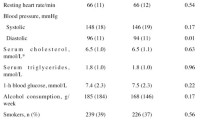
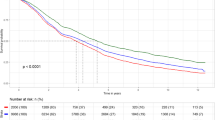
Abstract
Objectives
The aim was to study whether the effects of a population-based health check and lifestyle intervention differed according to study participation rate.
Methods
All persons living in 73 areas of Copenhagen County, Denmark, were included in the Inter99 randomized trial in 1999 (intervention group n = 11,483; control group n = 47,122). All persons in the intervention group were invited for health checks and were offered lifestyle counseling if they were at high risk of ischemic heart disease. Areas were divided into low 35–49%, middle 50–54% and high ≥ 55% health check participation. All persons were followed in registers for 10-year cause-specific mortality.
Results
In high-participation areas, there was a significantly higher risk of lifestyle-(HR 1.37 [1.04, 1.79]) and cancer-related deaths (HR 1.47 [1.08, 2.02]) among women in the intervention group than control group. Regarding smoking-related cancer deaths, differences were even more pronounced. Among men, no significant difference in mortality was seen between control and intervention groups.
Conclusions
The results of this paper suggest that among women, the health check and lifestyle intervention may increase the risk of lifestyle and cancer-related deaths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The premise of population-based general health checks is that early detection of risk factors or preclinical manifestations of diseases improve the prognosis (Wilson et al. 1968). In several countries, ministries of health have (typically in collaborations with non-governmental organizations, insurance companies, private health providers and industry) (Abdalrahman and Soljak 2015) introduced nationwide preventive health checks followed by lifestyle interventions of high-risk persons. The evidence that supports population-based health checks is founded on extensive amount of scientific literature including longitudinal observational studies showing strong associations between unhealthy lifestyle and development of cardiovascular disease (CVD) (Perk et al. 2012). Therefore, it makes sense to believe that the majority of deaths that are attributed to CVD can be prevented through early identification of risk factors followed by lifestyle interventions of high-risk persons (Rose 1987). In addition, at individual level there is good evidence that some interventions as, e.g., assistance to quit smoking given by health professionals does increase quit rates significantly (Stead et al. 2013).
In population-based multifactorial preventive interventions, however, no effect can be detected in intention-to-treat analyses (Krogsboll et al. 2012). These results have been confirmed by the large Danish Inter99 study, a population-based randomized lifestyle intervention consisting of health checks, risk assessment and repeated lifestyle interventions offered to high-risk persons (Jørgensen et al. 2014). And recently, a paper found no effect on mortality or ischemic heart disease (IHD), while higher incidence of stroke was observed in the group offered health checks (Skaaby et al. 2017). One argument for the interventions finding no effects at the population level has been low participation rates and difficulties with attracting those who benefit the most (NHS Health Check Expert Scientific and Clinical Advisory Panel 2014). Participation and adherence are vital to create a better foundation for reaching a tailored and effective lifestyle intervention approach. Participation rates in epidemiological studies in Western countries have declined during the past decades, and today participation rates in studies involving health checks are typically around 40–55% (Mindell et al. 2015).
Therefore, in a previous paper we investigated how different participation rates in 73 census districts of the Inter99 study area influenced the effect of the intervention on mortality (Bender et al. 2017). Surprisingly, among women living in high-participation areas (≥ 55%) a significantly higher risk of all-cause mortality (HR 1.32 [1.03–1.69]) was found in the intervention group (ref = controls). There was no difference between the intervention and control groups in incident IHD/stroke. In a previous paper by Jørgensen et al. [appendix 3 of the paper (Jørgensen et al. 2014)], among women of the Inter99 intervention a nonsignificant higher CVD risk has likewise been detected.
This raised the question why mortality was higher in women in the intervention group in areas with high participation. Comparing the risk of death from specific causes will shed light on the disease etiology and will support the evidence of potential pathways between participation and death. Ultimately, the aim is to investigate whether the previous results on higher risk of death is explained by harmful effects of the intervention or merely is due to chance.
In this paper, the aim was to study differences in disease-specific death among men and women in the intervention and control group of the Inter99 study, across areas with low, middle and high participation.
