Abstract
The honey bee Apis mellifera is a model organism for studying learning and memory in insects. Although much work has been done to study olfactory learning using the proboscis extension reflex paradigm, we currently lack a high throughput method to study spatial learning and memory under controlled conditions. Here we outline a new paradigm to study spatial learning and memory in honey bees based on a food search task adopted from the vertebrate literature. After establishing that honey bees are able to learn and recall the location of artificial flowers inside the testing arena, we used the procedure to compare the performance of young nurse bees, who stay in the colony and nurse the brood, and old forager bees who forage outside. We also compared the spatial learning and memory of age-staged bees that were experimentally treated with cyclic guanosine monophosphate (cGMP), which causes precocious foraging, and cyclic adenosine monophosphate (cAMP), which does not alter behavioural state. Although foragers and nurses from typical colonies had similar learning curves, we found that foragers were significantly more accurate when recalling the location of previously rewarding artificial flowers inside the testing arena. This effect was also observed when comparing age-staged bees that have been chronically exposed to cGMP vs. those chronically exposed to cAMP. The Food Search Box paradigm is a simple and effective way to study spatial learning and memory in honey bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The honey bee, Apis mellifera, has served as a model organism for the study of learning and memory in insects. Honey bees are generalists and must learn to forage and manipulate different plants that vary in their resource availability and quality over short (i.e., hours), intermediate (i.e., days to weeks), and long (i.e., different seasons) timescales (von Frisch 1967). Honey bees explore their surroundings to find profitable flowers, develop spatial maps (Menzel et al. 2005), and communicate the spatial information to nestmates via the waggle dance (von Frisch 1967). These life history traits likely contributed to the development of the honey bee’s remarkable capacity for learning.
There is an extensive body of literature on the genetics and molecular biology of associative olfactory learning in honey bees that is mostly derived from the proboscis extension reflex (PER) paradigm (Giurfa 2007; Menzel 1990, 2001). In olfactory PER, an odour is presented to a harnessed honey bee in tandem with sugar water, which she drinks by extending her proboscis. After several such pairings, the honey bee extends her proboscis to the odour alone, without the sugar, which indicates that the bee has learned to associate the odour with the sugar reward. The PER paradigm allowed biologists to dissect the genetic, molecular, and neuronal basis of olfactory learning in honey bees (Menzel and Muller 1996). In comparison to olfactory learning, we know little about the genetics and molecular biology of the honey bee’s spatial learning and memory. Spatial learning and memory is often studied using displacement experiments (Dyer et al. 1993; Menzel et al. 1998, 2000, 2005), where a honey bee is taken from its hive or usual feeding location, displaced, and then tracked to see if she will successfully return to either the hive or the feeding location. Such studies have revealed that honey bees can navigate using specialized route memory and general landscape memory (Menzel et al. 2000), and that they are able to integrate different vector memories to take novel routes (Menzel et al. 1998). Other test paradigms, like mazes, have also been used to study spatial learning and memory in bees. These studies revealed that honey bees are able to navigate complex mazes (Zhang et al. 1996), and that they can use symbolic (Zhang et al. 1998b), olfactory (Srinivasan et al. 1998), and colour (Zhang et al. 1998a) cues for spatial orientation. These studies have demonstrated that honey bees have a great capacity to learn and remember spatial information. Unfortunately, these types of behavioural tests take a long time to perform and are difficult to standardize, rendering them impractical for genetic and molecular studies that require large sample sizes. Moreover, the previous paradigms cannot study the acquisition and recall of spatial information as separate processes. Further, the existing paradigms require free-flying bees, which precludes studies of the ontogeny of spatial learning that is associated with the behavioural maturation of worker bees from in-hive tasks to outside tasks. Clearly, the development of a fast and standardized behavioural test would greatly facilitate research on spatial learning in bees.
In vertebrates, the food search task is often used to test spatial learning and memory (Chauvin and Thierry 2005; Clayton and Krebs 1994; MacDonald and Wilkie 1990; Mench and Andrew 1986; Oades and Isaacson 1978). The task usually involves several vessels distributed in space, where only one or a few contain food consistently. The animal is given several training trials to learn the location of a food reward before being tested by removing the food and observing the search pattern of the animal.
