Abstract
Habitat heterogeneity has a complex effect on ant species richness and community structure (both alpha- and beta-diversity). Savannahs and forests are thought to have distinct species assemblages, but studies comparing savannah–forest mosaics produced conflicting results, with savannah (the less complex ecosystem) poorer in species, equally rich, or richer than forest (the most complex habitat). We compared subterranean and above ground ant assemblages in savannah and forests of Lamto Reserve (Côte d’Ivoire) to examine how habitat heterogeneity and vertical stratification drive species composition and structure patterns. The Ants of the Leaf Litter (ALL) protocol and soil monolith were used to sample ants along 16 transects of 200 m in the three main habitats (gallery forest, forest islands, and savannah). In total, 138 species from 44 genera were collected. Forested habitats held assemblages that matched those found in savannah in terms of species density and richness; however, they differed in composition. Taxonomic structure varied among habitats, with Formicinae and Amblyoponinae prevailing in savannah, and Myrmicinae numerically dominant in forests. Ants were clearly vertically stratified. Subterranean assemblages had a less even distribution of occurrences among species as compared to above ground ones. The patterns observed agree with the heterogeneity-diversity theory relative to beta-diversity but not alpha-diversity, emphasizing the difficulty to generalize across continents about factors structuring ant assemblages. Our results support the need to conserve both forest and savannah to maintain biodiversity at landscape level in Côte d’Ivoire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species coexistence at landscape scale is usually facilitated by habitat heterogeneity and associated variations in climatic conditions, resource availability, vegetation structure and complexity, or edaphic properties (Tews et al. 2004; Fahr and Kalko 2011; Pacheco and Vasconcelos 2012a; Stein et al. 2014; Stein and Kreft 2015; and references therein). More heterogeneous habitats indeed provide a higher number of ecological niches, resulting in a finer spatial segregation of species (Stein et al. 2014). Accordingly, savannahs and forests in tropical landscapes support distinct faunas and floras, even when both habitats are geographically close to each other (Verhaag and Rościszewski 1994; Fisher and Robertson 2002; Vasconcelos and Vilhena 2006; Andersen et al. 2007; van Ingen et al. 2008; Bond and Parr 2010; Calcaterra et al. 2010). Although it is often assumed that savannahs support lower species diversity because of lower vegetation complexity (Bond and Parr 2010), studies using ants as model organism have provided diverging results. In the savannah–forest mosaic of Beni ecoregion in Bolivia and of the Brazilian cerrado, forests harbor more ant species than savannahs (Verhaag and Rościszewski 1994; Vasconcelos and Vilhena 2006, respectively), in Madagascar ant species richness is similar in both habitats (Fisher and Robertson 2002), and in the Paranean ecoregion in Argentina and monsoonal northern Australia, richness is higher in savannahs (Calcaterra et al. 2010; Andersen et al. 2007, respectively). Moreover, savannahs of central Brazil have generally 60% more ant species than those of northern Australia despite very similar climate (Campos et al. 2011). These contrasting patterns might be explained by differences in biogeographical and historical factors during fauna evolution (Campos et al. 2011). For instance, ant assemblages currently found in Australian and Brazilian savannahs derive primarily from faunas associated with arid and forest conditions, respectively (Andersen et al. 2007; Campos et al. 2011). Moreover, other factors, such as contrasting temperature or net primary productivity among sites at different latitudes, might (have) influence(d) differently the ecology and/or evolution of ant assemblages from forests and savannahs (Kaspari et al. 2000, 2003, 2004; Dunn et al. 2010).
In West Africa, a natural savannah–forest mosaic occurs along the contact zone between the evergreen forest (near the coast in the South) and the dry intertropical savannah in the North. The vegetation of this zone predominantly consists of savannah that is compartmentalized by gallery forests running along permanent or temporary watercourses; many forest islands are also often found in the savannah (Adjanohoun 1964; Menaut 1971). This mosaic is termed Guinean or humid savannah (Adjanohoun 1964). The Guinean savannah is derived from the rainforest as suggested by its occurrence under a climate favorable to forest (Devineau 1984). There is growing agreement that it originated during the succession of humid and dry periods during the Quaternary. This climatic alternation has caused the progression of the forest at the expense of the savannah in humid periods and the inverse during dry periods (Adjanohoun 1964; Avenard 1969; Guillaumet 1967; Mangenot 1955; Peltre 1977; Portère 1950; Spichiger 1977). Studies of West African forest–savannah boundaries have generally focused on plant communities (e.g., Adjanohoun 1964; Spichiger 1977) while little is known about how animals, ants included, respond to this habitat heterogeneity. The history of this mosaic led us to expect that the savannah ant assemblage is derived from the forest-associated fauna as observed in the Neotropics.
At finer spatial scale (within-habitat level), ant species coexistence is generally facilitated by vertical segregation of species, with some taxa foraging on the ground and others in the vegetation (Campos et al. 2011). This stratification is often very strict, with low overlap of species among strata (Itino and Yamane 1995; Brühl et al. 1998; Yanoviak and Kaspari 2000, but see Hashimoto et al. 2006). In savannah–forest mosaics, such stratification of ant species was found in both habitats (Vasconcelos and Vilhena 2006 but see Campos et al. 2008).
Although the subterranean ant fauna is seldom integrated in biodiversity studies, mainly due to sampling difficulties (Berghoff et al. 2003; Ryder Wilkie et al. 2007; Schmidt and Solar 2010), it was recently demonstrated to be highly diverse and stratum specific (Berghoff et al. 2003; Silva and Silvestre 2004; Ryder Wilkie et al. 2007; Schmidt and Diehl 2008; Schmidt and Solar 2010; Andersen and Brault 2010; Neves et al. 2013; but see Lynch et al. 1988; Berman and Andersen 2012; Pacheco and Vasconcelos 2012b). Indeed, recent dramatic discoveries in ant taxonomy concern mostly subterranean species (e.g., Martialis heureka (Rabeling et al. 2008), Simopelta minima (Brandão et al. 2008), Anillidris bruchi (Schmidt et al. 2013), Simopelta anomma (Fernandes et al. 2015)). Diversity and distribution patterns of subterranean ant assemblages remain however largely unknown. Pioneer studies indicated that some subterranean ant assemblages are up to twice as species rich as leaf litter assemblages (Delabie and Fowler 1995; Fowler et al. 2000). Also, it seems that both species richness and species composition usually vary strongly between localities, even in sites with similar soils and vegetation (Andersen and Brault 2010; Jacquemin et al. 2012). Lastly, Berman and Andersen (2012) noted that subterranean and above ground ant faunas can respond differently to abiotic factors and habitat properties.
