Abstract
Objectives
The aim of this paper was to assess the diarrhea risks caused by various pathogens among those exposed to biogas wastewater through different activities.
Methods
Application of quantitative microbial risk assessment (QMRA) of biogas wastewater was conducted in Hanam Province, Vietnam. A total of 451 representatives from households that use biogas were interviewed about their practices of handling biogas plant and reuse of biogas effluent for irrigation. In addition, 150 samples of biogas wastewater were analyzed for Escherichia coli, Cryptosporidium parvum, and Giardia lamblia. Risk characterization was calculated using Monte Carlo simulation.
Results
The annual diarrhea risk caused by exposure to biogas effluent through irrigation activities ranged from 17.4 to 21.1% (E. coli O157:H7), 1.0 to 2.3% (G. lamblia), and 0.2 to 0.5% (C. parvum), while those caused through unblocking drains connected to biogas effluent tanks were 22.0% (E. coli), 0.7% (G. lamblia), and 0.5% (C. parvum).
Conclusions
Further measures are needed to reduce the risk by either improving the microbial quality of biogas effluent or by ensuring the use of personal protective measures when exposed to biogas wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global increase in demand for livestock products has led to many concerns about the associated negative impacts of livestock rearing on the environment and on human health. In Vietnam, especially the management of animal waste has become a considerable challenge due to the rapid increase of swine production. A common method to treat animal waste in Vietnam is anaerobic digestion, also called biogas technology. This is a microbiological process whereby organic matter is decomposed in the absence of oxygen. Animal manure as well as human feces can be used as feedstock. The outputs of anaerobic digestion are biogas (a mix of methane and CO2) and a digestate wastewater, which is the digested slurry exiting the biogas reactor. Biogas is a renewable energy source, and its use also contributes to avoiding burning of fossil or wood, therefore reducing deforestation (Seadi et al. 2008). With these many proven benefits, biogas technology has become widespread throughout Asia.
In recent years, Vietnam has installed about 20,000 biogas reactors annually; reaching more than 100,000 by 2010 (REN21 2011). Biogas plants in Vietnam have often been installed by farmers individually, and mostly at household scale without much technical support or advice. This frequently leads to biogas plants which are not properly designed, constructed, operated or maintained. This limits the efficiency of microbial removal and thereby affects biogas production. Previous studies on biogas treatment have also shown that the hygienic quality of the biogas effluent does not meet required quality values for discharge into surface water bodies and reuse for irrigation (Huong et al. 2014; Kobayashi et al. 2003; Lohri et al. 2014; Phi et al. 2009). For example biogas digestion only reached 1–2 log reduction for Escherichia coli, giving 3.70 ± 0.84 (log10) CFU/mL E. coli on average in effluent as compared with raw slurry, whereas the requirement by WHO for wastewater used in agriculture is 103–105 CFU/100 mL. Nevertheless, a large majority of Vietnam farmers still discharge the biogas effluent into the environment or use it as a valuable source of fertilizer (Huong et al. 2014). Such activities and resulting exposure to pathogens pose potential health risk to humans as diarrhea still remains one of the most important health problems (WHO 2006a). The health risks for communities in Vietnam with biogas plants, when handling or being exposed to biogas wastewater have so far not been assessed.
Ha Nam is a province in the North of Vietnam where there is frequent use of biogas plants with farming households raising pigs. Many of these farmers, most of whom lack understanding around issues of biogas effluent quality, use this effluent for irrigation of vegetables, crops and fruit trees, or then discharge it into drains. This lack of knowledge and awareness are reasons that during these activities of wastewater handling most local people do not pay attention to protective measures and expose themselves to biogas wastewater putting themselves at risk of diseases, especially diarrhea. Poor personal hygiene when in contact with wastewater-based on a theoretical exposure pathway would increase the risk of infection or disease; however, no epidemiological data are available. It is also known that some excreta-related pathogens, such as E. coli, Cryptosporidium, and Giardia can survive in the environment long enough to pose health risks (WHO 2006a).
The aim of this study was to assess the diarrhea risk of people exposed to biogas wastewater using the quantitative microbial risk assessment (QMRA) method. The results will provide estimations of the health risks associated with exposure to biogas wastewater through different activities. This aims to better understand the current sanitation of biogas plants for further research and interventions; which are geared towards enhancing the environmental and health conditions of communities with biogas plants.
Methods
Study site
The study was carried out in three communities of Ha Nam Province, namely Hoang Tay, Le Ho, and Chuyen Ngoai. Ha Nam is a peri-urban province in Vietnam, situated 60 km South of Hanoi. The three communities comprise a population of 6300 (1700 households), 8800 (2200 households), and 9300 (2300 households), respectively. The economic basis of these three communities relies on agriculture, with equal development of both livestock and crops. The total swine population (not including piglets) in all three communities was around 17,600, and almost all of them are raised at small household scale. The increase of livestock and crop production has unfortunately led to many clearly visible environmental unhygienic conditions with related health issues. These issues have become the concern of local citizens and authorities.