Here, we present a new procedure to study spatial learning in honey bees that is modelled after the vertebrate food search task. We developed the Food Search Box (FSB) paradigm, which allows for rapid analysis of spatial learning and memory while permitting researchers to manipulate the age, behavioural state, and experience of individual bees. The procedure involves three training trials, whereby a bee is presented with four different artificial flowers, one of which has a sugar reward. The location of the rewarding flower does not change during these trials. The training trials are followed by a memory test involving four non-rewarding artificial flowers. Learning and memory is evidenced by a bee non-randomly visiting the focal flower, which contained a reward in the training trials, but not in the memory trial.
After we confirmed that honey bee workers subjected to the FSB procedure exhibited learning and recollection, we used the FSB to study the learning performance of nurse bees (typically young workers), who feed the brood, and foragers (typically old bees), who forage for nectar and pollen for the colony. Finally, we studied the effect of chronic oral treatment of age-staged honey bees with 8-Br-cyclic guanosine monophosphate (a membrane permeable analog of cGMP) and cyclic adenosine monophosphate (cAMP). Ben-Shahar et al. (2002) showed that chronic oral treatment of honey bees with 8-BR-cGMP elevated the cGMP-dependent protein kinase (PKG) and caused precocious foraging in young bees, while cAMP treatments did not. The cGMP/cAMP treatment experiments thereby allow us to partially disentangle differences in spatial learning and memory caused by age (i.e., young vs. old), versus those caused by behavioural state (i.e., nurse vs. forager).
Method
Experimental subjects
We used bee colonies located at York University’s Research Apiary (Toronto, Ontario, Canada). The colonies, like most bees found in North America, have a mixed genetic ancestry with major contributions from the East European population group (C group: A. m. ligustica and A. m. carnica) and minor contributions from the West European population group (M group: A. m. mellifera) (Harpur et al. 2012, 2013, 2015).
Testing arena
Artificial flowers were placed upright in a row in front of a 1 cm diameter entrance in a 908 mL plastic container (12.5 cm × 12.5 cm × 6.5 cm) with ventilation holes and a removable lid. The artificial flowers were made out of cotton swabs, cut in half (4 cm long) and were supported by red plasticine 1.5 cm in diameter. Artificial flowers were placed 2.5 cm apart and 2.5 cm away from the right and left wall, 4 cm away from the back wall and 8.5 cm from the entrance (Fig. 1). The procedure was performed in a room without windows and with florescent lighting.
Experiment 1: establishing the FSB paradigm
We collected foragers returning to the colony with pollen on their legs and kept them overnight at 33 °C with 30% sucrose (Sigma) and pollen supplement (Bee-Pro Patties, Mann Lake LTD) in excess. The bees were then tested on the following day (N = 39).
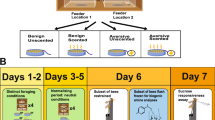
First, we tested whether the workers were motivated to learn by placing a bee in an individual petri dish with air holes containing a drop of 1 µL dyed (methyl blue) 30% sucrose solution. Only the bees which consumed the sucrose solution were considered motivated and were tested. At the onset of training each bee, we randomly picked a focal flower, whose cotton tip was saturated with a 30% sucrose solution for the first three training trials. A bee was then placed inside the testing arena and we measured the time elapsed between her introduction and her visit to the focal flower, hereafter called the discovery time. We also recorded the number of visits she made to the other flowers (i.e., errors). Repeated visits to the same flower were not recorded as additional errors. After she successfully found the focal flower and fed for 10 s, she was removed and allowed to rest in the petri dish for 20 min. Bees that landed on the focal flower and did not feed were removed from the experiment, which represented less than 1% of the bees tested. The procedure was repeated for a total of three training trials, where the focal flower was always in the same location for any given bee. We then conducted the memory test, where the focal flower did not contain a sucrose reward (Fig. 2). We wiped the testing arena with 70% ethanol and replaced all the plasticine and artificial flowers between trials to remove any scent trails.
