Abstract
Animals communicate with each other using a variety of signal modalities, any of which can provide useful information to non-intended receivers, or eavesdroppers. Eavesdropping on chemical signals is a widespread phenomenon but its role in shaping the behavior of multi-species assemblages is poorly known. Here, we tested the hypothesis that workers of multiple Neotropical ant species change their behaviors when exposed to odors of the common canopy ant, Azteca trigona. We exposed workers of 16 canopy ant species (five subfamilies) to A. trigona alarm pheromones and compared their behavioral responses to the behavior of ants in control treatments (ambient air). Seven species showed distinct responses to A. trigona odors relative to the control. The most common behavioral responses were increased antennation and running. The results of this study suggest that eavesdropping on heterospecific alarm signals allows ants to avoid generalized threats or negative interactions with aggressive A. trigona workers. Such eavesdropping presumably is selectively advantageous and may determine local arboreal ant species distributions and interspecific differences in access to resources in the forest canopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals use a variety of signaling modalities (e.g., visual, mechanical, chemical) to communicate vital information to nestmates, conspecifics, and heterospecifics (Endler 1992; Wyatt 2014). Chemical signaling is widespread in nature, in part, because pheromones and other chemical messages often are relatively inexpensive to produce, and can provide important contextual physiological and ecological information over long distances (Bossert and Wilson 1963; Symonds and Elgar 2008; Wyatt 2014). For insects, and especially social insects such as ants (Hymenoptera: Formicidae), chemical signaling is a ubiquitous part of life. Ants use a variety of compounds to organize individual behaviors and maintain colony structure (Hölldobler and Wilson 1990; Roitberg and Isman 1992). Ants also leverage volatile chemical signals as alarm pheromones in part because they are detectable over longer distances than are non-volatile chemicals (Blum 1969; Wyatt 2014; Leonhardt et al. 2016).
Alarm pheromones in some ant species occur as relatively conspicuous plumes of volatile compounds (Blum 1969; Attygalle and Morgan 1984; Keeling et al. 2004). These volatile chemical signals can be problematic because the strength and distribution of the message cannot be controlled once it is emitted (Bossert and Wilson 1963; Wyatt 2014). Such signals become freely available "public" information that is a reliable cue for exploitation by unintended receivers (i.e., eavesdroppers; Peake 2005) including ant-associated eavesdroppers, especially parasitoids and predators (Feener et al. 1996; Mathis et al. 2011; Cárdenas et al. 2012; reviewed by Adams et al. 2020). Indeed, chemical eavesdropping is common in diverse groups of animals and is well-studied in insects (Stowe et al. 1995; Wyatt 2014). However, to our knowledge, no studies have explored the possibility that co-occurring ants eavesdrop on heterospecific ant alarm pheromones.
Interspecific eavesdropping on alarm pheromones is likely to be advantageous for co-occurring competitive or antagonistic species. Given that ant workers are valuable to their colonies directly as biomass (Wilson 1968) and indirectly via foraging and defensive behaviors (Carroll and Janzen 1973), selection should favor eavesdropping on any warning signals that consistently prevent worker loss by inducing avoidance behaviors in submissive species. Eavesdropping on heterospecific alarm pheromones could provide such a mechanism because alarm pheromones are reliable indicators of nearby dangers, including negative interspecific interactions among competing ants, the presence of predators and parasitoids, or any generalized threat or disturbance (Blum 1969).
Azteca chartifex/trigona (Dolichoderinae) is a common Neotropical canopy ant species complex that has conspicuous nests (Fig. S1), large polydomous colonies, distinct alarm pheromones, and aggressive workers (Wheeler 1986; Adams 1994; Longino 2007). Hereafter, we refer to this species complex as A. trigona for simplicity. In some forests, A. trigona influences local ant community structure via territorial behaviors creating a mosaic of species distributions (Adams 1994). Alarm pheromones coordinate A. trigona defensive behaviors (Adams 1994), but the effects of A. trigona alarm pheromones on other ant species are unknown.
