Abstract
Objectives
To analyse the age, period and cohort effects on the mean body mass index (BMI) and obesity over the past two decades in Estonia.
Methods
Study used data from nationally representative repeated cross-sectional surveys on 11,547 men and 16,298 women from 1996 to 2018. The independent effects of age, period and cohort on predicted mean BMI and probability of obesity (BMI ≥ 30 kg/m2) were modelled using hierarchical age–period–cohort analysis.
Results
Curvilinear association between age and mean BMI was found for men, whereas the increase in mean BMI was almost linear for women. The predicted mean BMI for 40-year-old men had increased by 6% and probability of obesity by 1.8 times over 1996–2018; the period effects were slightly smaller for women. Men from the 1970s birth cohort had higher mean BMI compared to the average, whereas no significant cohort effects were found for obesity outcome.
Conclusions
Population-level BMI changes in Estonia during 1996–2018 were mostly driven by period rather than cohort-specific changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A steady increase in the population body mass index (BMI) has been witnessed globally with 39% of adults being overweight (BMI 25.0–29.9 kg/m2) and 13% obese (BMI ≥ 30 kg/m2) in 2016 (World Health Organisation 2020). In 24 out of 36 OECD countries, the obesity epidemic has resulted in about 50 million additional obese individuals since 2010 with more than half of the population being overweight and almost one in four people being obese (OECD 2019). Excess body weight constitutes major risk of cardiovascular disease, hypertension, type 2 diabetes, hyperlipidemia, sleep apnoea, stroke, certain cancers and other non-communicable diseases (Centers for Disease Control and Prevention 2018). As these conditions account for the majority of global disease burden (Benziger et al. 2016), the consequences of excess body weight present a serious challenge for health care systems.
The causes of increasing population-level BMI are complex and cover physiological, environmental, genetic and behavioural factors. Possible explanations include the increasingly sedentary lifestyle and respective changes in dietary and physical activity patterns (Townshend and Lake 2017), hormonal and metabolic changes related to biological ageing (Johnson et al. 2015), but also the impact of “obesogenic environment” that marks the intertwined behavioural and environmental pathways to obesity (Townshend and Lake 2017). As these mechanisms are often time-varying, the age–period–cohort (APC) analysis that disaggregates the specific contributions of age, period and cohort, can be a useful tool for studying long-term BMI changes. In this context, age-related effects on BMI refer to various individual-level changes in weight trajectories from childhood (Robinson et al. 2019) to older ages (Murayama and Shaw 2017). Period effects characterise the external influence of social, economic, cultural and physical environments that may affect individual BMI. For example, such effects have been demonstrated in the context of the late 2000s economic recession (Jofre-Bonet et al. 2018) with recession resulting in higher BMI among general population. The BMI may also vary by birth cohorts as recent birth cohorts may have been more exposed to the lack of physical exercise, heightened sedentary behaviour and poor quality diet than earlier cohorts (Hoare et al. 2014). It is therefore plausible that different exposures may result in cohort-specific effects on BMI.
Most previous APC studies (Allman-Farinelli et al. 2008; An and Xiang 2016; Diouf et al. 2010; Jaacks et al. 2013; Keyes et al. 2010; Reither et al. 2009; Wilson and Abbott 2018) have found age and period effects related to BMI changes. A substantial curvilinear relationship with age is generally reported in these studies with prevalence of overall overweight/obesity increasing until the mid-60s followed by a decline at older ages. Most of the studies also report an almost linear increase in BMI as a period effect. For example, the predicted probability of obesity for a 25-year-old adult in USA has increased almost threefold from 7.5% in 1976 to 21% in 2002 (Reither et al. 2009). Regarding cohort effects, the results are less consistent. Some studies (Allman-Farinelli et al. 2008; An and Xiang 2016; Wilson and Abbott 2018) have reported no significant cohort effects, whereas most other studies have reported nonlinear increase in prevalence of overweight/obesity for more recent cohorts.