An overview of the Food Search Box testing procedure. Before testing a bee, we randomly picked a ‘focal’ flower (grey circle), which receives a sucrose reward in the first three training trials. The training trial ends when the bee visits the focal flower and feeds for 10 s. After the training trials, we subjected the bee to a memory test where the focal flower did not contain a sucrose reward. During all of the trials, visits to the non-focal flowers (white) are scored as errors (X) and the time to discover the focal flower is recorded
Experiment 2: influence of worker behavioural state
Nurses were collected from a field colony with a naturally mated queen. Honey bees that inserted their heads into a cell containing larvae were considered nurses. After collection, they were placed overnight in separate boxes, identical to the testing arena, but without the artificial flowers. The bees were provided 30% sucrose and pollen supplements in excess. We tested the nurses (N = 40) the following day using the same procedure described in Experiment 1. We compared the performance of nurse bees to that of foragers (N = 39) from Experiment 1—both were collected from the same colony at the same time.
Experiment 3: effect of cGMP and cAMP treatments
We collected newly emerged bees from a sealed brood frame stored in a 33 °C incubator, housed them as in Experiment 1, and randomly allocated them into 3 treatment groups. Following Ben-Shahar et al. (2002), the cGMP group (N = 38) received 500 µM 8-Br-cGMP dissolved in a 30% sucrose solution for 5 days, the cAMP group (N = 38) received 1000 µM of 8-Br-cAMP dissolved in a 30% sucrose solution for 5 days, while the control (N = 81) received a 30% sucrose solution without any additives. These concentrations are sufficient to elicit an increase in cGMP-dependent kinase activity and cAMP dependent kinase activity within the bee head (Ben-Shahar et al. 2002). The 6 day old bees from all three groups were tested using the same procedure described in Experiment 1.
Statistics
Statistical analyses were performed using R (version 3.1.1) (Team 2005) with the package Analysis of Overdispersed Data (aod). We used the R package sciplot to generate the figures. The time data in Experiment 1 was normalized using a log transformation. We used non-parametric tests to analyze data from Experiments 2 and 3.
Results
Experiment 1: establishing the FSB paradigm
Honey bees introduced to the spatial learning arena exhibited learning and retention of information. The discovery time significantly declined from trial 1 to all subsequent trials and the memory test (Fig. 3a; Within Subject ANOVA, Tukey–Kramer adjustment, df = 114, F = 12.21, p < 0.0001). The performance of bees in trials 2, 3 and the memory test did not statistically differ with respect to discovery time (Within Subject ANOVA, Tukey–Kramer adjustment, p > 0.05). The location of the focal flower did not affect discovery time (Kruskal–Wallis, p > 0.05 for all). The average number of errors a bee can make at random is 1.5, \(\left( {{\text{i}}.{\text{e}}.,\,\frac{{0+1+2+3}}{4}} \right)\). We found that the number of errors the bees made is not statistically different from chance in trial 1 (Wilcoxon signed rank test with continuity correction, p-adj method = holm, V = 265.5, p = 0.074), but were statistically different in all subsequent trials and the memory test (Fig. 3b; trial 2 V = 111, p < 0.001; trial 3 V = 149.5, p = 0.002; memory test V = 148.5, p = 0.002). The location of the focal flower did not affect the number of errors the bees made (Kruskal–Wallis, p > 0.05 for all). The rapid and non-random visits to focal flowers in the memory test indicate that workers are able to recall spatial information acquired from the three learning trials.
Foragers exhibit learning and memory in the FSB. a The time it took foragers to find the focal flower is statistically greater in the first trial than in trials 2, 3 and the memory test (Within Subject ANOVA, Tukey–Kramer adjustment, df = 114, F = 12.21, p < 0.0001). b The number of errors the bees make on average is statistically different from chance in all instances, except trial 1 (Wilcoxon signed rank test with continuity correction, p-adj method = holm, trial 1 V = 265.5, p = 0.074; trial 2 V = 111, p < 0.001; trial 3 V = 149.5, p = 0.002; memory test V = 148.5, p = 0.002)
Experiment 2: influence of worker behavioural state
Nurses and foragers from typical colonies exhibited similar learning curves over the course of the three training trials (Fig. 4a; trial 1 Kruskal–Wallis chi-squared = 0.364, df = 1, p value = 0.546; trial 2 Kruskal–Wallis chi-squared = 1.408, df = 1, p value = 0.235; trial 3 Kruskal–Wallis chi-squared = 0.070, df = 1, p value = 0.791). They also did not differ in the number of errors made during these trials (Fig. 4b; trial 1 Kruskal–Wallis chi-squared = 0.206, df = 1, p value = 0.650; trial 2 Kruskal–Wallis chi-squared = 0.002, df = 1, p value = 0.965; trial 3 Kruskal–Wallis chi-squared = 2.626, df = 1, p value = 0.105). However, during the memory test, foragers found the focal flower significantly faster than nurses (Kruskal–Wallis chi-squared = 6.083, df = 1, p value = 0.014) and made fewer errors (Kruskal–Wallis chi-squared = 4.497, df = 1 p = 0.034).