Chemical components of alarm pheromones frequently are conserved within genera or subfamilies (Blum 1969; Wheeler et al. 1975; Norman et al. 2017), thus eavesdropping species are likely to be closely related to the emitting species. Additionally, some behaviorally dominant ants competitively exclude other ant species that are ecologically or morphologically similar (Hölldobler and Wilson 1990; Andersen and Patel 1994; Adams 2016). If ants respond to the alarm pheromones of closely related heterospecific species, and if A. trigona similarly influences local species assemblages via competitive exclusion, we expect eavesdropping on A. trigona pheromones to be best developed in phylogenetically and behaviorally similar subordinate or codominant species. Our personal observations in the forests of Panama (e.g., Yanoviak and Kaspari 2000, Adams et al. 2017) indicate that most canopy ants avoid direct interactions with A. trigona workers while foraging. We also frequently observe strong negative behavioral responses (i.e., fleeing) by some arboreal species when presented with forceps contaminated with A. trigona alarm pheromones. These observations suggest that chemical cue recognition and eavesdropping on A. trigona are common among canopy ants.
The principal objective of this study was to determine if tropical canopy ants eavesdrop on the alarm pheromones of A. trigona ants. We asked if canopy ants change their behaviors when exposed to A. trigona alarm pheromones, how their behaviors change, and whether behavioral responses are more common in certain subfamilies. We predicted that differences in behavioral responses would be associated with ant subfamily identity, and species that are more phylogenetically similar to A. trigona would exhibit the greatest frequency of responses. We experimentally tested these predictions in Panama with freshly captured worker ants of 16 species.
Methods
This study was conducted on Barro Colorado Island (BCI) in Panama (9.15 °N, 79.85 °W) between July 2016 and October 2018. BCI is a seasonally moist lowland forest with a wet season spanning May to December. All data for this study were collected during wet season months. More details about the site are provided elsewhere (Croat 1978; Leigh et al. 1996).
All worker ants used in experiments were collected by hand or with forceps from tree trunks and branches on BCI. Workers of a given species were collected from multiple colonies, and ants collected from a given colony (usually < 5 workers) were housed together in a vial until the start of an experiment. All ants were used in experiments within 48 h of collection and were provided with water and honey during the holding period. The 16 focal ant species (Table 1) were chosen to maximize phylogenetic diversity and to include species that commonly co-occur in trees with Azteca trigona at the study site (Adams et al. 2017).
Alarm pheromone trials
To determine which ant species responded to A. trigona alarm pheromones and how they responded, we placed a single ant (hereafter recipient ant) in a small glass arena (25.4 × 76.2 × 76.2 mm; Figs. S2, S3) and allowed it to acclimate for 3–5 min before each trial. At the beginning of each trial, A. trigona volatile odors (hereafter alarm pheromones) were obtained by aggravating ca. 30 workers inside a plastic vial (i.e., by continuously shaking the vial for 5 s) and then drawing air from the vial into a 20 mL polypropylene syringe (Norm-Ject, Henke Sass Wolf GmbH, Germany). Twelve milliliters of air containing A. trigona alarm pheromones were then injected into the chamber via the syringe. Control treatments followed the same protocol using a new conspecific recipient ant worker for each trial and a clean syringe filled only with ambient air. All trials were conducted at temperatures ranging 26–30 °C.
We recorded recipient ant behavior for multiple individual workers of 16 different species (Table 1). In each case, ant behavior was recorded 5 s before and 5 s immediately after exposure to A. trigona alarm pheromones using the video function of a compact digital camera (Canon-PowerShot ELPH 180, Canon Inc., Japan). We observed the videos blindly (i.e., odor treatments were unknown to the viewer) and noted the following conspicuous changes in behavior of recipient ants: increased or decreased running speed, increased or decreased frequency of antennal movement, mandible flaring, and gaster flagging/tucking (i.e., positioning the gaster approximately orthogonal to the body axis either dorsally or ventrally; Curtis 1985; Obin and van der Meer 1985; Fig. 1). Changes in behavior were visually estimated. If individuals exhibited two or more of the behavior changes listed above, we recorded a behavioral change in response to the odor source. We similarly quantified the behavioral responses of recipient ants in control treatments.