We will use the APC framework to study the long-term BMI trends among adult population in Estonia, where over half of the adult population is either overweight or obese (Reile and Leinsalu 2019). Although the estimated impact of obesity on overall life expectancy in Estonia is smaller compared to some Central and Eastern European countries (Vidra et al. 2019), the considerable increase in obesity prevalence from the 1990s, particularly among men (Klumbiene et al. 2004; Pomerleau et al. 2000) underpins the rising importance of excess body weight as a public health problem. While these earlier studies have highlighted some age- and period-specific patterns, the age–period–cohort perspective might shed further light on evolving overweight/obesity epidemic. This study aimed to examine the long-term changes in BMI among Estonian adults by disentangling the independent effects of age, period and cohort on (a) mean BMI and (b) on probability of obesity.
Methods
Data
Data from the Health Behaviour Surveys among Estonian Adult Population, a series of biennial cross-sectional postal surveys, were used for this study. These nationally representative surveys have been carried out since 1990 using similar methodology. Each survey was based on individual random sample of Estonian residents aged 16–64 years obtained from population registry. Survey response rates varied from 77% in 1996 to 51% in 2018. Detailed information about the survey can be found elsewhere (Reile et al. 2019). All studies were approved by Tallinn Medical Research Ethics Committee and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
This study covers data from 12 consecutive surveys between 1996 and 2018 with 11 547 men and 16,298 women aged 16–64 years in total. The extended period of time and even spacing between the survey years make this data series suitable for APC analyses on long-term trends in BMI. The dependent variables of (a) mean BMI and (b) prevalence of obesity were calculated from self-reported height and weight data (BMI = weight (kg)/[height (m)]2.
Respondents’ age, year of birth and the survey year are key temporal variables in APC analyses. To reduce the linear dependency between these variables (e.g. period − age = birth cohort), different interval lengths were used. For descriptive analysis, age was aggregated into six groups of 16–19-, 20–29-, 30–39-, 40–49-, 50–59- and 60–64-year-olds. The respondents’ age in full years was included both as a continuous and as a squared variable in the models to address the identification problem, i.e. the linear dependency between temporal variables of age, period and cohort (Yang and Land 2013). Similar approach has been used in several other APC studies (Reither et al. 2009; Wilson and Abbott 2018). Additionally, age was centred on 40 years to allow easier interpretation of the intercept.
Period was represented by the year of the survey. To increase the statistical power for analysis, two consecutive survey years were merged resulting in six study periods: 1996/1998, 2000/2002, 2004/2006, 2008/2010, 2012/2014 and 2016/2018. Cohort was defined by respondents’ birth decade and classified as: 1930s–1940s, 1950s, 1960s, 1970s, 1980s and 1990s–2000s. For example, a 30-year-old respondent in 1996 survey and a 50-year-old respondent in 2016 survey were born in 1966 and belong to the 1960s birth cohort.
Earlier studies (An and Xiang 2016; Reither et al. 2009; Wilson and Abbott 2018) have demonstrated that APC effects on BMI may vary by educational attainment and ethnicity/race. Among other demographic changes during the study period (1996–2018) in Estonia, the proportion of ethnic Estonians (from 67 to 73%) and those with tertiary education (from 16 to 36%) have increased considerably. As both indicators are powerful behavioural and socio-economic determinants, the changing distributions in ethnicity and education variables could confound the APC effects on BMI and obesity. The models were therefore additionally adjusted for ethnicity and educational level of the respondents. Self-reported ethnic identity was categorised as Estonians and non-Estonians. The highest educational level obtained was dichotomised into categories of tertiary and less-than-tertiary education.
Statistical analysis
Hierarchical age–period–cohort (HAPC) analysis with cross-classified random effects modelling (CCREM) was used in this study. Same technique has been used earlier by Reither et al. (2009) and more recently by Wilson and Abbott (2018). Age and age squared were specified (along with ethnicity and education in Model 2) as fixed effects and variables of period and cohort as random effects in these models. All analyses were performed separately for men and women. For the ease of interpretation, random effects coefficients were converted to predicted probabilities as suggested by Yang and Land (2013). The tables present the initial model estimates (with p values compared to model intercept) and predicted mean BMI values for the 40-year-olds with 95% confidence intervals (Table 2) and predicted probabilities of obesity for the 40-year-olds with 95% confidence intervals (Table 3). The robustness of model estimates was validated by additional sensitivity analysis using two-factor models (e.g. age–period model) that resulted in very similar random effects coefficients (data not shown). All analyses were performed in SAS Studio 3.8 using PROC MIXED and PROC GLIMMIX commands.