Foragers recall spatial information more accurately relative to nurses. a The learning curves for foragers and nurses are similar, but foragers found the focal flower significantly faster in the memory test (p = 0.014). b Foragers made a similar number of errors to nurses during the training trials, but made significantly fewer errors in the memory test (p = 0.034)
Experiment 3: effect of cGMP and cAMP treatments
Age-staged workers that were chronically treated with cGMP and cAMP exhibited similar learning curves over the course of the three training trials (Fig. 5a; Pairwise comparisons using Wilcoxon rank sum test, p > 0.05). However, in the memory test, cGMP-treated bees took significantly less time to visit the focal flower relative to the control treatment (Pairwise Wilcoxon rank sum test, p = 0.027). cGMP-treated bees were also marginally faster at visiting the focal flower relative to cAMP-treated bees (Pairwise Wilcoxon rank sum test, p = 0.064). Further, cGMP-treated bees made significantly fewer errors in the memory test relative to both the control and cAMP-treated groups (Fig. 5b; cGMP v Control p = 0.001, cGMP v cAMP p < 0.001). Controls and cAMP-treated bees did not differ from each other during the three learning trials and the memory test with respect to discovery time (Pairwise Wilcoxon rank sum test p > 0.05) and errors made (Pairwise Wilcoxon rank sum test p > 0.05).
cGMP-treated bees resemble typical foragers in the FSB. a The learning curves for cGMP-treated, cAMP-treated, and control bees are similar, but cGMP-treated bees found the focal flower significantly faster in the memory test than controls (p = 0.027). b cGMP-treated, cAMP-treated, and control bees made a similar number of errors during the training trials, but cGMP-treated bees made significantly fewer errors in the memory test relative to cAMP-treated and control bees (p < 0.001)
Discussion
The FSB procedure has several advantages over the traditional ways for testing spatial learning and memory in honey bees. For example, many bees can be tested within a short time, and the entire FSB procedure takes about 1.5 h, while mazes take anywhere from 5 to 20 h for training (Zhang et al. 1996, 1999, 2000) and displacement studies usually take days (Menzel et al. 1998, 2005). The training duration of the maze and the displacement studies often lead to low samples sizes. For example, 61 bees were tested by Menzel et al. (2000), 59 by Zhang et al. (1996), while 236 bees were tested by us using the FSB. Another advantage of the FSB is that the procedure is conducted within the lab, allowing researchers to control many variables that influence bee behaviour and flight activity in the field. Displacement studies are often limited by weather and resource availability (Menzel et al. 1998, 2005), while maze studies require a flight room (Zhang et al. 1996, 1999, 2000). An additional benefit of the FSB is it allows researchers to study the ontogeny of spatial learning and memory by examining bees with different behavioural roles and ages—experiments that were virtually impossible to carry out using the old paradigms that relied on testing free-flying bees.
Honey bee workers clearly exhibited signs of learning and memory in the FSB paradigm. In Experiment 1, we found that the workers were able to discover the focal flower faster and were better at avoiding non-rewarding flowers over the course of training. On average, the largest improvement in a worker’s performance in the FSB occurred between the first and second training trials, suggesting that most bees are able to associate the location of the focal flower with the reward after a single training session. After three training trials, we asked if trained bees were conditioned to visit the focal flower in the absence of a reward. In the memory test, the bees in Experiment 1 visited the focal flower much faster than the first training trial and made fewer errors than expected by chance, which is indicative of learning and memory. The memory trial rules out the possibility that the honey bees were simply visiting ‘wet’ artificial flowers in the testing arena. While ‘wetness’ of the focal artificial flower may have offered an additional cue for learning, all artificial flowers were dry during the memory test.