Analyses
Analyses were performed in the R statistical environment (R Core Team 2019). We used Fisher’s Exact tests to determine if the frequency of behavioral responses to A. trigona alarm pheromones differed from controls. A separate test was conducted for each species. We used a mixed-effect generalized linear model to compare subfamily responses to A. trigona and control odor treatments (glmer, package lme4). Responses of A. trigona workers to A. trigona and control odors were excluded from this analysis. The response term was a binomial variable (behavioral response or no response), subfamily and odor treatment were fixed effects, and species was a random effect. Stepwise model reduction with likelihood ratio tests removed the non-significant interaction between subfamily and odor source. To compare subfamily responses to A. trigona alarm pheromones and control odors separately, we used a mixed-effect generalized linear model comparing subfamily responses using only recipient ants exposed to A. trigona alarm pheromones and a separate model with only recipient ants exposed to control odors. The response term was again a binomial variable, subfamily was the only fixed effect, and species was a random effect. Subfamily-level differences in behavioral responses were determined with post-hoc Tukey HSD tests.
Results
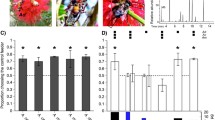
Seven of the 16 focal ant species responded more frequently to A. trigona odors than to ambient air (Fig. 2). Those seven species represented four distinct subfamilies; three were dolichoderines. Ants in all five focal subfamilies showed at least some behavioral response to A. trigona pheromones. Specifically, when worker responses were pooled at the subfamily level, response frequencies for A. trigona odors were consistently higher than for controls (χ2 = 68.82, df = 1, p < 0.001) and differed among subfamilies (χ2 = 13.61, df = 4, p = 0.009). However, only dolichoderines responded to A. trigona pheromones at a higher frequency than myrmicines and pseudomyrmecines (Table S1, Fig. S4). Response frequencies to the ambient air control also differed among subfamilies (χ2 = 15.53, df = 4, p = 0.004), with dolichoderines and formicines responding to control odors at a higher frequency than myrmicines and pseudomyrmecines (Table S1, Fig. S4).
The proportion (with ± 95% Clopper-Pearson CI) of trials in which workers of 16 recipient ant species exhibited behavioral responses when exposed to the alarm odors of Azteca trigona (black bars) and ambient air controls (gray bars). Ecta. = Ectatomminae. Significant differences between responses to A. trigona alarm pheromones and control ambient air are indicated as *p < 0.05, **p < 0.01, and ***p < 0.001
In all, 334 individual ants (out of 833) changed their behaviors when exposed to pheromones or ambient air. Half (53%) of the 444 ants that were exposed to A. trigona pheromones showed a behavioral response, whereas only 25% of the 389 ants exposed to control odors responded. Most (75%) of the responding ants exhibited a combination of altered running speed and increased antennating frequency (Table 2, A + R). The least common behavioral change was gaster flagging, although dolichoderines exhibited combined behaviors that included gaster flagging in 26% of responses (Table S2).
Discussion
Here we show that multiple Neotropical canopy ant species across a broad distribution of subfamilies change their behaviors when exposed to Azteca trigona alarm pheromones. Four of the five subfamilies tested included species that were both responsive and non-responsive to A. trigona pheromones (except for Ectatomminae, in which only one species was tested), suggesting that eavesdropping is a selective phenomenon among coexisting ant species that is not based on phylogenetic relatedness. Eavesdropping species occur across all domains of life (Stowe et al. 1995; Joint et al. 2007; Frost et al. 2008) and eavesdropping specifically on heterospecific alarm signals in animals is likely to be advantageous in many circumstances (Stowe et al. 1995; Adams et al. 2020). In this study, aggressive responses to A. trigona odors were uncommon (77% of responders exhibited what appeared to be flight behaviors—more frequent antennation and faster running speeds—which was similar to the 72% response frequency to control air including these behaviors). This suggests that avoidance of a potentially threatening species is the basis for eavesdropping behaviors among the focal ants. Such an "ecology of fear" occurs among many animal taxa (Pfeiffer 1962; Apfelbach et al. 2005; Goodale and Nieh 2012).