In this study, we compared vertical and horizontal patterns of subterranean and above ground ant assemblages in Lamto Scientific Reserve, a pristine Guinean savannah in Côte d’Ivoire. It is the first time that such a comparison is carried out in a West African savannah–forest mosaic. We aimed to test the following hypotheses: (1) ant species richness, diversity, and composition differ among habitats (savannahs, gallery forests, and forest islands) and strata (soil and above ground); (2) the less complex habitat (savannah) supports lower ant species diversity; forest islands harbor both forest and savannah-associated ant species and are therefore the most species-rich; species richness of gallery forest is intermediate; (3) savannah ant assemblage is derived from the forest-associated fauna; (4) species turnover of subterranean ants is higher in savannahs because, in Lamto, edaphic conditions are more heterogeneous than in forests. Lastly, we identified ant species that could be indicator of a specific habitat, stratum, or soil type.
Methods
Study site
We carried out our research in Lamto Scientific Reserve (2,500 ha) situated in central Côte d’Ivoire at 5°02′W, 6°13′N. Annual rainfall in Lamto averages 1210 mm (Lamotte and Tireford 1988), with a long rainy season from February to November usually interrupted by a short dry season in August, and a long dry season in December and January. The temperature is constant year-round (annual mean 27 °C).
We distinguished three main habitat types (Figs. 1, 2). (1) The gallery forest borders the Bandama river (the main watercourse of the reserve and the longest in the country). This riverine forest has much less contact with the savannah than the forest islands. It has a closed canopy and open undergrowth with deep leaf litter. The tree species are dominated by Croton scarciesii and Cynometra megalophylla (Devineau 1975). (2) The forest islands are forest fragments entirely surrounded by savannah or connected to the gallery forest by a narrow band of vegetation. These forests are less dense than the gallery forest, and canopy cover is often discontinuous. Climbers mainly dominate the undergrowth and the soil is generally covered with a shallow layer of leaf litter. The main tree species belong to genera Triplochiton and Celtis (Devineau 1975). Most of these forest patches are too small for our 200-m-long transects, so we included some of the deep extensions of gallery forest into savannah. (3) The savannah is tropical grassland in which scattered trees, mostly the palm Borassus aethiopum, tower above the high and dense grass layer dominated by Loudetia simplex and Hyparrhenia spp. (Adjanohoun 1964). Many subtypes of savannah have been distinguished in relation to tree density (Gauthier 1990), but we considered the savannah to be one unit. Litter was very sparse between the grass clumps due to annual burning, as in all the savannahs in West Africa.
Vegetation map of Lamto Scientific Reserve (2.500 ha) (modified from Gauthier 1990) and localization of the transects within the different habitats: gallery forest in black (GF: 5 transects), bordering the Bandama river; forest islands in black (FI: 5 transects), scattered in the savannah; savannah (Sa: 6 transects)
Sampling design
We sampled ants along 16 transects of 200 m length. Five transects were cleared in the gallery forest, five in forest islands and six in savannah (Fig. 1). Starting points of transects were random but direction was selected to maximize the sampling of heterogeneity within a habitat. In Lamto, three soil types occur in savannah (black clay, hydromorphic, and ferruginous soils) so we sampled two transects in each soil type to include this heterogeneity. In contrast, both the gallery forest and forest islands occur on ferruginous soils only, and hence there was no need to stratify our sampling. Transects in the same habitats were at least 200-m distant from one another.
We used two collection protocols aimed at different ant fauna. (1) Ants of the Leaf Litter protocol (ALL) is a standard method for measuring and monitoring the biodiversity of ground and leaf litter ants (Agosti and Alonso 2000; Fisher et al. 2000). It combines two techniques: mini-Winkler extractors and pitfall traps. Along each transect, twenty litter samples were collected inside a 1 m2 quadrat, spaced at 10-m intervals. The litter was sieved (mesh size 4 mm) and taken back to the field laboratory where it was hung in mini-Winkler bags for 48 h in order to extract the ants. Next to each quadrat (1 m away), a pitfall trap, containing ethanol and glycerin, was left for 48 h. As both Winkler and pitfall traps aim to collect above ground ant species, we combined the samples from these two methods for each sampling point (Fisher 1999). (2) Soil monolith extraction is used to sample subterranean ants. We dug a soil cube (30 × 30 × 30 cm) 10 m from each litter sample, and exhaustively searched for individual ants as well as their nests (Fisher and Robertson 2002). This yielded a total of 100 samples for both subterranean and above ground ant assemblages in each forest habitat (gallery forest and forest islands) and 120 samples in savannah. All transects were sampled between February and July 2003.
Data analysis
Ant identification
Ant specimens were sorted and identified to species level when possible or to morphospecies. We compared our specimens to the collections of the Muséum National d’Histoire Naturelle (Paris), the Natural History Museum (London), and the Museum of Comparative Zoology (Cambridge, USA). Voucher specimens are deposited in the entomological collection of Lamto ecology station. Our estimates of species abundance are based on the occurrence of individual workers (presence–absence) rather than total numbers of individuals (Andersen 1991). We excluded queens and males because most ant taxonomy is based on the worker caste. Furthermore, the presence of a worker is clear evidence of an established colony while this is seldom the case for queens and males (Fisher 1997; Fisher and Robertson 2002; Longino et al. 2002). We defined “common” species as those whose occurrence equals or exceeds the mean number of occurrences (=22) in the combined data from the three habitats and two strata. These species are assumed to be numerically dominant.