Results
Sample sizes by age groups, periods and cohorts along with observed mean BMI values and obesity prevalence (with 95% confidence intervals) are presented in Table 1. Among men, the mean BMI varied from 22.2 in the 16–19-year-olds to 27.4 in the 60–64-year-olds; among women, it varied from 21.1 to 28.5 in respective age groups. The prevalence of obesity ranged from 3.1% (men) and 1.7% (women) in the 16–19 age group to 24.0% (men) and 34.7% (women) in the 60–64 age group. Although both indicators had increased significantly over the study period, the confounding age gradient in mean BMI and prevalence of obesity is visible in univariate cohort data.
APC analysis of mean BMI
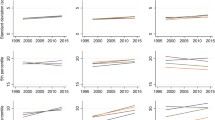
Table 2 presents the results of the HAPC mixed effects model on predicted mean BMI. As demonstrated by descriptive data, age was significantly associated with BMI for both men and women. The adjustment for ethnicity and education in Model 2 had only minor effect on age coefficients; the results of the adjusted analysis are graphically presented in Fig. 1.
Predicted mean BMI for the 40-year-olds increased over the study period from 25.6 to 27.0 among men and from 24.8 to 25.1 among women. Compared to the model intercept, statistically significant period effects were found for 1996/1998, 2004/2006 and 2016/2018 for men and 2004/2006 and 2012/2014 for women. After adjustment for ethnicity and education in Model 2, the period effects on predicted mean BMI values retained their statistical significance.
Independent cohort effects in mean BMI were found only for men born in the 1970s. For men, the mean predicted BMI of the 1970s cohort was significantly higher than overall mean represented by the model intercept. After adjusting for ethnicity and education, the 1970s cohort effect retained its statistical significance. For women, no statistically significant cohort effects were found in our data.
APC analysis of obesity
Table 3 presents the results of the HAPC with generalised linear mixed models on obesity. As with the mean BMI, older age significantly increased the predicted probability of obesity for both men and women. The estimates varied only slightly when ethnicity and education were added as fixed effects in Model 2. The results of adjusted models are graphically presented in Fig. 2.
Predicted probability of obesity for the 40-year-old men increased over the study period from 0.11 in 1996/1998 to 0.20 in 2016/2018 with significant period effects compared to model intercept found for 1996/1998 and 2016/2018. The effects persisted after adjustment for ethnicity and education in Model 2. Among women, the period effects were smaller with statistically significant effects found only for 2012/2014 period in the adjusted model.
No statistically significant cohort effects were found for obesity outcome. The predicted probability of being overweight at the age of 40 ranged from 0.16 to 0.18 among men across cohorts and was reduced to 0.14 to 0.17 in Model 2. For women, the predicted probability of obesity was 0.14 for all cohorts in Model 1 and ranged from 0.17 to 0.18 after adjustment for education and ethnicity in Model 2.
Discussion
This study examined the long-term BMI changes among Estonian adult population by focusing on the independent effects of age, period and cohort. The findings are generally consistent with previous studies (Allman-Farinelli et al. 2008; An and Xiang 2016; Diouf et al. 2010; Jaacks et al. 2013; Keyes et al. 2010; Reither et al. 2009; Wilson and Abbott 2018) with respect to age and period effects, but differ slightly when cohort effects are considered. A distinctive age gradient, where advancement in age resulted in higher predicted mean BMI and probability of obesity, was found for both men and women. Independently from age, statistically significant period effects in mean BMI and obesity risk were found with higher mean BMI and predicted probability of obesity found for recent study periods. The cohort effects in mean BMI were found for the men born in 1970s who had slightly higher mean BMI compared to period average, but no significant differences were found for obesity outcome.