Methods
Inter99 study population and study design
The analyses of this paper are post hoc analyses based on the entire Inter99 study population. The Inter99 study is a large population-based randomized lifestyle intervention, which took place in the southwestern part of Copenhagen County, Denmark, in the years 1999–2006. The study was approved by the Regional Scientific Ethics Committee (KA 98 155) and the Danish Data Protection Agency. The study is registered at ClinicalTrials.Gov (NCT00289237). The study population was selected on December 2, 1998, and comprised all inhabitants living in 73 census districts within 11 municipalities in the South Western part of Copenhagen County, Denmark. Selected age groups were persons born in 1939–1940, 1944–1945, 1949–1950, 1954–1955, 1959–1960, 1964–1965 and 1969–1970 (n = 61,301). Details of the study have previously been published (Jørgensen et al. 2003). The study population consisted of all inhabitants in selected age groups. Before baseline, men and women were randomized by computer-generated random numbers to either control group (n = 48,285) or intervention group (n = 13,016) based on power calculations (Jørgensen et al. 2003). A small random sample (10%) of the intervention group (n = 1308) was allocated to a low-intensity intervention group. (This group will not be dealt with in this paper.) Between the date of randomization and the baseline examination date, 184 persons emigrated and 187 persons died. In addition, persons moving to a municipality outside the study area and persons with no identifiable census district code were excluded (n = 1011); leaving 11,483 persons in the intervention group and 47,122 persons in the control group for analyses.
Persons in the intervention group were invited to participate in a health check at the Research Centre for Prevention and Health, Glostrup, Denmark. Invitations included easy-to-change pre-arranged dates for the health check, and in order to increase the participation rate, one reminder was sent if no response was obtained. A total of 6090 persons accepted and participated in the health check (52%) where each participants’ 10-year risk of fatal and non-fatal IHD was calculated on the basis of physical measurements from the health examination and other predefined criteria by use of the Copenhagen Risk Score (Thomsen et al. 2001; Jørgensen et al. 2003). In total 60% of the participants in the intervention group met the high-risk criteria (Jørgensen et al. 2003). All participants received lifestyle counseling, and high-risk persons were additionally offered group-based counseling with six sessions over a 4- to 6-month period. After one and 3 years, all high-risk participants were re-invited to health checks and individual lifestyle counseling. If they still met the high-risk criteria they were once more offered group-based lifestyle counseling. Finally, after 5 years all participants who attended the baseline health check were re-invited to a health check. Protocol can be downloaded at www.inter99.dk. Except for a small random sample of the control group receiving questionnaires, no contact was taken to the control group.
A start date was noted for each person indicating the onset of the 10-year observational period. The date of the baseline health check was the start date for those who participated in the intervention group. The start date for non-participants and for persons in the control group was defined as the median examination date of the majority of the participants born in the same month (Jørgensen et al. 2014).
Data
Persons in the intervention group were categorized as participating if they attended the baseline health check. All persons were grouped into their respective census district and area participation rate for each census district was calculated as the number of participants in the intervention group divided by the number of all invited persons. The participation rate varied substantially between areas; participation ranged between 35 and 84%. We ranked the areas according to their participation rate and divided them into tertiles defining three groups: low (35–49%)-, middle (50–54%)- and high (55–84%)-participation area. Area participation rate thus reflects the participation rate of persons assigned to the intervention group before baseline.
Information on date of emigration, sex, age and ethnicity was obtained from the central personal register at baseline. Ethnicity (Danish/Western or other origins) was based on each person’s and their parents’ nationality. Education was categorized into basic education (up to high school), low education (< 2 years of vocational training/education), middle education (2–4 years of vocational training/education) and high education (> 4 years of education; academic degree). Income (equalized disposable) was calculated as the 5-year average household income after taxation and interest, divided by the number of equivalent adults in the household and corrected for inflation by adjusting to the year 2000 price index (European Commission 2014). In the descriptive analyses, income was divided into quartiles. Employment status was categorized as wage earners, not in work or retired. Cohabitation was categorized as living with a partner or being single. Housing tenure was categorized as either tenant or homeowner. Severe morbidity was included as a continuous variable and covered potentially life-shortening diseases from all organ systems comprising hospital contacts in the period from 1978 to start. (More details on the variable can be obtained from Jørgensen et al. 2014.) In the descriptive analyses, the variable was coded “yes” if a person had any record within this given period.