Assemblage structure (alpha-diversity)
We compared ant species richness found in each stratum within each habitat (1) after rarefaction to a common number of species occurrences (Coleman method), (2) after extrapolation (Chao2 estimation), and (3) comparison of occurrence-based or sample-based rarefaction, and extrapolation curves for sample-based incidence data under the Bernoulli product model (Colwell et al. 2012). The latter estimates the number of species found in both smaller and larger numbers of samples. Because extrapolation based on doubling sample size may become unreliable (Colwell et al. 2012), we estimated the number of species found in up to 200 samples, therefore doubling (for forest islands and gallery forest) or almost doubling sample size (savannah). Accumulation curves of uniques (species collected only once) and duplicates (species collected only twice) were also examined. Three diversity indices were computed: Fisher’s alpha standard deviation (a measure affected by the number of unique species), exponential Shannon (this measure gives the number of species that would have been found if all species had been equally common), and inverse Simpson (this index increases as the assemblage becomes more even; it is affected by the number of occurrences of most frequent species) (Maguran 2004). Curves and indices were computed with EstimateS 9.1 (Colwell 2013). Similar analyses were carried out (1) at the stratum level, to compare subterranean and above ground ant assemblages independently of the habitat in which they were sampled, (2) at the habitat level, and (3) at the scale of the Lamto Scientific Reserve. In those two last cases, the three sampling methods (pitfall traps, Winkler, and soil samples) were combined for each sampling point.
We computed k-dominance curves to graphically observe the diversity and evenness of the distribution of occurrences among species found in each habitat and stratum. The more elevated the curve, the less diverse the ant assemblage, and the more rapidly the curve increases, the less even is the species distribution. To test for differences in k-dominance curves, the distance between every pair of cumulative curves was calculated with the Manhattan distance measure, and analyses of similarity (ANOSIM tests) were carried out afterwards (Clarke 1990; Clarke and Gorley 2006).
Lastly, relative percentages of ant subfamilies were calculated from occurrences for each stratum within each habitat.
Comparison of assemblage composition (beta-diversity)
The similarity of above ground and subterranean ant assemblages among each habitat was expressed by the Morisita-Horn index (calculated with the BiodivR 1.0 software; Hardy 2005). Assemblages were ordinated by non-metric multidimensional scaling (NMDS) and two-way crossed ANOSIM was carried out with the PRIMER 6.1.6 software to test for differences in the composition of ant assemblages between strata and habitats. Last, Mantel tests were carried out to assess the relationship between assemblage similarity among above and below ground transects, using zt (Bonnet and van de Peer 2002).
Indicator species
We evaluated associations between ant species and stratum, habitat, stratum within habitat, and savannah soil type using the Indicator Value Method (IndVal) of Dufrêne and Legendre (1997), calculated with the PC-ORD 5.0 software (McCune and Mefford 1999). The IndVal ranges from 0% (no association) to 100% (perfect association). A species was considered to be a good indicator species when its IndVal was ≥70% (McGeoch et al. 2002). The significance of the IndVal for each species was evaluated using Monte Carlo randomization tests (5000 iterations).
Results
Overview
In total, the ALL protocol and soil monolith extractions yielded 3040 occurrences of workers of 138 ant species belonging to 44 genera and 7 subfamilies (Table 1). The most species-rich genera were Tetramorium (19 species), Strumigenys (13), Pheidole, Carebara (9 each), Camponotus, Crematogaster, Dorylus (7 each), Monomorium, and Hypoponera (6 each) (Appendix).
At the scale of Lamto Reserve, occurrence-based accumulation curves of ant species and uniques number began to decrease in slope, indicating that most species were found with our sampling effort (i.e., 16 transects of 20 sampling points combining three complementary sampling methods) (Fig. 3). On average, only 16 additional species (10%) would have been recorded if the sampling size had been doubled (Table 1).
Occurrence-based curves of accumulated species richness and 95% confidence intervals, Chao2 estimated species richness, uniques and duplicates at the scale of the Lamto Scientific Reserve. These curves were obtained using data from our 320 sampling points. We considered a species occurred at a sampling point if at least one individual was collected by at least one of the three sampling techniques (pitfall trap, Winkler, and soil sample)
Species density, richness, and diversity
Species density and species richness were not significantly different among habitats as indicated by the overlap of the 95% confidence intervals of each curves (Fig. 4a, b; Table 1). Species richness tended to be higher for the subterranean ant fauna than for the above ground fauna but this difference was also not significant (Fig. 4d; Table 1).
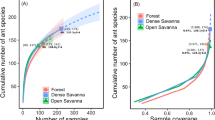
Sample-based and occurrence-based interpolation (rarefaction) and extrapolation for observed references (larger symbols) for ant assemblages from the three habitats (a, b) and two strata (c, d) studied in the Lamto Scientific Reserve, under the Bernoulli product model (Colwell et al. 2012), with 95% unconditional confidence intervals. Sample-based curves (a, c) are estimates of species density, and occurrence-based curves (b, d) estimate species richness (Colwell et al. 2012). The habitats are gallery forest, forest islands, and savannah. The strata are soil and above ground
In terms of rarefied species richness, the subterranean and above ground ant fauna from savannah were significantly less rich than the fauna from these strata in forest islands and gallery forest (Coleman method, Table 1). Nevertheless, no significant differences were observed in terms of extrapolated species richness (Chao 2; Table 1) or with the Bernoulli product model (Fig. 5).
Occurrence-based interpolation (rarefaction) and extrapolation for observed references (larger symbols) for ant assemblages from the two strata (soil and above ground [ALL]) within the three habitats (gallery forest, forest islands, and savannah) studied in the Lamto Scientific Reserve, under the Bernoulli product model (Colwell et al. 2012). Note that, 95% unconditional confidence intervals are not shown for clarity, but they all overlap indicating no significant differences among curves
For each of the three habitats and for the three habitats combined, exponential Shannon and inverse Simpson indices were higher for above ground ant assemblages than for subterranean assemblages (Table 1). However, the opposite pattern was obtained with Fisher’s alpha due to the higher number of uniques collected in soil samples.
In addition, k-dominance curves of subterranean assemblages were significantly different from those of above ground assemblages (one-way ANOSIM: R = 0.42; P < 0.001), with lower species richness and lower evenness in assemblages from that stratum (Fig. 6). Structure of above ground ant assemblages from forested habitats and savannah were also significantly different (one-way ANOSIM: R = 0.36; P = 0.039), with forested habitats in general more species-rich and more even.