Before discussing these findings in detail, some potential considerations regarding the data and the methods used need to be addressed. First, respondents’ height and weight were based on self-reports and can potentially be underestimated. Such social desirability bias has been generally low in self-administered postal surveys (Bowling 2005). This is supported by findings from a previous study (Aasvee et al. 2015) where the mean difference between self-reported and measured overweight prevalence among Estonian adolescents was 3.6%. While this cannot be controlled for in our data, the underestimation of BMI could differ by age as demonstrated by Ikeda (2016) and confound the association between age and BMI. Second, declining response rates of the surveys may have affected the data representativeness due to possible systematic differences in characteristics between responders and non-responders. Its effects are most likely limited as an earlier study (Helasoja et al. 2002) using the same survey data did not find systematic differences in prevalence estimates between respondents and late respondents, suggesting minimal response bias if late respondents were used as proxies for non-respondents. Model-related considerations are foremost related to the APC methodology used in this study as validity of HAPC modelling has been disputed by some authors. While the detailed description of the issue can be found elsewhere (Bell and Jones 2014, 2018), the main argument dwells on the identification problem that HAPC approach does not necessarily resolve. Reither and colleagues (2015) have already addressed this critique and provided additional support for the use of HAPC modelling when analysing data from repeated cross-sectional surveys. The major strengths of the study are the long, nationally representative data series. The standardised methodology of initial surveys enables to use comparable measures over long time period that is necessary for APC analysis. To the best of our knowledge, this is the first study on age, period and cohort effects on BMI trends from Eastern Europe providing new insights into the problem in this region.
Study found that increasing age resulted in higher mean BMI and obesity risk for both men and women. Association between individual age and excess BMI and obesity risk could be explained by a range of interlinked metabolic and environmental factors. These include the change in body composition due to decline in resting energy expenditure at older ages (Siervo et al. 2015) and increasing sedentary lifestyle (Wullems et al. 2016) that are also associated with BMI and other weight-related biomarkers (Wirth et al. 2017). However, the age effects on BMI differed by gender in our data. For men, the mean BMI increased until the late 40s, after which the growth slowed and plateaued (or declined in case of obesity) around the age 60. Among women, the age and BMI presented nearly a linear association. Similar gender differences were found for obesity where the predicted probability of obesity was comparable between men and women up to the age of 40 after which obesity risk among women continued to increase. These patterns are somewhat different from previous APC studies (An and Xiang 2016; Jiang et al. 2013; Reither et al. 2009) where the decline in BMI and obesity is visible in late 60s. One likely explanation relates to the narrower age range in our study (16–64 years) compared to earlier studies that have also included respondents in their 70s and 80s. It is therefore possible that the slightly later declines in BMI for women could be right censored by the age range of our sample. The gender differences in BMI’s age gradient could also relate to other external factors such as differences in adulthood stress levels (Liu and Umberson 2015) or lower socio-economic position (Clarke et al. 2009) among others. Although our cross-sectional data did not allow analysing past exposure to stress, the variable of education proved to be statistically significant contributor to both BMI and obesity outcomes with stronger effects found for women.
Independently from age, statistically significant period effects in mean BMI and obesity risk were found in our data. In the adjusted model, the predicted mean BMI for men was 1.4 points higher in 2016/2018 compared to 1996/1998, whereas the respective increase among women was 0.64 points. Similarly, the probability of being obese at the age of 40 was 12% for men in 1996/1998 data, but had increased to 19% by 2016/2018. For women, the probability of being obese at the age of 40 was highest (20%) in 2012/2014 period. Although the slight decline in mean BMI and probability of obesity in the latest period was statistically insignificant for women, it contrasts to the consistent increase that is visible for men since 2008/2010 data. Although gender differences in the period effects have also been reported in some earlier studies (Jaacks et al. 2013; Tu et al. 2011), one potential explanation in our case could relate to differences in weight-related behaviour among overweight persons. A recent study among adults with excess body weight (Reile and Leinsalu 2019) demonstrated that women were on average 1.5 times more likely to change their diet or improve physical activity than men. Although respective changes increased at higher rate for men, the gender differences in weight-related behavioural change remained significant. Another period effect, visible for both men and women, is the increase in mean BMI between 2004/2006 and 2008/2010. This corresponds to the period of rapid economic growth (Brixiova et al. 2010). While increasing wealth is generally associated with health improvement, it can also promote unfavourable changes in dietary structure and lifestyle habits that may eventually lead to a number of diseases linked with prosperity (Lange and Vollmer 2017). For example, factors such as median income, alcohol price and restaurant density, all related to economic growth, have been associated with BMI increases (Courtemanche et al. 2016). As period effects were compared to overall mean in our models, the respective changes remained statistically non-significant. However, this particular explanation relating economic expansion to individual-level BMI changes in our data warrants further research including wider range of socio-economic and macro-level indicators.