The results of this study do not support the prediction that species responding to A. trigona alarm pheromones would be closely related (i.e., dolichoderines). The major components of alarm pheromones that elicit worker responses are often specific within ant subfamilies or genera (Blum 1969; Norman et al. 2017). For example, among ants, cyclopentanoid monoterpenes and sulcatone (6-methyl-5-hepten-2-one) apparently occur only in Azteca alarm pheromones (Wheeler et al. 1975; McCann et al. 2013). These compounds elicit sustained alarm responses in Azteca workers (Blum 1969; Wheeler et al. 1975; McCann et al. 2013). Ants often respond to pheromones only with a specific compound ratio (Blum 1969; Pokorny et al. 2020), suggesting that species that do not respond more to A. trigona odors either do not detect the odors of A. trigona, or ignore them. Additional research is needed to isolate the specific compound or compounds within the A. trigona alarm pheromone that elicit behavioral responses from heterospecifics.
Given the lack of strong phylogenetic signal, it is likely that the observed ant responses to A. trigona alarm pheromones reflect ecological pressures. It is potentially advantageous for foraging workers (regardless of their identity) to recognize the presence of potential heterospecific competitors, or to detect the existence of a nearby disturbance that is provoking an alarm response by one or more species. Indeed, in a related pilot study, we found that a subset of the species used in this study also responded to alarm pheromones from ants other than A. trigona, although with different frequencies. Thus, we hypothesize that the species responding to A. trigona alarm pheromones are those that are more likely to have negative interactions with A. trigona workers (i.e., competing species). For example, A. trigona tend to have non-overlapping foraging territories with the responding species Atta colombica and Ectatomma tuberculatum (Jutsum et al. 1981; Armbrecht et al. 2001). We also cannot exclude the possibility that co-occurring species eavesdrop on other A. trigona cues, such as trail pheromones or chemical footprints, similar to the trail parasites Cephalotes maculatus and Camponotus beebei (Wilson 1965; Adams 1990; Wüst and Menzel 2017). Ultimately, additional field-based studies and natural history observations are needed to clarify links between pheromone eavesdropping and foraging decisions among potentially competing ants (Adams et al. 2020).
The results of this study are consistent with observations of eavesdropping in a variety of non-ant systems (Pfeiffer 1962; Goodale and Nieh 2012). However, the specific compounds that elicit responses, and the ecological consequences of responding to A. trigona alarm pheromones (e.g., potential loss of access to food resources) remain unknown. Understanding such patterns will clarify the role of chemical eavesdropping on species interactions, foraging behavior, and community structure in arboreal ants.
Availability of data and material
Raw data for this project are available on FigShare with the following https://doi.org/10.25422/azu.data.13963886.
Code availability
Not applicable.