After demonstrating that bees can learn and recall spatial information in the FSB paradigm, we examined if nurses and foragers have different capacities for learning and recalling spatial information. In typical colonies, nurse bees specialize on feeding the brood and do not engage in tasks outside of the colony. Foragers, on the other hand, venture outside the colony to collect pollen and nectar from flowers that can be up to 5 km away from the colony (Winston 1991). If the behavioural repertoire of nurses and foragers are associated with different capacities for spatial learning and memory, then we may expect foragers to be better at learning and recalling spatial information within the FSB paradigm. Our data showed that foragers and nurses have similar learning curves, but that foragers significantly outperform nurses when recalling spatial information.
Differences between the performance of nurses and foragers in the FSB memory test may reflect differences in age between these two behavioural roles in typical colonies (i.e., young vs. old, respectively), or it may stem from differences in their behavioural states. Foragers and nurses differ in many aspects that can influence their spatial learning abilities. Forgers have a substantially different pattern of brain gene expression relative to nurses (Zayed and Robinson 2012), and some of the differentially expressed genes are known to be invovled in learning and memory, inlcuding genes within the Notch pathway (Zayed et al. 2012). Foragers also exhibit an increase in the volume of their mushroom bodies relative to nurses (Durst et al. 1994; Withers et al. 1993), an area of the insect brain thought to be responsible for learning and memory. Additionally, foragers have higher sucrose responsiveness than nurses, which is known to influence olfactory learning (Scheiner et al. 2004).
One way to disentangle the effects of age and behavioural state, which are confounded when studying workers from typical colonies, is to compare the learning performance of bees of similar age but different behavioural states. There are several ways to generate precocious foragers (young foragers) and overage nurses (old nurses) by manipulating colony conditions or manipulating individual bees using pharmacology. It was previously shown that young bees treated with cGMP, which elevates the cGMP-dependent protein kinase (PKG), leads to precocious foraging (Ben-Shahar et al. 2002), but does not increase sucrose responsiveness (Ben-Shahar et al. 2004). Precocious foragers have a ‘forager’ neurogenomic state even in the absence of any foraging experience (Whitfield et al. 2006). As such, we used cGMP-treatments to manipulate the behavioural state of individual honey bees that have the same age. We found that cGMP-treated young bees were virtually identical to typical foragers in the FSB paradigm. cGMP-treated bees had similar learning curves relative to cAMP-treated bees and controls, but they were quicker and made fewer errors in the memory test.
Taken together, Experiment 2 and Experiment 3 suggest that, although workers from both behavioural roles learn at the same rate, foragers are better able to recall spatial information over short-timescales (20 min) relative to nurses. More work is needed to understand the molecular and neurobiological basis of this intriguing result. We believe that the FSB paradigm will be instrumental in dissecting the honey bee’s amazing capacity to learn and recall spatial information and could potentially be extended to other insects.