We retrieved information on total and disease-specific mortality from date of randomization and 10 years onward from the Danish Register of Causes of Death. Disease-specific deaths were categorized into deaths from cancer (neoplasms); CVD (cardiovascular disease; diseases of the circulatory system); other primarily lifestyle-related diseases including all deaths from (1) diseases of the digestive system and liver, (2) endocrine, nutritional and metabolic diseases and (3) respiratory disease; and primarily not-lifestyle-related causes including deaths from 11 distinctive causes of death (e.g., accidents, infections, congenital disease). Analyses on cancer-related deaths were additionally divided into smoking-related cancers and other cancer deaths (Office of the Surgeon General (US) and Office on Smoking and Health (US) 2004; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health 2014). (ICD-10 codes for each disease category can be found in web-based Appendix A.)
Statistical analyses
Descriptive statistics include the baseline distribution of potential confounders in the low-, middle- and high-participation area for men and women separately. We accounted for higher age (planned randomization) in the intervention group by conducting age-adjusted Chi-square tests. Supplementary analyses (web-based appendix B) stratified the baseline analyses into control and intervention groups of each participation area, and again Chi-square tests were used to assess differences in the distribution of categorical variables between control and intervention groups.
Cox proportional hazard analyses were conducted to estimate the differences in disease-specific mortality between persons in the intervention group and the control group. For each participation area (low, middle and high), HR of death was estimated in the intervention group with reference group defined as controls living within the same area. All analyses were conducted separately for men and women as a previously published paper showed important sex differences (Bender et al. 2017). Several previous analyses from the Inter99 study have shown a successful randomization with little and no difference in the distribution of potential confounders between the intervention and control groups (Jørgensen et al. 2014). Still, as randomization of study populations is not possible in non-participation analyses, it was important to include potential confounders in the statistical analyses, which could differ between the intervention and control groups. Therefore, in adjusted analyses we included five socioeconomic factors, ethnicity and age as confounders.
There was no indication of violation of the proportional hazards assumption, tested by Schoenfeld residual plots and Log–Log curves. We also tested for nonlinearity of age by including both the continuous variable age and age squared into the statistical model. Statistical significance was taken as a two-tailored p value < 0.05, and all analyses were performed using the statistical software program SAS 9.3.
Results
Baseline analyses of men and women in low-, middle- and high-participation areas (Table 1) show that persons living in high-participation areas in general were older, more were of Danish origin, and they had better socioeconomic position and a smaller proportion had a severe disease. Supplementary analyses (web-based appendix B) showed no significant differences in socioeconomic position between the intervention and control groups in high-participation areas. Likewise, no large differences were seen in low- and middle-participation areas. During the 10-year follow-up period 595 (5.2%) persons in the intervention group and 2568 (5.4%) persons in the control group died.
The distribution of causes of deaths (crude percentage) among controls and intervention group is shown for men and women and for each participation area separately in Fig. 1a–c. The figures among men and women show a larger proportion of cancer deaths and fewer deaths from not-lifestyle-related deaths among persons of high-participation areas when compared to middle- and low-participation areas. Among women living in high-participation areas, no clear difference existed in the distribution of causes of deaths compared to the control group. Among men in high-participation areas, the largest difference between the intervention and control groups is seen for CVD-related deaths, in which there was a higher proportion in the intervention group dying compared to men in the control group, and on the other hand a larger proportion of men in the intervention group died from not-lifestyle-related deaths and other lifestyle-related deaths.
In survival analyses (Table 2) in high-participation areas, in all disease-specific death categories we see a higher risk of death among women in the intervention group compared to controls and they have a significantly higher risk of dying from cancer in the intervention group compared to controls. Especially, a difference regarding smoking-related cancer deaths is higher in the intervention compared to control group, although not significant. No notable difference in the risk of death from not-lifestyle-related causes is found between the intervention and controls in high-participation areas (men CI 95% 0.41, 1.11; women CI 95% 0.52, 1.97).
Among men, no significant differences in risk of disease were seen between the intervention and control groups.
Discussion
In a randomized trial with health checks and lifestyle intervention in a general population, the Inter99 study, we found a significantly higher risk of death for lifestyle-related causes for women in areas with high participation rate in the intervention group. This seemed in large part to be driven by a higher risk of cancer-related deaths, and especially those related to smoking. No notable differences were seen for primarily not-lifestyle-related causes of death. Among men, no significant differences in cause-specific death were seen between the intervention and control groups in either area with low, medium or high participation rates.