Structure of the subterranean (blue triangles) and epigean (empty green triangles) ant assemblages collected in the Lamto Scientific Reserve; k-dominance curves were computed for each of the 16 monolith or Ants of Leaf Litter (ALL) transects. Note log scale used on x axis. Curves from the two strata significantly differ, indicating that subterranean assemblages were less even than epigean assemblages (one-way ANOSIM: R = 0.42; P < 0.001)
Similarity and taxonomic structure of ant assemblages
At the Lamto scale (i.e., three habitats combined), transect similarity was on average lower for subterranean than for above ground ant assemblages (Morisita-Horn similarity index 0.37 ± 0.25 [mean ± SD; n = 120 pairwise comparisons], and 0.51 ± 0.21, respectively, Table 2). This pattern was also observed within each habitat (Table 2). In general, above ground and subterranean ant fauna were clearly dissimilar [Morisita-Horn similarity index 0.34 ± 0.19 (mean ± SD; n = 256 pairwise comparisons)]. Similarity pattern among transects for above ground and subterranean ant fauna was strongly correlated (Mantel test: r = 0.84; P < 0.0001).
NMDS ordination illustrated a clear separation between ant assemblages from savannah and forested habitats (gallery forests and forest islands), as well as between subterranean and above ground ant assemblages (Fig. 7). Consistently, species composition differed among strata (two-way crossed ANOSIM: R = 0.69; P < 0.001) and among habitats (R = 0.8; P < 0.001; all pairwise comparisons with P < 0.001). No significant differences were observed among subterranean ant assemblages from different soil types in the savannah (R = 0; P = 0.53).
Non-metric multidimensional scaling (NMDS) plot (two dimensions) showing differences in species composition between epigean (empty symbols) and subterranean ( filled symbols) ant assemblages in the three habitats of the Lamto Scientific Reserve: gallery forest (triangles), forest islands (squares), and savannah (circles). Each symbol represent a different transect. The ordination is based on a similarity matrix calculated with the Morisita-Horn index. The low stress value (0.13) indicates a high goodness-of-fit of the ordination
Subfamily composition in savannah contrasted with that in forested habitats, with Formicinae and Amblyoponinae well represented in terms of relative occurrences in both above ground and subterranean assemblages (Fig. 8). In forested habitats, ponerine ants were proportionally more frequently collected in the soil than above ground. In every stratum and habitat, but especially in forested habitats, Myrmicinae was the most represented subfamily.
Ubiquitous, common, and indicator species
Only 13 of the 138 species collected (9.4%) were found at least once in the two strata of the three habitats, and could thus be considered as ubiquitous (Carebara thoracica WEBER 1950, Parasyscia nitidulus BROWN 1949, Crematogaster striatula EMERY 1892, Hypoponera inaudax SANTSCHI 1919, Hypoponera sp. 1, Monomorium sp. 5, Euponera brunoi FOREL 1913, Mesoponera caffraria SMITH 1858, Bothroponera soror EMERY 1899, Paltothyreus tarsatus FABRICIUS 1798, Pheidole sp. 6, Pheidole sp. 9 (termitophila group), and Tetramorium zambezium SANTSCHI 1939).
Fifty species (36.2%) were only collected in forested habitats, among which 25 and 10 were restricted to gallery forest and forest islands, respectively. In contrast, 25 species (18.1%) were only collected in savannahs. Similarly, 18 species (13%) and 42 species (30.4%) were only found in the soil and above ground, respectively.
Our criterion for “common” species corresponds to 33 out of the 138 species collected (24%) (Appendix), representing 2496 out of 3040 species occurrences (82%). The four most common species, C. thoracica (233 occurrences), Pheidole sp. 6 (198 occurrences), C. striatula (149 occurrences), and T. zambezium (145 occurrences), represented together nearly one-fourth of the total number of species occurrences.
Among the 33 common species, 19 were significantly associated with a particular habitat, stratum, or soil type (=indicator species) (IndVal method; Table 3). Only two indicator species were not categorized as common: Tetramorium calinum BOLTON 1980 and Hypoponera sp. 6 (19 and 18 occurrences, respectively). So, a total of 21 ant species out of the 138 species collected were identified as “indicator species” by the IndVal analysis.
Five of these indicator species were significantly associated with the savannah habitat (Table 3). Among them, Carebara sp. 2 was associated with savannah soil, and two species, T. calinum and the widespread Pheidole megacephala FABRICIUS 1793, were typical above ground species. Three indicator species were associated with gallery forests, but no subterranean species were specifically associated with that habitat (Table 3). Last, no indicator species were identified for forest islands.
Eight indicator ant species, including the invasive Monomorium pharaonis LINNAEUS 1758, were associated with the above ground stratum in Lamto (i.e., specifically found in ALL samples), but no ant species could be identified as indicator of the subterranean stratum (Table 3). Nevertheless, when the IndVal analysis was restricted to soil samples collected in savannahs, some ants appear to be indicator of clay (Plagiolepis mediorufa FOREL 1916), ferruginous (C. thoracica, M. caffraria), or hydromorphic soils (Hypoponera sp. 6) (Table 3).
Discussion
This study is the first to compare diversity and distribution of subterranean and above ground ant assemblages in a forest–savannah mosaic of West Africa. Our sampling provided a thorough estimate of the number of ant species present in Lamto Scientific Reserve (138 observed species; 159 ± 10.2 estimated species; Table 1). Although this number is a minimum because we did not include arboreal ant assemblages, it can already be considered high. In comparison, Fisher and Robertson (2002), based on five sampling techniques (pitfall traps of narrow or wide diameter, Winkler samples, soil monoliths, and vegetation beating) resulting in 400 samples, collected a total of 59 species from one forest and one savannah in Madagascar. Neves et al. (2013), using vegetation beating and baited pitfall traps placed in the subterranean, above ground, and arboreal strata, found 113 ant species in three adjacent habitats (cerrado, riparian forest, and dry forest) in Brazil. Campos et al. (2011), using 640 baited pitfall traps placed in the above ground and arboreal strata, collected 150 and 93 ant species in savannah woodlands of Brazil and Australia, respectively.
Interestingly, our estimation of species richness in forest islands (Chao2: 92.2 ± 6.5 species; Table 1) closely matches the estimation of another Ivorian forest located 65 km away from Lamto (Chao2: 95 species), where ants were sampled along three transects combining the ALL and monolith protocols (Kone et al. 2012).
We found no significant difference in species richness among the three habitats studied (forest islands, gallery forest, and savannah). We had expected the ant community in forest islands to be more species rich than in savannah, and that in gallery forest to be intermediate. We assessed subjectively that the savannah is less complex than the gallery forest; thus, our results contradict the general pattern that species richness correlates with habitat complexity (McCoy and Bell 1991; Stein et al. 2014; Stein and Kreft 2015). An example of this general pattern is given by Vasconcelos and Vilhena (2006) who found that forests in the Brazilian Amazon supported about twice as many ant species as the nearby savannah (125 vs. 64 species). However, exceptions exist as Lassau and Hochuli (2004) found that sites of low habitat complexity supported much greater ant species richness in Sydney sandstone ridgetop woodlands (Australia). Also, Australian savannahs support more ant species than forests (Andersen et al. 2007). Our results are similar to those of Fisher and Robertson (2002) who collected similar number of species in Malagasy mountain grassland and nearby forest.