References
Ben-Shahar Y, Dudek NL, Robinson GE (2004) Phenotypic deconstruction reveals involvement of manganese transporter malvolio in honey bee division of labor. J Exp Biol 207:3281–3288
Ben-Shahar Y, Robichon A, Sokolowski M, Robinson G (2002) Influence of gene action across different time scales on behavior. Science 296:741–744
Chauvin C, Thierry B (2005) Tonkean macaques orient their food search from olfactory cues conveyed by conspecifics. Ethology 111:301–310
Clayton N, Krebs J (1994) Memory for spatial and object-specific cues in food-storing and non-storing birds. J Comp Physiol A 174:371–379
Durst C, Eichmüller S, Menzel R (1994) Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behav Neural Biol 62:259–263
Dyer FC, Berry NA, Richard AS (1993) Honey bee spatial memory: use of route-based memories after displacement. Anim Behav 45:1028–1030
Giurfa M (2007) Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J Comp Physiol A 193:801–824
Harpur B et al (2015) Assessing patterns of admixture and ancestry in Canadian honey bees. Insectes Soc 62:479–489
Harpur BA, Minaei S, Kent CF, Zayed A (2012) Management increases genetic diversity of honey bees via admixture. Mol Ecol 21:4414–4421
Harpur BA, Minaei S, Kent CF, Zayed A (2013) Admixture increases diversity in managed honey bees: reply to De la Rúa et al. (2013). Mol Ecol 22:3211–3215
MacDonald SE, Wilkie DM (1990) Yellow-nosed monkeys’ (Cercopithecus ascanius whitesidei) spatial memory in a simulated foraging environment. J Comp Psychol 104:382
Mench J, Andrew R (1986) Lateralization of a food search task in the domestic chick. Behav Neural Biol 46:107–114
Menzel R (1990) Learning, memory, and “cognition” in honey bees. In: Kesner RP, Olton DS (eds) Neurobiology of Comparative Cognition. Lawrence Erlbaum Associates, Hillsdale, pp 237–292
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Menzel R, Brandt R, Gumbert A, Komischke B, Kunze J (2000) Two spatial memories for honey bee navigation. Proc R Soc Lond B Biol Sci 267:961–968
Menzel R, Geiger K, Joerges J, Müller U, Chittka L (1998) Bees travel novel homeward routes by integrating separately acquired vector memories. Anim Behav 55:139–152
Menzel R et al (2005) Honey bees navigate according to a map-like spatial memory. Proc Natl Acad Sci USA 102:3040–3045
Menzel R, Muller U (1996) Learning and memory in honeybees: from behavior to neural substrates. Annu Rev Neurosci 19:379–404
Oades RD, Isaacson RL (1978) The development of food search behavior by rats: the effects of hippocampal damage and haloperidol. Behav Biol 24:327–337
Scheiner R, Page RE, Erber J (2004) Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35:133–142
Srinivasan M, Zhang S, Zhu H (1998) Honeybees link sights to smells. Nature 396:637–638
Team R (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2008. ISBN 3-900051-07-0
von Frisch K (1967) The dance language and orientation of bees. Harvard University, Cambridge
Whitfield CW et al (2006) Genomic dissection of behavioral maturation in the honey bee. Proc Natl Acad Sci USA 103:16068–16075
Winston ML (1991) The biology of the honey bee. Harvard University Press, Cambridge
Withers GS, Fahrbach SE, Robinson GE (1993) Selective neuroanatomical plasticity and division of labour in the honey bee. Nature 364:238–240
Zayed A, Naeger N, Rodriguez-Zas S, Robinson G (2012) Common and novel transcriptional routes to behavioral maturation in worker and male honey bees. Genes Brain Behav 11:253–261
Zayed A, Robinson GE (2012) Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee. Annu Rev Genet 46:591–615
Zhang S, Bartsch K, Srinivasan M (1996) Maze learning by honeybees. Neurobiol Learn Mem 66:267–282
Zhang S, Lehrer M, Srinivasan M (1998a) Eye-specific learning of routes and “signposts” by walking honeybees. J Comp Physiol A 182:747–754
Zhang S, Lehrer M, Srinivasan M (1998b) Stimulus-conditioned sequence learning in honeybees. In: Proceedings of the 26th Göttingen neurobiology conference. Thieme, Stuttgart
Zhang S, Mizutani A, Srinivasan MV (2000) Maze navigation by honeybees: learning path regularity. Learn Mem 7:363–374
Zhang SW, Lehrer M, Srinivasan MV (1999) Honeybee memory: navigation by associative grouping and recall of visual stimuli. Neurobiol Learn Mem 72:180–201
Acknowledgements
This study was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada and an Early Researcher Award from the Ontario Ministry of Research and Innovation to AZ. NT was supported by a Graduate Scholarship from the Province of Ontario and a York University Graduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsvetkov, N., Madani, B., Krimus, L. et al. A new protocol for measuring spatial learning and memory in the honey bee Apis mellifera: effects of behavioural state and cGMP. Insect. Soc. 66, 65–71 (2019). https://doi.org/10.1007/s00040-018-0641-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-018-0641-8