31 (renal cancer) and IGROV1 (ovarian cancer) with GP of 51.27, 67.58 and 77.85, while compound 12f showed maximum sensitivity on SNB-75 (CNS cancer) with GP of 60.89. The compounds 8a, 12d and 12f were found to be highly sensitive on SNB-75 and UO-31. The GP and percent growth inhibition (GI) of these compounds are shown in Fig. 3. The maximum percent growth inhibition was recorded for SNB-75 with GP of 51.27 and percent GI of 48.73.
Structure activity relationship (SAR)
On the basis of our findings, we could analyse the following points for antibacterial and anticancer activities:
-
The compounds having 1,3,4-oxadiazole carrying benzimidazole moiety have better antibacterial activity (8a and 8c) and anticancer activity (8a) than those of the compounds having only 1,3,4-oxadiazole moiety.
-
Groups like 2-phenoxy-methyl/2-naphthoxymethyl and naphthoxymethyl benzimidazole shown good antibacterial activity.
-
The antibacterial activity was improved due to the presence of electron withdrawing groups viz. 4-nitro, 3,5-dinitro and 4-chloro (12a, 12c and 12h)-substituted phenyl on 1,3,4-oxadiazole ring against B. cereus MTCC1305 and K. pneumonia NCTC7447.
-
The compound having electron-releasing substituent like 3,5-dimethoxy and nicotinyl (12d and 12f) has better anticancer activity, i.e. GP of the most sensitive cell lines.
Conclusion
Two series of 2,5-disubstitutedoxadiazole (8a–c and 12a–i) were synthesized successfully by the Schiff base (7a–c and 11a–i) with chloramine-T. Among the synthesized compound mainly 1,3,4-oxadiazole derivatives (8a, 8c, 12a, 12b, 12c and 12h) emerged as lead compounds, these compounds showed good antibacterial activity with MIC 12.5 and 25 µg/ml. The compounds 8a, 12d and 12f showed significant anticancer activity which can be further modified to exhibit better potency.
References
Ali MA, Shaharyar M (2007) Oxadiazole mannich bases: synthesis and antimycobacterial activity. Bioorg Med Chem Lett 17:3314–3316
Almasirad A, Tabatabai SA, Faizi M, Kebriaeezadeh A, Mehrabi N, Dalvandi A, Shafiee A (2004) Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem Lett 14:6057–6059
Amir M (2007) Synthesis of some 1,3,4-oxadiazole derivatives as potential anti-inflammatory agents. Indian J Chem B 46:1014–1019
Ansari KF, Lal C (2009) Synthesis and evaluation of some new benzimidazole derivatives as potential antimicrobial agents. Eur J Med Chem 44:2294–2299
Asamenew G, Bisrat D, Mazumder A, Asres K (2011) In-vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytother Res 25:1756–1760
Aziz MA, Abou-Rahama GEA, Hassan AA (2009) Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur J Med Chem 44:3480–3487
Bhandari SV, Bothara KG, Raut MK, Patil AA, Sarkate AP, Mokale VJ (2008) Design, synthesis and evaluation of anti-inflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as non ulcerogenic derivatives. Bioorg Med Chem 16:1822–1831
Boyd MR, Paull KD (1995) Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 34:91–109
Dabiri M, Salehi P, Baghbanzadeh M, Bahramnejad M (2006) A facile procedure for the one-pot synthesis of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett 47:6983–6986
Dewangan D, Pandey A, Sivakumar T, Rajavel R, Dubey RD (2010) Synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazole and its analgesic, anti-inflammatory, antibacterial and anti-tubercular activity. Int J Chemtech Res 3:1397–1412
Gilani SJ, Khan SA, Siddiqui N (2010) Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives of isoniazid. Bioorg Med Chem Lett 16:4762–4765
Holla BS, Poojary NK, Bhat KS, Ashok M, Poojary B (2005) Synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazole and its analgesic, anti-inflammatory, anti-bacterial and anti-tubercular activity. Indian J Chem B 44:1669–1673
http://dtp.nci.nih.gov. Accessed 22 Sep 2013
Isikdag I, Ozkay Y, Incesu Z (2011) Synthesis and anticancer activity of some bisquinoxaline derivatives. Turk J Pharm Sci 8:179–188
Jayashankar B, Lokanath KM, Baskaran N, Satish SH (2009) Synthesis and pharmacological evaluation of 1,3,4-oxadiazole bearing bis(heterocycle) derivatives as anti-inflammatory and analgesic agents. Eur J Med Chem 44:3898–3902
Jha KK, Samad A, Kumar Y, Shaharyar M, Khosa RL, Jain J, Kumar V, Singh P (2010) Design, synthesis and biological evaluation of 1,3,4-oxadiazole derivatives. Eur J Med Chem 45:4963–4967