As the analysis of the Inter99 study is the first to compare disease-specific mortality rates of an intervention in areas with different participation rates, no basis exists for comparison with other studies. Previous studies comparing the entire intervention and control group in intention-to-treat analyses of population-based lifestyle interventions (Jørgensen et al. 2014; Si et al. 2014) have shown no clear association between participation rates and effects of the intervention, supporting the results found in men. The hypothesis of a possible harm was also detected in a general practice setting, where a meta-analysis has shown higher risk of CVD among persons of the intervention group receiving health checks (Si et al. 2014).
The results seen in women bring new insight into the complex process of lifestyle changes and response to health checks. The analyses indicate that women in the intervention group died to a larger degree from smoking-related diseases, which is counterintuitive as higher quit rates at 5-year follow-up were observed among smoking participants in the intervention group than those in the small sample of the control group answering a questionnaire (23.9% vs. 13.9%) (Pisinger et al. 2008). Also quit rates in the smoking cessation groups offered as part of the intervention were lower for women than for men (Pisinger et al. 2008). Randomized smoking cessation interventions (Stead et al. 2013) and programs offering smoking cessation assistance free of charge likewise show high quit rates (Richard et al. 1996). We therefore expected if not a lower, then at least a nonsignificant tendency toward a lower risk of death in the intervention, compared to the control group. Nevertheless, we saw the same tendency across all lifestyle-related disease groups, but not notable difference regarding not-lifestyle-related causes of death. In this paper, we find significant worse health and lower socioeconomic position among persons living in low compared to middle- and high-participation areas. Previous analyses on the Inter99 study population show that analyses restricted to participants are subject to selection bias as people accepting the invitation and participating in the study have better health and socioeconomic profile (Bender et al. 2015). However, the supplementary analyses (Appendix B) of this paper suggest that selection bias is not violating the results as there are no important differences in socioeconomic position between persons in the control and intervention groups within low-participation areas, middle-participation areas and high-participation areas. Likewise, the supplementary analyses (web-based appendix B) show that the results do not seem to be explained by selection bias (e.g., differences in socioeconomic position between the intervention and control groups at baseline). Hence, there is a reason to believe our results are reflecting a true negative effect of the intervention. There are no obvious explanations for our finding, but we hypothesize three possible: (1) Smoking rates are low in areas with high participation. Those who continue smoking despite living in a higher SES area where anti-smoking is the norm are probably very dependent and might get stressed when they are told how important it is to stop smoking as they feel unable to do so. This might be explained by an increase in smoking intensity (McDermott et al. 2013). On the other hand, research points in the opposite direction, showing that persons who reported greater feelings of stigmatization about their smoking were more likely to report having made recent quit attempts (O’Connor et al. 2017) and those who have a relapse on average decrease their number of daily smoked cigarettes when compared to before their quit attempt (Yong et al. 2008). (2) People in areas with high participation seem to be more health conscious and we hypothesize that female smokers unable or to stop after they have been advised to do so, instead compensate by buying nutritional supplements. This will lead to higher number of cancer cases, as there is evidence that beta-carotene which is believed to protect smokers from cancer instead increases the risk of smoking-related cancer (Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group 1994). (3) Finally, we hypothesize that a normal lung function test might undermine the wish to quit in some female smokers.