References
Adams ES (1990) Interaction between the ants Zacryptocerus maculatus and Azteca trigona: interspecific parasitization of information. Biotropica 22:200–206. https://doi.org/10.2307/2388413
Adams ES (1994) Territory defense by the ant Azteca trigona: maintenance of an arboreal ant mosaic. Oecologia 97:202–208. https://doi.org/10.1007/BF00323150
Adams ES (2016) Territoriality in ants (Hymenoptera: Formicidae): a review. Myrmecological News 23:101–118. https://doi.org/10.1016/0095-8956(72)90015-9
Adams BJ, Schnitzer SA, Yanoviak SP (2017) Trees as islands: canopy ant species richness increases with the size of liana-free trees in a Neotropical forest. Ecography 40:1067–1075. https://doi.org/10.1111/ecog.02608
Adams RMM, Wells RL, Yanoviak SP et al (2020) Interspecific eavesdropping on ant chemical communication. Front Ecol Evol 8:24. https://doi.org/10.3389/fevo.2020.00024
Andersen AN, Patel AD (1994) Meat ants as dominant members of Australian ant communities: an experimental test of their influence on the foraging success and forager abundance of other species. Oecologia 98:15–24. https://doi.org/10.1007/BF00326085
Apfelbach R, Blanchard CD, Blanchard RJ et al (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144. https://doi.org/10.1016/j.neubiorev.2005.05.005
Armbrecht I, Jiménez E, Alvarez G et al (2001) An ant mosaic in the Colombian rain forest of Chocó (Hymenoptera: Formicidae). Sociobiology 37:491–509
Attygalle AB, Morgan ED (1984) Chemicals from the glands of ants. The Royal Society of Chemistry London 13:245–278. https://doi.org/10.1039/CS9841300245
Blum MS (1969) Alarm Pheromones. Annu Rev Entomol 14:57–80. https://doi.org/10.1146/annurev.en.14.010169.000421
Bossert WH, Wilson EO (1963) The analysis of olfactory communication among animals. J Theor Biol 5:443–469. https://doi.org/10.1016/0022-5193(63)90089-4
Cárdenas M, Jiroš P, Pekár S (2012) Selective olfactory attention of a specialised predator to intraspecific chemical signals of its prey. Naturwissenschaften 99:597–605. https://doi.org/10.1007/s00114-012-0938-9
Carroll CR, Janzen DH (1973) Ecology of foraging by ants. Annu Rev Ecol Syst 4:231–257. https://doi.org/10.1146/annurev.es.04.110173.001311
Croat TB (1978) Flora of Barro Colorado Island. Stanford University Press, Stanford
Curtis BA (1985) Observations on the natural history and behaviour of the dune ant, Camponotus detritus Emery, in the central Namib Desert. Modoqua 3:279–289
Endler JA (1992) Signals, signal conditions, and the direction of evolution. Am Nat 139:S125–S153. https://doi.org/10.1086/285308
Feener DH, Jacobs LF, Schmidt JO (1996) Specialized parasitoid attracted to a pheromone of ants. Anim Behav 51:61–66. https://doi.org/10.1006/anbe.1996.0005
Frost CJ, Mescher MC, Carlson JE, de Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824. https://doi.org/10.1104/pp.107.113027
Goodale E, Nieh JC (2012) Public use of olfactory information associated with predation in two species of social bees. Anim Behav 84:919–924. https://doi.org/10.1016/j.anbehav.2012.07.016
Hölldobler B, Wilson EO (1990) The ants. Springer, Berlin
Joint I, Downie JA, Williams P (2007) Bacterial conversations: talking, listening and eavesdropping. An introduction. Phil Trans R Soc B 362:1115–1117. https://doi.org/10.1098/rstb.2007.2038
Jutsum AR, Cherrett JM, Fisher M (1981) Interactions between the fauna of citrus trees in Trinidad and the ants Atta cephalotes and Azteca sp. J Appl Ecol 18:187–195. https://doi.org/10.2307/2402488
Keeling CI, Plettner E, Slessor KN (2004) Hymenopteran semiochemicals. Top Curr Chem 239:133–177. https://doi.org/10.1007/b95452
Leigh EG, Rand AS, Windsor DM (1996) The ecology of a tropical rain forest: seasonal rhythms and long term changes. Smithsonian Press, Washington D.C.