One possible explanation is that we underestimated the heterogeneity present in our six savannah sites. Indeed, we considered the Lamto’s savannah habitat as a whole although many subtypes have been distinguished, mainly in relation to tree density and soil properties (Bonvallot et al. 1970; Menaut 1971; Menaut and Cesar 1979). In fact, we expressly aimed to increase heterogeneity by sampling ants in savannahs of three different soil types. Forested sites were more homogeneous since all of them occurred on ferruginous soils only. Further studies with finer measures of habitat heterogeneity and vegetation complexity are required to confirm the pattern observed. Another nonexclusive explanation is that the disturbance caused by annual fires in Lamto’s savannahs may limit ant abundance and reduce interspecific competition, allowing a higher number of species to exploit the ecosystem resources (Philpott et al. 2010; Maravalhas and Vasconcelos 2014; Parr et al. 2014; Veldman et al. 2015). In comparison, forests are more stable habitats, allowing some species to dominate the ecosystem, which in turn limit ant diversity (Parr et al. 2005). Consistent with this idea, the four most “common” species (C. thoracica, Pheidole sp. 6, C. striatula, and T. zambezium) were numerically dominant in forested habitats (Appendix). Finally, it is possible that the evolutionary history of the savannah biota in West Africa, with its dynamics of expansion and retraction (Adjanohoun 1964; Avenard 1969; Guillaumet 1967; Mangenot 1955; Peltre 1977; Portère 1950), allowed the evolution of a distinct ant fauna.
Contrary to our hypothesis, the composition of the savannah ant assemblage was not simply derived from the forest assemblage but was significantly different, with distinct taxonomic structure (i.e., subfamily relative occurrences), 18% of species exclusively found in savannah, and recognition of six indicator species such as Camponotus acvapimensis MAYR 1862 and Stigmatomma sp. 2 (Table 3). The distinctness of savannah ant assemblages was also observed in different savannah–forest mosaics worldwide (Verhaag and Rościszewski 1994; Fisher and Robertson 2002; Vasconcelos and Vilhena 2006; Andersen et al. 2007; van Ingen et al. 2008; Calcaterra et al. 2010). However, the proportion of species exclusive to the savannah was much higher in the Brazilian cerrado (35.9%; Vasconcelos and Vilhena 2006), and in Australia (90.7%; Andersen et al. 2007).
Ant assemblage composition also differed significantly between gallery forests and forest islands perhaps because of differences in vegetation structure, composition or density (Devineau 1975), in quantity/quality of the leaf litter layer (nesting site of the majority of epigean ant species), in temperature, humidity, insolation or net primary productivity (e.g., Kaspari et al. 2000, 2003, 2004), or in their contact with the savannah, forest islands having more contact than the gallery forests. Nevertheless, the similarity between these habitats was higher than between savannah and forested habitats. This may be explained by microhabitat conditions that differ from savannah to forest but are more similar between the two forest types, for instance savannah ants are much more exposed to sunlight. Furthermore, most of the ants in the two forest habitats are cryptic species that live in the litter and rotten logs; in contrast savannah species nest mainly underground because annual fires burn the litter and other dead wood above ground.
Subterranean ant assemblages tended to be less species rich than above ground assemblages, although the difference was not significant. Taxonomic structure and composition of ant assemblages were however strikingly different, with 43.4% of ant species found only in one of the two strata. In addition, soil stratum had less even assemblages and higher number of rare species (uniques and duplicates), among which the rarely sampled and poorly known Parasyscia lamborni CRAWLEY 1923, Concoctio concenta BROWN 1974, and Dolioponera fustigera BROWN 1974. However, we did not identify any indicator species of the subterranean stratum (at the scale of the Lamto Scientific Reserve). It is perhaps due to the way the IndVal index identifies indicator species, because it considers both the specificity and the fidelity of the species to a particular habitat or stratum (Dufrêne and Legendre 1997); this means the species should ideally be found in all samples of a single habitat or stratum to receive a high and significant IndVal value. When the species turnover is high, the species fidelity may seem erroneously low, resulting in a low IndVal value. Here, we observed that species turnover was particularly high for subterranean ant assemblages (Table 3). It was consistently even higher than the turnover observed for above ground ant assemblages, whatever the habitat studied. This result is consistent with the high species turnover observed in subterranean ant assemblages in Australia (Andersen and Brault 2010) and Ecuador (Jacquemin et al. 2012). Additional studies are needed to determine if it is a characteristic of subterranean ant assemblages or a sampling artifact due to the difficulty to collect ants in the soil (Berghoff et al. 2003; Ryder Wilkie et al. 2007; Schmidt and Solar 2010).
We did not observe any significant difference among ant assemblages according to edaphic conditions in savannah. Nevertheless, we were able to identify indicator species for the three soil types found in the habitat (black clay, hydromorphic, and ferruginous). This gives support to the finding of Lévieux (1971) that ant fauna differed according to edaphic conditions in Lamto’s savannah. However, we lack biological data to understand this divergent response to soil conditions. This pattern could be partly linked to the ability of some ant species to withstand temporal flooding since black clay and hydromorphic soils retain an important amount of water during the rainy season.
Overall, most of the collected species belonged to subfamilies Myrmicinae (numerically dominant in forest habitats), Formicinae (prevailing in savannah), and Ponerinae (more dominant in the soil). This is consistent with Lévieux’s (1983) observation that Formicinae are more abundant in dry and open areas such as savannah in northern Côte d’Ivoire. Ant species collected in Lamto include an important proportion of the regional pool since at least 46 species (33.3% of the 138 species [morphospecies could not be compared]) were also recorded in Ghana (≥350 km eastwards from Lamto), by Belshaw and Bolton (1994) who surveyed leaf litter ants in semi-deciduous forests similar to those occurring in Lamto (Appendix). An even higher proportion (46%; 63 species) was shared with assemblages from forests and cocoa plantations located closer (65 km to the North; Kone et al. 2012).