The strengths of this paper are the large size of the multifactorial health check followed by individual- and group-based lifestyle intervention. The intervention is in large part comparable to the population-based health checks implemented at the national level in several countries (NHS Health Check Expert Scientific and Clinical Advisory Panel 2014; Kohro et al. 2008; Amoroso et al. 2009; Boytsov and Potemkina 2014). Apart from the qualities regarding study design, the possibility to merge the data from the Inter99 with those from national registers by means of a personal identification number is a strength. From the registers, we included information on each person in the study population; both participants, non-participants and controls eliminating social desirability bias, minimizing missing information and achieving complete blinding of a large majority of the control group who were not aware that they took part in the study. The entire study population was followed from date of randomization and 10 years onward, eliminating selective participation and loss to follow-up. The high completeness (only 1–2% were unknown) of the death register made it possible to compare mortality according to disease-specific causes. Despite the large size of the study, we did not have power to estimate deaths from smaller disease categories. For example, comparing differences in lung cancer would have strengthened our conclusions. One way to increase the power would be to extend the follow-up time in order to obtain more cases and precise estimates for rare disease. However, we assume the potential effect (if any) of the health check to diminish over time. Differences between the intervention and control groups may dilute when followed for more than 10 years. Though the entire control group was compared to the entire intervention group, the population was grouped and compared across participation areas, hereby losing the randomization regarding potential confounders. It is not possible to conduct non-participation research using randomization methods, because one cannot assign various participation rates to different areas. Still, the prospective component of the study assures that the temporal relationship is established. Additionally, the supplementary analyses showed in none of the participation areas any notable differences in socioeconomic position and health at baseline between the intervention and control groups. Apart from cancer and CVD, most disease-specific deaths were relatively rare; and we therefore chose to categorize them into brought disease categories. Likewise, some of the disease categories continue to have relatively few events which may have increased the confidence intervals and this should be taken into account when interpreting the results. In the future, additional analyses comparing death rates among participants (high- and low-risk participants) with non-participants in high-participation areas will tell if the intervention in its self is harmful or if the higher risk rather is explained by compensatory behavior.
Population-based preventive health checks are expensive and ineffective approaches to tackle non-communicable diseases in Western countries (Jørgensen et al. 2012; Holland 2009). In times of scarce resources, health care services should be spent cautiously. It has been argued that reasons for the interventions finding no effects at the population level might be low participation rates and difficulties with attracting those who benefit the most (Jørgensen et al. 2014). Based on the results from this paper, we found that when participation is high, participants are healthier, better off, more resourceful and thus have the lowest need of a health check. Higher participation rates in population-based health checks are probably unlikely to improve the effects of these. Most importantly, the results presented propose a serious concern; a higher risk of death from cancer among female participants living in the areas with high participation rates, indicating a harmful effect of the intervention in subgroups of the population.
References
Abdalrahman B, Soljak M (2015) NHS health checks. J Ambul Care Manag 38:5–9. https://doi.org/10.1097/JAC.0000000000000070
Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330:1029–1035. https://doi.org/10.1056/NEJM199404143301501
Amoroso C, Harris MF, Ampt A et al (2009) The 45 year old health check—feasibility and impact on practices and patient behaviour. Aust Fam Physician 38:358–362
Bender AM, Jørgensen T, Pisinger C (2015) Is self-selection the main driver of positive interpretations of general health checks? The Inter99 randomized trial. Prev Med 81:42–48. https://doi.org/10.1016/j.ypmed.2015.07.004
Bender AM, Jørgensen T, Pisinger C (2017) Do high participation rates improve effects of population-based general health checks? Prev Med. https://doi.org/10.1016/j.ypmed.2017.05.008
Boytsov S, Potemkina RA (2014) Perspectives: preventive measures for public health in Russian Federation. Eur Heart J Suppl 16:A84–A86. https://doi.org/10.1093/eurheartj/sut018
European Commission E (2018) Glossary: equivalised disposable income. https://ec.europa.eu/eurostat/statisticsexplained/index.php/Glossary:Equivalised_disposable_income
Holland W (2009) Periodic health examination—a brief history and critical assessment. Eurohealth 15(4):16–20