Quantitative microbial risk assessment
QMRA is a method for assessing risks from microbial agents in a framework that defines the statistical probability of an infection from environmental pollution (Haas et al. 2014). QMRA can estimate very low levels of infection or disease risks, and can estimate risks from different exposures pathways and/or from different pathogens that would be difficult to measure using epidemiological studies given the high cost and the large sample sizes needed (WHO 2006a). The QMRA application includes four steps: hazard identification, exposure assessment, dose–response assessment, and risk characterization (Haas et al. 2014). A more updated method of QMRA is presented in WHO (2016), but the essential steps remain similar to Haas et al. (2014).
Hazard identification
Enteric pathogens related to human waste and animal manure cause diarrhea and are transmitted from animal to human waste. It is reported as the second largest contributor to the global burden of diseases, causing an estimated 1.5 million deaths among children under 5 years of age every year (WHO and UN-Water 2010). Our study focused on E. coli O157:H7, G. lamblia, and C. parvum. All three pathogens cause waterborne diseases, especially diarrhea and are very resistant to adverse environmental conditions (Haas et al. 1999). Previous studies carried out in Vietnam showed high load of these pathogens in biogas effluent (Huong et al. 2014; Kobayashi et al. 2003) and wastewater (Phuc 2012) and reported high prevalence of diarrhea in communities (Phuc 2012; Trang et al. 2007).
Exposure assessment
The aim of this step was to determine the intensity and duration of the exposure to biogas wastewater of the populations. In order to achieve this, a survey was conducted in the three above-mentioned communities. From a total of about 1500 households with biogas plants, 451 households were randomly selected. In each of these selected households, a random adult who consented to participate in this research was interviewed about his/her practices of and exposure situation to biogas wastewater. The person was supposed to know best how biogas operates. Therefore, the survey recorded basic characteristics of biogas such as their age, material used (animal manure or with human feces), residency time. The survey results helped quantify the number of people exposed and estimate the frequency of exposure regarding each practice. Among the potential means of exposure to biogas effluent, this study focused on four scenarios as follows: (1) irrigating crops, (2) irrigating fruit trees, (3) irrigating vegetables, and (4) unblocking the open drains connected to effluent tanks. Popular crops and vegetables in the study area included maize, leafy vegetable such as morning glory, coriander, and some herbs that are eaten raw. Answers of each interview may include multiple practices by one individual.
During these scenarios, accidental ingestion of wastewater—by splashing directly into the mouths or indirectly on hands and then to the mouth—was assumed. This assumption was confirmed by observations of most local people using rudimentary tools such as a round scoop with a bucket or better a watering can for irrigation, and a hoe, rake or a wooden stick to unblock the drains without having proper personal protective measures. For this study, we assumed the volume of wastewater of 1 mL that each person involuntarily ingested during one single-exposure while conducting any of these practices (Hoglund et al. 2002; Ottoson and Stenstrom 2003).
Dose–response assessment
The dose–response assessment describes the relationship between the dose (number of pathogens entering the body to cause infection) received and the resulting health effects (diarrhea infection or disease). An exponential and β-Poisson model are the two dose–response models widely used in literature because they fit well to several microorganisms (Haas et al. 2014).
The β-Poisson dose response model (Eq. 1) was applied to estimate the risks of E. coli O157:H7:
where P inf is the probability of infection, d is the ingested dose (d = C × V, where C is the concentration of microorganism, and D is the amount of wastewater which a person has involuntarily ingested), ID50 = average infecting dose (214.94), and α = parameter of probability function (0.373) (Teunis et al. 2008).
The exponential dose response model (Eq. (2)) was used for G. lamblia and C. parvum:
where r = organism specific infectivity (0.0199 for G. lamblia, and 0.0042 for C. parvum) (Haas and Eisenberg 2001).
Risk characterization
This last step of the QMRA application integrates the information from the three previous steps (hazard identification, exposure assessment, and dose–response assessment) into a single mathematical model to calculate risks as a probability of infection or illness.
Annual infection risk is shown in Eq. (3):
where n is the number of exposures per year.
Annual risk of diarrhea disease is calculated by Eq. (4):
where P ill/inf is probability of illness given infection: P ill/inf (E. coli) = 0.25 (Howard et al. 2006); P ill/inf (G. lamblia) = 0.67 (Rose et al. 1991); P ill/inf (C. parvum) = 0.7 (WHO 2006b).
Sampling strategy
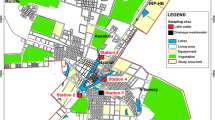
From three communities, 15 households with biogas plants were randomly selected for the study. At each household, two sampling points were identified: the first one at the effluent tank of the biogas plant and the second at the open household drain into which biogas effluent, wastewater and other runoff flow (Fig. 1a). The effluent tank of the biogas plant is a point of exposure as that is where farmers collect effluent for irrigation of fields (see Fig. 1b). The household open drain is also considered a potential point of exposure as this drain often needs to be unblocked by users (see Fig. 1c). Three wastewater samples were collected at each sampling point. Thus, five rounds of sampling gave a total of 150 wastewater samples collected from April to December 2014.
a Scheme of a biogas plant and the two sampling points [Source adapted from (Tilley et al. 2014)]. b An open effluent tank of biogas plant in Hoang Tay, Hanam Province, Vietnam, 2014 (Source Center for Public Health and Ecosystem Research—CENPHER). c Open drain receiving biogas effluent in Hoang Tay, Hanam Province, Vietnam, 2014 (Source Center for Public Health and Ecosystem Research—CENPHER)
All samples from the biogas effluent tanks were collected at 20 cm depth, and in the center of the tank. Wastewater samples from household drains were collected where the wastewater from the effluent tanks enters the drain. One liter of wastewater was collected for each sample, stored on dry ice (4–8 °C) during transport to the laboratory, and processed within the same day. The samples were analyzed for E. coli, G. lamblia, and C. parvum.