Cohort effects were found only for men born in the 1970s, who had higher mean BMI compared to the average. This cohort loosely matches the boundaries of “the winners’ generation” (Titma et al. 1998), the men and women who were in their 30s when Estonia underwent the transformation from Soviet system to liberal democracy in the first half of the 1990s. Their age and up-to-date education proved beneficial when starting their careers in new and rapidly changing circumstances. Although these advantages could have resulted in higher socio-economic status which in general is associated with lower BMI (Vieira et al. 2019), opposite association was found for men born in the 1970s. It is plausible that the link between higher SES and lower BMI could be reversed in settings with high material deprivation (Haregu et al. 2018). Also, the possible overall beneficial effects of upward social mobility for some could have been mitigated by the drastic rise in overall social disparities (Henderson et al. 2008) and severely affected health outcomes (Leinsalu et al. 2004). With respect to obesity, no cohort differences were found in our data. Similar findings were reported in previous studies from Australia (Allman-Farinelli et al. 2008) and USA (An and Xiang 2016), whereas some other studies have found increased obesity rates for later cohorts. For example, Reither and colleagues (2009) demonstrated that probability of obesity at age 25 had increased by 30% for cohorts born between 1955 and 1975. Similar obesity risk across birth cohorts in our data suggests that the overall increase in obesity rates in Estonia is more likely due to period rather than cohort-specific exposures to behavioural and environmental determinants of excess body weight.
Conclusions
The findings on age and period effects in BMI trends were generally consistent with previous studies, providing further support for the claim that BMI and obesity risk increase at older ages and in more recent study periods. The strong period but limited cohort effects indicate that the rise in population-level mean BMI and prevalence of obesity are most likely driven by overall behavioural and environmental changes that affect the whole population rather than being influenced by cohort-specific factors. Being the first study on age, period and cohort effects on BMI in Eastern Europe, these findings elucidate the temporal patterns of increasing excess body weight and its public health challenge in this region.
References
Aasvee K, Rasmussen M, Kelly C, Kurvinen E, Giacchi MV, Ahluwalia N (2015) Validity of self-reported height and weight for estimating prevalence of overweight among Estonian adolescents: the Health Behaviour in School-aged Children study. BMC Res Notes 8(606):6. https://doi.org/10.1186/s13104-015-1587-9
Allman-Farinelli MA, Chey T, Bauman AE, Gill T, James WP (2008) Age, period and birth cohort effects on prevalence of overweight and obesity in Australian adults from 1990 to 2000. Eur J Clin Nutr 62:898–907. https://doi.org/10.1038/sj.ejcn.1602769
An R, Xiang X (2016) Age–period–cohort analyses of obesity prevalence in US adults. Public Health 141:163–169. https://doi.org/10.1016/j.puhe.2016.09.021
Bell A, Jones K (2014) Don’t birth cohorts matter? A commentary and simulation exercise on Reither, Hauser, and Yang’s (2009) age–period–cohort study of obesity. Soc Sci Med 101:176–180. https://doi.org/10.1016/j.socscimed.2013.09.004
Bell A, Jones K (2018) The hierarchical age–period–cohort model: Why does it find the results that it finds? Qual Quant 52:783–799. https://doi.org/10.1007/s11135-017-0488-5
Benziger CP, Roth GA, Moran AE (2016) The Global Burden of Disease Study and the preventable burden of NCD. Glob Heart 11:393–397. https://doi.org/10.1016/j.gheart.2016.10.024
Bowling A (2005) Mode of questionnaire administration can have serious effects on data quality. J Public Health-UK 27:281–291. https://doi.org/10.1093/pubmed/fdi031
Brixiova Z, Vartia L, Wörgötter A (2010) Capital flows and the boom-bust cycle: the case of Estonia. Econ Syst 34:55–72. https://doi.org/10.1016/j.ecosys.2009.11.002