Jørgensen T, Borch-Johnsen K, Thomsen TF et al (2003) A randomized non-pharmacological intervention study for prevention of ischaemic heart disease: baseline results Inter99. Eur J Cardiovasc Prev Rehabil Off J Eur Soc Cardiol Work Groups Epidemiol Prev Card Rehabil Exerc Physiol 10:377–386. https://doi.org/10.1097/01.hjr.0000096541.30533.82
Jørgensen T, Capewell S, Prescott E et al (2012) Population-level changes to promote cardiovascular health. Eur J Prev Cardiol. https://doi.org/10.1177/2047487312441726
Jørgensen T, Jacobsen RK, Toft U et al (2014) Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: inter99 randomised trial. BMJ 348:g3617
Kohro T, Furui Y, Mitsutake N et al (2008) The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int Heart J 49:193–203
Krogsboll LT, Jorgensen KJ, Gronhoj Larsen C, Gotzsche PC (2012) General health checks in adults for reducing morbidity and mortality from disease: cochrane systematic review and meta-analysis. BMJ 345:e7191. https://doi.org/10.1136/bmj.e7191
McDermott MS, Marteau TM, Hollands GJ et al (2013) Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study. Br J Psychiatry 202:62–67. https://doi.org/10.1192/bjp.bp.112.114389
Mindell JS, Giampaoli S, Goesswald A et al (2015) Sample selection, recruitment and participation rates in health examination surveys in Europe—experience from seven national surveys. BMC Med Res Methodol. https://doi.org/10.1186/s12874-015-0072-4
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health (2014) The health consequences of smoking—50 years of progress: a report of the surgeon general. Centers for Disease Control and Prevention (US), Atlanta (GA)
NHS Health Check Expert Scientific and Clinical Advisory Panel (2014) ESCAP (2014) Inter99 trial: a statement from the NHS Health Check Expert Scientific and Clinical Advisory Panel
O’Connor RJ, Rees VW, Rivard C et al (2017) Internalized smoking stigma in relation to quit intentions, quit attempts, and current e-cigarette use. Subst Abuse. https://doi.org/10.1080/08897077.2017.1326999
Office of the Surgeon General (US), Office on Smoking and Health (US) (2004) The health consequences of smoking: a report of the surgeon general. centers for disease control and prevention (US), Atlanta (GA)
Perk J, De Backer G, Gohlke H et al (2012) European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 223:1–68. https://doi.org/10.1016/j.atherosclerosis.2012.05.007
Pisinger C, Glümer C, Toft U et al (2008) High risk strategy in smoking cessation is feasible on a population-based level. The Inter99 study. Prev Med 46:579–584. https://doi.org/10.1016/j.ypmed.2008.02.026
Richard L, Potvin L, Kishchuk N et al (1996) Assessment of the integration of the ecological approach in health promotion programs. AJHP 10:318–328
Rose G (1987) European collaborative trial of multifactorial prevention of coronary heart disease. Lancet 1:685
Si S, Moss JR, Sullivan TR et al (2014) Effectiveness of general practice-based health checks: a systematic review and meta-analysis. Br J Gen Pract J R Coll Gen Pract 64:e47–e53. https://doi.org/10.3399/bjgp14X676456
Skaaby T, Jørgensen T, Linneberg A (2017) Effects of invitation to participate in health surveys on the incidence of cardiovascular disease: a randomized general population study. Int J Epidemiol 46:603–611. https://doi.org/10.1093/ije/dyw311
Stead LF, Buitrago D, Preciado N et al (2013) Physician advice for smoking cessation. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd000165.pub4
Thomsen TF, Davidsen M, Ibsen H et al (2001) A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD and the Copenhagen Risk Score. J Cardiovasc Risk 8:291–297
Wilson JMG, Jungner G, Organization WH (1968) Principles and practice of screening for disease. Available from: http://apps.who.int.ep.fjernadgang.kb.dk//iris/handle/10665/37650
Yong H-H, Borland R, Hyland A, Siahpush M (2008) How does a failed quit attempt among regular smokers affect their cigarette consumption? Findings from the International Tobacco Control Four-Country Survey (ITC-4). Nicotine Tob Res Off J Soc Res Nicotine Tob 10:897. https://doi.org/10.1080/14622200802023841
Funding
The Inter99 study was funded by the Danish Health Foundation (Grant No. 2010 B 131).
Author information
Authors and Affiliations
Contributions
TJ conceived and designed the experiments. CP performed the experiments. AMB, TJ, CP analyzed the data. AMB wrote the paper. All authors approved the final version of the manuscript and the submission to Clinical Epidemiology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from all the participants. In Denmark, researchers are entitled to use registers for research purposes (regarding the control group and non-participants of the intervention group) without persons’ informed consent as long as the researchers comply with predefined research regulations.
Ethics approval
The study was approved by the Regional Scientific Ethics Committee (KA 98 155) and the Danish Data Protection Agency. The study is registered at ClinicalTrials.Gov (NCT00289237).
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bender, A.M., Jørgensen, T. & Pisinger, C. Higher mortality in women living in high-participation areas of a population-based health check and lifestyle intervention study. Int J Public Health 64, 107–114 (2019). https://doi.org/10.1007/s00038-018-1179-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-018-1179-2