In conclusion, our results indicate that West African savannah and forested habitats hold ant assemblages that match in terms of species density and richness but differ in composition. Moreover, soil-above ground stratification constitutes an important factor driving composition patterns of ant assemblages in both savannah and forest (also observed by Neves et al. 2013). The relatively small proportion of species shared by forest and savannah emphasizes the importance of each habitat type within the mosaic. The co-occurrence of the two habitats promotes species coexistence and thus the maintenance of biodiversity at the landscape scale. Our results join a set of cumulative evidence that savannahs have high biological value (Bond and Parr 2010; Parr et al. 2014; Veldman et al. 2015). Unfortunately, these habitats are also highly threatened worldwide (Bond and Parr 2010; Beale et al. 2013; Parr et al. 2014; Veldman et al. 2015). In Côte d’Ivoire, both savannahs and forests are often degraded or converted to plantations (Fischer, 2004; Dosso et al. 2012, 2013; Gonedelé Bi et al. 2012). As a result, natural habitats of Lamto are more isolated and under pressure. To maintain the biodiversity and ecosystem functionality of the reserve (2,500 ha), important and immediate efforts are needed to preserve both savannahs and forests not only within the reserve but also its surroundings.
References
Adjanohoun E (1964) Végétation des savanes et des rochers découverts en d’Ivoire centrale. Memoir ORSTOM 7, Paris, p 178
Agosti D, Alonso LE (2000) The ALL protocol. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) ANTS: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington DC, pp 204–206
Andersen AN (1991) Sampling communities of ground-foraging ants: pitfall catches compared with quadrat counts in an Australian tropical savannah. Aust J Ecol 16:273–279
Andersen AN, Brault A (2010) Exploring a new biodiversity frontier: subterranean ants in northern Australia. Biodivers Conserv 19:2741–2750. doi:10.1007/s10531-010-9874-1
Andersen AN, van Ingen LT, Campos RI (2007) Constrasting rainforest and savanna ant faunas in monsoonal northern Australia: a rainforest patch in a tropical savanna landscape. Aust J Zool 55:363–369
Avenard JM (1969) Réflexions sur l’état de la recherche concernant les problèmes posés par le contact forêt-savane. Essai de mise au point et de la bibliographie. ORSTOM series Initiations et Documentations Techniques 14, Paris, p 149
Beale CM, van Rensberg S, Bond WJ, Coughenour M, Fynn R, Gaylard A, Grant R, Harris B, Jones T, Mduma S, Owen-Smith N, Sinclair ARE (2013) Ten lessons for the conservation of African savannah ecosystems. Biol Conserv 167:224–232. doi:10.1016/j.biocon.2013.08.025
Belshaw R, Bolton B (1994) A survey of the leaf litter ant fauna in Ghana, West Africa (Hymenoptera: Formicidae). J Hymenopt Res 3:5–10
Berghoff SM, Maschwitz U, Linsenmair KE (2003) Hypogaeic and epigaeic ant diversity on Borneo: evaluation of baited sieve buckets as a study method. Trop Zool 16:153–163. doi:10.1080/03946975.2003.10531192
Berman M, Andersen AN (2012) New Caledonia has a depauperate subterranean ant fauna, despite spectacular radiations above ground. Biodivers Conserv 21:2489–2497. doi:10.1007/s10531-012-0309-z
Bond WJ, Parr CL (2010) Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biol Conserv 143:2395–2404. doi:10.1016/j.biocon.2009.12.012
Bonnet E, Van de Peer Y (2002) zt: a software tool for simple and partial Mantel tests. J Stat Softw 7:1–12
Bonvallot J, Dugerdil M, Duviard D (1970) Recherches écologiques dans la savane de Lamto (Côte d’Ivoire): répartition de la végétation dans la savane pré-forestière. La Terre et la Vie 24:3–21
Brandão CRF, Feitosa RM, Schmidt FA, Solar RRC (2008) Rediscovery of the putatively extinct ant species Simopelta minima (Brandão) (Hymenoptera, Formicidae), with a discussion on rarity and conservation status of ant species. Rev Bras Entomol 52:480–483
Brühl CA, Gunsalam G, Linsenmair KE (1998) Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J Trop Ecol 14:285–297
Calcaterra LA, Cuezzo F, Cabrera SM, Briano JA (2010) Ground ant diversity (Hymenoptera: Formicidae) in the Iberá Nature Reserve, the largest wetland of Argentina. Ann Entomol Soc Am 103:71–83
Campos RI, Lopes CT, Magalhães WCS, Vasconcelos HL (2008) Estratificação vertical de formigas em Cerrado strictu sensu no Parque Estadual da Serra de Caldas Novas, Goiás, Brasil. Iheringia Sér Zool 98:311–316
Campos RI, Vasconcelos HL, Andersen AN, Frizzo TLM, Spena KC (2011) Multi-scale ant diversity in savanna woodlands: an intercontinental comparison. Austral Ecol 36:983–992. doi:10.1111/j.1442-9993.2011.02255.x
Clarke K (1990) Comparisons of dominance curves. J Exp Mar Biol Ecol 138:143–157
Clarke K and Gorley R (2006) Primer v6: User manual/tutorial.Plymouth: Primer-E
Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. Version 9.1. User’s Guide and application published at: http://purl.oclc.org/estimates
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5(1):3–21. doi:10.1093/jpe/rtr044
Delabie JHC, Fowler HG (1995) Soil and litter cryptic ant assemblages in Bahian cocoa plantations. Pedobiologia 39:423–433
Devineau J-L (1975) Etude quantitative des forêts galeries de Lamto (Moyenne Côte d’Ivoire). Thèse de Doctorat 3è cycle, Université Paris 6, p 190
Devineau J-L (1984) Structure dynamique de quelques forêts tropicales de l’Ouest africain (Côte d’Ivoire). Thèse de Doctorat d’Etat de l’Université Pierre et Marie Curie p 294
Dosso K, Yéo K, Konaté S and Linsenmair KE (2012) Importance of protected areas for biodiversity conservation in central Côte d’Ivoire: Comparison of termite assemblages between two neighbouring areas under differing levels of disturbance. J Insect Sci 12: 131. Available online 12:131. Available online: http://www.insectscience.org/12.131