Leonhardt SD, Menzel F, Nehring V, Schmitt T (2016) Ecology and evolution of communication in social insects. Cell 164:1277–1287. https://doi.org/10.1016/j.cell.2016.01.035
Longino JT (2007) A taxonomic review of the genus Azteca (Hymenoptera: Formicidae) in Costa Rica and a global revision of the aurita group. Zootaxa 1491:1–63
Mathis KA, Philpott SM, Moreira RF (2011) Parasite lost: chemical and visual cues used by Pseudacteon in search of Azteca instabilis. J Insect Behav 24:186–199. https://doi.org/10.1007/s10905-010-9247-3
McCann S, Moeri O, Jones T et al (2013) Strike fast, strike hard: the red-throated caracara exploits absconding behavior of social wasps during nest predation. PLoS ONE 8:e84114. https://doi.org/10.1371/journal.pone.0084114
Norman VC, Butterfield T, Drijfhout F et al (2017) Alarm pheromone composition and behavioral activity in fungus-growing ants. J Chem Ecol 43:225–235. https://doi.org/10.1007/s10886-017-0821-4
Obin MS, vander Meer RK, (1985) Gaster flagging by fire ants (Solenopsis spp.): functional significance of venom dispersal behavior. J Chem Ecol 11:1757–1768. https://doi.org/10.1007/BF01012125
Peake TM (2005) Eavesdropping in communication networks. In: McGregor PK (ed) Animal communication networks. Cambridge University Press, Cambridge
Pfeiffer W (1962) The fright reaction of fish. Biol Rev Camb Philos Soc 37:495–511. https://doi.org/10.1111/j.1469-185X.1962.tb01333.x
Pokorny T, Sieber LM, Hofferberth JE, et al (2020) Age-dependent release of and response to alarm pheromone in a Ponerine ant. Journal of Experimental Biology 223:jeb218040. https://doi.org/10.1242/jeb.218040
Roitberg BD, Isman MB (1992) Insect chemical ecology: an evolutionary approach. Chapman and Hall, New York
Stowe MK, Turlings TCJ, Loughrin JH et al (1995) The chemistry of eavesdropping, alarm, and deceit. Proc Natl Acad Sci USA 92:23–28. https://doi.org/10.1073/pnas.92.1.23
Symonds MRE, Elgar MA (2008) The evolution of pheromone diversity. Trends Ecol Evol 23:220–228. https://doi.org/10.1016/j.tree.2007.11.009
Wheeler DE (1986) Polymorphism and division of labor in Azteca chartifex laticeps (Hymenoptera: Formicidae). J Kansas Entomol Soc 59:542–548
Wheeler JW, Evans SL, Blum MS, Torgerson RL (1975) Cyclopentyl ketones: identification and function in Azteca ants. Science 187:254–255. https://doi.org/10.1126/science.1111099
Wilson EO (1965) Trail sharing in ants. Psyche 72:2–7. https://doi.org/10.1155/1965/24875
Wilson EO (1968) The ergonomics of caste in the social insects. Am Nat 102:41–66. https://doi.org/10.1086/282522
Wüst M, Menzel F (2017) I smell where you walked—how chemical cues influence movement decisions in ants. Oikos 126:149–160. https://doi.org/10.1111/oik.03332
Wyatt TD (2014) Pheromones and animal behavior: chemical signals and signatures, 2nd edn. Cambridge University Press, Cambridge
Yanoviak SP, Kaspari M (2000) Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89:259–266. https://doi.org/10.1034/j.1600-0706.2000.890206.x
Acknowledgements
Noah Gripshover and Aspen Workman assisted in the lab; Benjamin Adams, Jelena Bujan, and Daniella Prince assisted in the field; Evan Gora provided statistical advice. Comments from Benjamin Adams, Natalie Christian, Perri Eason, Evan Gora, Kane Lawhorn, Daniella Prince, and Jeannine Richards improved the manuscript. We thank Melissa Cano and the staff of the Smithsonian Tropical Research Institute for logistical support in Panama. This work was supported by the National Science Foundation grants GRF-2018265609 to RLW, IOS-1656625 and IOS-2101059 to CJF, and DEB-1252614 to SPY.
Funding
This work was supported by the National Science Foundation grants GRF-2018265609 to RLW, IOS-1656625 and IOS-2101059 to CJF, and DEB-1252614 to SPY.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analyses were performed by RLW. All authors contributed to the writing, and read and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
None.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wells, R.L., Frost, C.J. & Yanoviak, S.P. Effects of Azteca trigona alarm pheromones on heterospecific ant behavior. Insect. Soc. 68, 359–365 (2021). https://doi.org/10.1007/s00040-021-00836-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-021-00836-2