Centers for Disease Control and Prevention (2018) Adult obesity causes & consequences. https://www.cdc.gov/obesity/adult/causes.html. Accessed 7 Feb 2020
Clarke P, O’Malley PM, Johnston LD, Schulenberg JE (2009) Social disparities in BMI trajectories across adulthood by gender, race/ethnicity and lifetime socio-economic position: 1986–2004. Int J Epidemiol 38:499–509. https://doi.org/10.1093/ije/dyn214
Courtemanche CJ, Pinkston JC, Ruhm CJ, Wehby GL (2016) Can Changing economic factors explain the rise in obesity? South Econ J 82:1266–1310. https://doi.org/10.1002/soej.12130
Diouf I, Charles MA, Ducimetiere P, Basdevant A, Eschwege E, Heude B (2010) Evolution of obesity prevalence in France: an age–period–cohort analysis. Epidemiology 21:360–365. https://doi.org/10.1097/ede.0b013e3181d5bff5
Haregu TN, Mohamed SF, Muthuri S, Khayeka-Wandabwa C, Kyobutungi C (2018) Body mass index and wealth index: positively correlated indicators of health and wealth inequalities in Nairobi slums. Glob Health Epidemiol Genom 3:e11–e11. https://doi.org/10.1017/gheg.2018.10
Helasoja V, Prattala R, Dregval L, Pudule I, Kasmel A (2002) Late response and item nonresponse in the Finbalt Health Monitor survey. Eur J Public Health 12:117–123
Henderson DR, McNab RM, Rozsas T (2008) Did inequality increase in transition? An analysis of the transition countries of Eastern Europe and Central Asia. Eastern Eur Econ 46:28–49
Hoare E, Skouteris H, Fuller-Tyszkiewicz M, Millar L, Allender S (2014) Associations between obesogenic risk factors and depression among adolescents: a systematic review. Obes Rev 15:40–51. https://doi.org/10.1111/obr.12069
Ikeda N (2016) Validity of self-reports of height and weight among the general adult population in Japan: findings from National Household Surveys. PLoS ONE 11:e0148297. https://doi.org/10.1371/journal.pone.0148297
Jaacks LM, Gordon-Larsen P, Mayer-Davis EJ, Adair LS, Popkin B (2013) Age, period and cohort effects on adult body mass index and overweight from 1991 to 2009 in China: the China Health and Nutrition Survey. Int J Epidemiol 42:828–837. https://doi.org/10.1093/ije/dyt052
Jiang T, Gilthorpe MS, Shiely F, Harrington JM, Perry IJ, Kelleher CC, Tu YK (2013) Age–period–cohort analysis for trends in body mass index in Ireland. BMC Public Health 13:889. https://doi.org/10.1186/1471-2458-13-889
Jofre-Bonet M, Serra-Sastre V, Vandoros S (2018) The impact of the Great Recession on health-related risk factors, behaviour and outcomes in England. Soc Sci Med 197:213–225. https://doi.org/10.1016/j.socscimed.2017.12.010
Johnson W, Li L, Kuh D, Hardy R (2015) How has the age-related process of overweight or obesity development changed over time? co-ordinated analyses of individual participant data from five United Kingdom birth cohorts. PLOS Med 12:e1001828. https://doi.org/10.1371/journal.pmed.1001828
Keyes KM, Utz RL, Robinson W, Li G (2010) What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc Sci Med 70:1100–1108. https://doi.org/10.1016/j.socscimed.2009.12.018
Klumbiene J, Petkeviciene J, Helasoja V, Prattala R, Kasmel A (2004) Sociodemographic and health behaviour factors associated with obesity in adult populations in Estonia, Finland and Lithuania. Eur J Public Health 14:390–394. https://doi.org/10.1093/eurpub/14.4.390
Lange S, Vollmer S (2017) The effect of economic development on population health: a review of the empirical evidence. Br Med Bull 121:47–60. https://doi.org/10.1093/bmb/ldw052
Leinsalu M, Vågerö D, Kunst AE (2004) Increasing ethnic differences in mortality in Estonia after the collapse of the Soviet Union. J Epidemiol Commun Health 58:583–589. https://doi.org/10.1136/jech.2003.013755
Liu H, Umberson D (2015) Gender, stress in childhood and adulthood, and trajectories of change in body mass. Soc Sci Med 139:61–69. https://doi.org/10.1016/j.socscimed.2015.06.026
Murayama H, Shaw BA (2017) Heterogeneity in Trajectories of body mass index and their associations with mortality in old age: a literature review. J Obes Metab Syndr 26:181–187. https://doi.org/10.7570/jomes.2017.26.3.181