Jin Z (2003) Muscarine, imidazole, oxazole, and thiazole alkaloids. Nat Prod Rep 20:584–605
Jin L, Chen J, Song B, Chen Z, Yang S, Li Q, Hu D, Ruiqing X (2006) Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg Med Chem Lett 16:5036–5040
Kumar H, Javed SA, Khan SA, Amir M (2008) 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: synthesis and preliminary evaluation of biological properties. Eur J Med Chem 43:2688–2698
Manjunatha K, Poojary B, Lobo PL, Fernandes J, Kumari NS (2010) Synthesis and biological evaluation of some 1,3,4-oxadiazole derivatives. Eur J Med Chem 45:5225–5233
Mazumder R, Dasidar SG, Basu SP, Mazumder A, Singh SK (2004) Antibacterial potentiality of Mesua ferrea Linn. flowers. Phytother Res 18:824–826
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro WA, Gray GM, Campbell H, Mayo J, Boyd M (1991) Feasibility of a high-flux anticancer drug screen utilizing a diverse panel of human tumor cell lines in culture. J Natl Cancer Inst 83:757–766
Mulwad VV, Chaskar AC (2006) Synthesis and antibacterial activity of new oxadiazolo[1,3,5]triazine, 1,2,4-triazolo and thiadiazolo[1,3,4]oxadiazole derivatives. Indian J Chem B 45:1710–1715
Ogata M, Atobe H, Kushida H, Yamamoto K (1971) In vitro sensitivity of mycoplasmas isolated from various animals and sewage of antibiotics and nitrofurans. J Antibiot 24:443–451
Salahuddin, Mazumder A, Shaharyar M (2014) Synthesis, characterization, and in vitro anticancer evaluation of novel 2,5-disubstituted 1,3,4-oxadiazole analogue. BioMed Res Int. Article ID 491492, p 14. http://dx.doi.org/10.1155/2014/491492
Salahuddin, Shaharyar M, Mazumder A, Ahsan MJ (2014b) Synthesis, characterization and anticancer evaluation of 2-(naphthalen-1-ylmethyl/naphthalen-2-yloxymethyl)-1-[5-(substituted phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-1H-benzimidazole. Arab J Chem 7:418–424
Schlecker R, Thieme PC (1988) The synthesis of antihypertensive 3-(1,3,4-oxadiazol-2-yl) phenoxypropanolamines. Tetrahedron 44:3289–3294
Sengupta P, Dash DK, Yeligar VC, Murgesh K, Rajalingam D, Singh J, Maity T (2008) Evaluation of anticancer activity of some 1,3,4-oxadiazole derivatives. Indian J Chem B 47:460–462
Shailaja M, Anitha M, Manjula A, Rao BV (2010) Synthesis and biological activity of novel 2,5-disubstituted-1,3,4-oxadiazoles. Indian J Chem B 49:1088–1097
Shetgiri NP, Nayak BK (2005) Synthesis and antimicrobial activities of oxadiazoles, phthalazines and indolinones. Indian J Chem B 44:1267–1272
Shoemaker RH (2006) The NCI-60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6:813–823
Shui LH, Zhao BX, Li JK, Xia Y, Lian S, Liu WY, Gong ZL (2010) The synthesis, characterization and optical properties of novel substituted pyrazoly 1,3,4-oxadiazole derivatives. Dyes Pigment 86:25–31
Somani RR, Shirodkar PY (2009) Oxadiazole: a biologically important heterocycle. Der Pharma Chemica 1:130–140
Srivatava A, Singh RM (2005) Vilsmeier-Haack reagent: a facile synthesis of 2-chloro-3-formylquinolines from N-arylacetamides and transformation into different functionalities. Indian J Chem B 44:1868–1875
Steigbigel RT, Cooper DA, Kumar PN (2008) Raltegravir with optimized background therapy for resistant HIV-1 infection. New Engl J Med 4:339–354
Vardan S, Sulyan H, Mookherjee S, Eich R (1983) Effects of tiodazosin, a new antihypertensive, thermodynamics and clinical variables. Clin Pharmacol Ther 34:290–296
Acknowledgments
The authors are so much grateful to Managing Director NIET for providing the research facilities and IIT Delhi for providing the spectral data. The authors also wish to express their thanks to all the staffs of National Cancer Institute, Bethesda, MD, USA for in vitro evaluation of anticancer activity. We would also like to thanks Dr Jawed Ahsan, Dr. G.S. Chakraborthy, Mr. Vikas Rathore, Ms. Sangita Kumari and Mr. Rajnish Kumar for the kind support for preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salahuddin, Mazumder, A. & Shaharyar, M. Synthesis, antibacterial and anticancer evaluation of 5-substituted (1,3,4-oxadiazol-2-yl)quinoline. Med Chem Res 24, 2514–2528 (2015). https://doi.org/10.1007/s00044-014-1308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1308-2