Dosso K, Deligne J, Yéo K, Konaté S, Linsenmair KE (2013) Changes in the termite assemblage across a sequence of land-use systems in the rural area around Lamto Reserve in central Côte d’Ivoire. J Insect Conserv 17:1047–1057. doi:10.1007/s10841-013-9588-2
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Dunn RR, Guénard B, Weiser MD and Sanders NJ (2010). Geographic gradients. In Lach L, Parr CL, Abbott KL Ant Ecology. Oxford University Press, pp 38–58
Fahr J, Kalko EKV (2011) Biome transitions as centres of diversity: habitat heterogeneity and diversity patterns of West African bat assemblages across spatial scales. Ecography 34:177–195. doi:10.1111/j.1600-0587.2010.05510.x
Fernandes IO, Souza JLP, Fernández FC, Delabie JHC, Schultz TR (2015) A new species of Simopelta (Hymenoptera: Formicidae: Ponerinae) from Brazil and Costa Rica. Zootaxa 3956(2):295–300. doi:10.11646/zootaxa.3956.2.10
Fischer F (2004) Status of the Comoé National Park, Côte d’Ivoire, and the effects of war. Parks 14:17–23
Fisher BL (1997) A comparison of ant assemblages (Hymenoptera: Formicidae) on serpentine and non serpentine soils in northern California. Insect soc 44:23–33
Fisher BL (1999) Improving inventory efficiency: A case study of leaf-litter ant diversity in Madagascar. Ecol Appl 9:714–731
Fisher BL, Robertson HG (2002) Comparison and origin of forest and grassland ant assemblages in the High plateau of Madagascar (Hymenoptera: Formicidae). Biotropica 34:155–167
Fisher BL, Malsh AKF, Gadagkar R, Delabie JHC, Vasconcelos HL, Majer JD (2000) Applying the ALL protocol. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) ANTS: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington DC, pp 207–214
Fowler HG, Delabie JHC, Moutinho PRS (2000) Hypogaeic and epigaeic ant (Hymenoptera: Formicidae) assemblages of Atlantic costal rainforest and dry mature and secondary Amazon forest in Brazil: continuums or communities. Tropical Ecology 41:73–80
Gauthier L (1990) Carte du recouvrement ligneux de Lamto. Echelle: 1/10000. Conservatoire et Jardin Botanique de Genève, Genève
Gonedelé Bi S, Koné I, Bitty AE, Béné Koffi JC, Akpatou B, Zinner D (2012) Distribution and conservation status of catarrhine primates in Côte d’Ivoire (West Africa). Folia Primatol 83:11–23. doi:10.1159/000338752
Guillaumet JL (1967) Recherches sur la végétation et la flore de la région du Bas-Cavally (Côte d’Ivoire). Memoir ORSTOM 20, Paris, p 240
Hardy O (2005) BiodivR 1.0. A program to compute statistically unbiases indices of species diversity within samples and species similarity between samples using rarefaction principles. Université Libre de Bruxelles
Hashimoto Y, Morimoto Y, Widodo ES, Mohamed M (2006) Vertical distribution pattern of ants in a Bornean tropical rainforest (Hymenoptera: Formicidae). Sociobiology 47:697–710
Itino T, Yamane S (1995) The vertical distribution of ants on canopy trees in a Bornean lowland rain forest. Tropics 4:277–281
Jacquemin J, Drouet T, Delsinne T, Roisin Y, Leponce M (2012) Soil properties only weakly affect subterranean ant distribution at small spatial scales. Appl soil Ecol 62:163–169. doi:10.1016/j.apsoil.2012.08.008
Kaspari M, O’Donnel S, Kercher JR (2000) Energy, density, and constraints to species richness: ant assemblages along a productivity gradient. Am Nat 155:280–293
Kaspari M, Yuan M, Alonso L (2003) Spatial grain and gradients of ant species richness. Am Nat 161:459–477
Kaspari M, Ward PS, Yuan M (2004) Energy gradients and the geographic distribution of local ant diversity. Oecologia 140:407–413
Kone M, Konate S, Yeo K, Kouassi PK, Linsenmair KE (2012) Changes in ant communities along an age gradient of cocoa cultivation in the Oumé region, central Côte d’Ivoire. Entomol Sci 15:324–339. doi:10.1111/j.1479-8298.2012.00520.x
Lamotte M and Tireford JL (1988) Le climat de la savane de Lamto (Côte d’Ivoire) et sa place dans les climats de l’Ouest africain. Travaux de Recherche de Lamto vol. 8, p 146
Lassau SA, Hochuli DF (2004) Effects of habitat complexity on ant assemblages. Ecography 27:157–164
Lévieux J (1971) Données écologiques et biologiques sur le peuplement en fourmis terricoles d’une savane préforestière de Côte d’Ivoire. Thèse de Doctorat de la Faculté des Sciences de Paris, p 288
Lévieux J (1983) Soil fauna of tropical savannahs: 4 The ants. In: Bourlière F (ed) Tropical savannahs pp 525–540. Elsevier, Amsterdam
Longino JT, Coddington J, Colwell RK (2002) The ant fauna of a tropical rain forest: estimating species richness, three different ways. Ecology 83:689–702
Lynch JF, Johnson AK, Balinsky EC (1988) Spatial and temporal variation in the abundance and diversity of ants (Hymenoptera: Formicidae) in the soil and litter layers of a Maryland forest. Am Midl Nat 119:31–44
Maguran AE (2004) Measuring biological diversity. Blackwell Publishing, Oxford, p 256
Mangenot G (1955) Etude sur les forêts des plaines et plateaux de Côte d’Ivoire. Etudes Eburnéennes 6:5–61
Maravalhas J, Vasconcelos HL (2014) Revisiting the pyrodiversity–biodiversity hypothesis: long-term fire regimes and the structure of ant communities in a Neotropical savanna hotspot. J Appl Ecol 51:1661–1668
McCoy ED, Bell SS (1991) Habitat structure: the evolution and diversification of a complex topic. In: McCoy ED, Bell SS and Mushinsky HR (eds) Habitat structure: the physical arrangements of objects in space, Chapman & Hall, pp 3–27
McCune B and Mefford MJ (1999) PC-ORD – Multivariate Analysis of Ecological Data, Version 5.0. MjM Software, Gleneden Beach, Oregon, USA
McGeoch MA, van Rensburg BJ, Botes A (2002) The verification and application of bioindicators: a case study of dung beetles in a savannah ecosystem. J Appl Ecol 39:661–672