OECD (2019) The heavy burden of obesity: the economics of prevention, OECD Health Policy Studies, OECD Publishing, Paris. https://doi.org/10.1787/67450d67-en. Accessed 7 Feb 2020
Pomerleau J et al (2000) Patterns of body weight in the Baltic Republics. Public Health Nutr 3:3–10
Reile R, Leinsalu M (2019) Factors associated with improving diet and physical activity among persons with excess body weight. Eur J Public Health 29:1166–1171. https://doi.org/10.1093/eurpub/ckz170
Reile R, Tekkel M, Veideman T (2019) Eesti täiskasvanud rahvastiku tervisekäitumise uuring 2018 [Health Behaviour among Estonian Adult Population, 2018], Tervise Arengu Instituut, Tallinn. https://www.tai.ee/et/terviseandmed/uuringud/download/484. Accessed 7 Feb 2020
Reither EN, Hauser RM, Yang Y (2009) Do birth cohorts matter? Age–period–cohort analyses of the obesity epidemic in the United States. Soc Sci Med 69:1439–1448. https://doi.org/10.1016/j.socscimed.2009.08.040
Reither EN, Land KC, Jeon SY, Powers DA, Masters RK, Zheng H et al (2015) Clarifying hierarchical age–period–cohort models: a rejoinder to Bell and Jones. Soc Sci Med 145:125–128. https://doi.org/10.1016/j.socscimed.2015.07.013
Robinson HA, Dam R, Hassan L, Jenkins D, Buchan I, Sperrin M (2019) Post-2000 growth trajectories in children aged 4–11 years: a review and quantitative analysis. Prev Med Rep 14:100834. https://doi.org/10.1016/j.pmedr.2019.100834
Siervo M et al (2015) Age-related changes in resting energy expenditure in normal weight, overweight and obese men and women. Maturitas 80:406–413. https://doi.org/10.1016/j.maturitas.2014.12.023
Titma M, Tuma NB, Silver BD (1998) Winners and losers in the postcommunist transition: new evidence from Estonia. Post-Sov Aff 14:114–136. https://doi.org/10.1080/1060586X.1998.10641449
Townshend T, Lake A (2017) Obesogenic environments: current evidence of the built and food environments. Perspect Public Health 137:38–44. https://doi.org/10.1177/1757913916679860
Tu YK, Chien KL, Burley V, Gilthorpe MS (2011) Unravelling the effects of age, period and cohort on metabolic syndrome components in a Taiwanese population using partial least squares regression. BMC Med Res Methodol 11:82. https://doi.org/10.1186/1471-2288-11-82
Vidra N, Trias-Llimós S, Janssen F (2019) Impact of obesity on life expectancy among different European countries: secondary analysis of population-level data over the 1975–2012 period. BMJ Open 9:e028086. https://doi.org/10.1136/bmjopen-2018-028086
Vieira LS, Bierhals IO, Vaz JDS, Meller FO, Wehrmeister FC, Assuncao MCF (2019) Socioeconomic status throughout life and body mass index: a systematic review and meta-analysis. Cad Saude Publica 35:e00125518. https://doi.org/10.1590/0102-311x00125518
Wilson R, Abbott JH (2018) Age, period and cohort effects on body mass index in New Zealand, 1997–2038. Aust Nz J Publ Heal 42:396–402. https://doi.org/10.1111/1753-6405.12804
Wirth K et al (2017) Biomarkers associated with sedentary behaviour in older adults: a systematic review. Ageing Res Rev 35:87–111. https://doi.org/10.1016/j.arr.2016.12.002
World Health Organisation Obesity: Situation and Trends. http://www.who.int/gho/ncd/risk_factors/obesity_text/en. Accessed 7 Feb 2020
Wullems JA, Verschueren SMP, Degens H, Morse CI, Onambélé GLJB (2016) A review of the assessment and prevalence of sedentarism in older adults, its physiology/health impact and non-exercise mobility counter-measures. Biogerontology 17:547–565. https://doi.org/10.1007/s10522-016-9640-1
Yang Y, Land K (2013) Age–period–cohort analysis: new models, methods, and empirical applications. Chapmann and Hall, New York. https://doi.org/10.1201/b13902
Funding
No specific funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reile, R., Baburin, A., Veideman, T. et al. Long-term trends in the body mass index and obesity risk in Estonia: an age–period–cohort approach. Int J Public Health 65, 859–869 (2020). https://doi.org/10.1007/s00038-020-01447-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-020-01447-7