Menaut JC (1971) Etude de quelques peuplements ligneux d’une savane guinéenne de Côte d’Ivoire. Thèse de doctorat 3ème cycle (Faculté des sciences de Paris), 141 pp
Menaut JC, Cesar J (1979) Structure and primary productivity of Lamto savannas, Ivory Coast. Ecology 60:1197–1210
Neves FS, Queiroz-Dantas KS, da Rocha WD, Delabie JHC (2013) Ants of three adjacent habitats of a transition region between the Cerrado and Caatinga biomes: the effects of heterogeneity and variation in canopy cover. Neotrop Entomol 42:258–268. doi:10.1007/s13744-013-0123-7
Pacheco R, Vasconcelos HL (2012a) Habitat diversity enhances ant diversity in a naturally heterogeneous Brazilian landscape. Biodivers Conserv 21:797–809. doi:10.1007/s10531-011-0221-y
Pacheco R, Vasconcelos HL (2012b) Subterranean pitfall traps: is it worth including them in your ant sampling protocol? Psyche. doi:10.1155/2012/870794
Parr CL, Sinclair BJ, Andersen AN, Gaston KJ, Chown SL (2005) Constraint and competition in assemblages: a cross-continental and modeling approach for ants. Am Nat 165:481–494
Parr CL, Lehmann CER, Bond WJ, Hoffmann WA, Andersen AN (2014) Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol Evol 29:205–213
Peltre P (1977) Le « V Baoulé » (Côte d’Ivoire centrale). Héritage géomorphologique et paléoclimatique dans le tracé du contact forêt-savane. Travaux et Document de l’ORSTOM N°80, p 197
Philpott SM, Perfecto I, Armbrecht I, Parr CL (2010) Ant diversity and function in disturbed and changing habitats. In: Lach L, Parr C, Abbott K (eds) Ant ecology. Oxford University Press, Oxford, pp 137–156
Portère R (1950) Problèmes sur la végétation de la basse Côte d’Ivoire. Bulletin de la Société Botanique de France 97:153–156
Rabeling C, Brown JM, Verhaagh M (2008) Newly discovered sister lineage sheds light on early ant evolution. P Natl Acad Sci USA 105:14913–14917
Ryder Wilkie KT, Mertl AL, Traniello JFA (2007) Biodiversity below ground: probing the subterranean ant fauna of Amazonia. Naturwissenschaften 94:725–731. doi:10.1007/s00114-007-0250-2
Schmidt FA, Diehl E (2008) What is the effect of soil use on ant communities? Neotrop Entomol 37:381–388
Schmidt FA, Solar RRC (2010) Hypogaeic pitfall traps: methodological advances and remarks to improve the sampling of a hidden ant fauna. Insect Soc 57:261–266. doi:10.1007/s00040-010-0078-1
Schmidt FA, Feitosa RM, de Moraes Rezende F, Silva de Jesus R (2013) News on the enigmatic ant genus Anillidris (Hymenoptera: Formicidae: Dolichoderinae: Leptomyrmecini). Myrmecological News 19:25–30
Silva RR, Silvestre R (2004) Riqueza da fauna de formigas (Hymenoptera: Formicidae) que habita as camadas superficiais do solo em Seara, Santa Catarina. Papéis Avulsos Zoologia 44:1–11
Spichiger R (1977) Contribution à l’étude du contact entre flore sèche et humide sur les lisières des formations forestières humides sémi-décidues du V Baoulé et de son extension nord-ouest. Bulletin de liaison des chercheurs de Lamto, numéro spécial fascicule 1, Paris, p 261
Stein A, Kreft H (2015) Terminology and quantification of environmental heterogeneity in species-richness research. Biol Rev 90:815–836. doi:10.1111/brv.12135
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880. doi:10.1111/ele.12277
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
van Ingen LT, Campos RI, Andersen AN (2008) Ant community structure along an extended rain forest–savanna gradient in tropical Australia. J Trop Ecol 24:445–455. doi:10.1017/S0266467408005166
Vasconcelos HL, Vilhena JMS (2006) Species turnover and vertical partitioning of ant assemblages in the Brazilian Amazon: a comparison of forests and savannas. Biotropica 38:100–106. doi:10.1111/j.1744-7429.2006.00113.x
Veldman JW, Buisson E, Durigan G, Fernandes GW, Le Stradic S, Mahy G, Negreiros D, Overbeck GE, Veldman RG, Zaloumis NP, Putz FE, Bond WJ (2015) Toward an old-growth concept for grasslands, savannas, and woodlands. Front Ecol Environ 13:154–162. doi:10.1890/140270
Verhaag M, Rościszewski K (1994) Ants (Hymenoptera, Formicidae) of forest and savanna in the Biosphere Reserve Beni, Bolivia. Andrias 13:199–214
Wetterer J (2010) Worldwide spread of the pharaoh ant, Monomorium pharaonis (Hymenoptera: Formicidae). Myrmecological News 13:115–129
Wetterer J (2012) Worldwide spread of the African big-headed ant, Pheidole megacephala (Hymenoptera: Formicidae). Myrmecological News 17:51–62
Yanoviak SP, Kaspari M (2000) Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89:259–266
Acknowledgements
This Project was conducted at Lamto Ecology Station in Côte d’Ivoire and the authors are grateful to Kouakou Firmin and Kouassi Kouassi (Apo) for their help during field work. The authors thank Brian L. Fisher and anonymous referees for their constructive comments on previous versions of the manuscript. The authors are grateful to Barry Bolton, Stephan Cover, and Gary Alpert as well as Janine Casevitz-Weulersse for their help during ant identification at Natural History Museum (London), Museum of Comparative Zoology (Cambridge), and Muséum National d’Histoire Naturelle (Paris), respectively. This work was financed by the French government (co-tutelle de thèse 2002-2004), Conservation International (Rapid Assessment Programs), and the Ernst Mayr Grant (Harvard University). Thibaut Delsinne thanks the Secretary of Higher Education, Science, Technology and Innovation (SENESCYT) of the Government of Ecuador for financial support (Prometeo Grant) during the statistical analyses and writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yeo, K., Delsinne, T., Konate, S. et al. Diversity and distribution of ant assemblages above and below ground in a West African forest–savannah mosaic (Lamto, Côte d’Ivoire). Insect. Soc. 64, 155–168 (2017). https://doi.org/10.1007/s00040-016-0527-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-016-0527-6