Microbiology analysis
Brilliance E. coli/coliform Selective Agar (CM1046, Oxoid) was applied for the detection and enumeration of E. coli from wastewater samples. The wastewater samples were diluted 1:10 with 0.1% sterile Peptone Water. The surface of the agar plates was dried before pipetting 0.1 mL of the prepared sample onto each plate and spread over the surface with a sterile spreader. The plates were then incubated for 24 h at 37 °C. The growth of dark purple to indigo blue colonies on agar plates was determined positive for E. coli.
Immunofluorescence staining was performed to enumerate Giardia spp. and Cryptosporidium spp. (Crypto/Giardia CEL; Cellabs Pty Ltd, Australia). Each wastewater sample was vigorously shaken and dispensed into twenty 50-mL sterile test tubes which were centrifuged at 1500g for 5 min. The contents of each of the 20 sets of tubes were mixed together after discarding the supernatant, and centrifuged again under the same conditions. A flotation step followed with the remaining sediments in which 10 mL of wastewater volume was mixed with 5 mL of flotation fluid, and centrifuged for 1 min at 100g. The remaining flotation fluid in the sample was then removed before concentrating the sample volume to 2 mL. A volume of 200 μl sample was air-dried on Teflon-coated diagnostic slide fixed with acetone and stained with fluorescent monoclonal antibodies to detect Giardia spp. and Cryptosporidium spp.
In this study, we assumed that 8% of the total E. coli population in the wastewater were pathogenic (Haas et al. 1999; Howard et al. 2006). Furthermore, we assumed that all Giardia spp. and Cryptosporidium spp. found by this testing method were G. lamblia and C. parvum which are human pathogenic species. This assumption was applied in Mota’s study where no information on the strain or the genotype was available (Mota et al. 2009).
Statistical analyses
Data from the 451 household survey were double-entered into Epidata 3.1. The statistical data analysis was done using SPSS 16.0. Non-parametric Mann–Whitney test was applied to compare difference between pathogen concentrations in the effluent tanks and in the drains of the three communities. All the parameters from exposure assessment of the risk model were included as probability density functions (PDF) (Table 1). These PDF were calculated from the original data collected from microbial analyses and from the survey with the best fits. Risks were calculated using estimated PDF randomly sampled by Monte Carlo simulation (10,000 iterations). Monte Carlo analysis gives a full range of possible risks, including average and worst-case events. In the latter, the risks were presented by the mean of 10,000 simulation values of risk. @Risk software (Version 5.5) added on to Microsoft Excel was used to calculate the PDF and to run risk models.
Results
Microbial contamination in biogas effluent tanks and drains
The mean concentration of E. coli, G. lamblia, and C. parvum from two sampling points are presented in Table 2. All 75 samples from the effluent tanks and 75 samples from drains attached to effluent tanks were positive for E. coli, with the mean concentration of 14.7 × 105 CFU/100 mL and 9.3 × 105 CFU/100 mL, respectively. Both sampling points had the same highest concentration of E. coli which was 200 × 105/100 mL. G. lamblia and C. parvum were found to have higher mean concentrations in the effluent tanks (19 cysts/100 mL and 18 oocysts/100 mL) than in the drains connected to these effluent tanks (4 cysts/100 mL and 12 oocysts/100 mL). G. lamblia had the highest concentration in the effluent tanks (260 cysts/100 mL), whereas C. parvum was highest in drains (480 oocysts/100 mL).
Characteristics and exposure situation of the study population
A total of 451 individuals from 451 households having biogas plants were selected for the exposure survey. 261 (58%) of respondents were females, the mean age of all respondents was 47. The majority of respondents (84%) indicated that their main occupation was working in agriculture. The frequency of exposure, ingestion dose of wastewater, and percentage of the population who participated in each exposure event are shown in Table 3. Using biogas effluent for irrigation was prevalent in the community. These percentages ranged from 24 to 28% depending on each irrigation activity. Similarly, 30% of the people reported participating in unblocking drains. People who were exposed to wastewater in these practices reported performing these activities an average of 24–53 times per year.
Part of the interviewed participants used personal protective measures when handling biogas wastewater. Such personal protective measures would include wearing gloves, wearing face masks, wearing boots and washing hands with soap after work. Amongst all respondents engaging in irrigation activities, 10% indicated that they always use gloves during the practices, 14% always use a face mask, 13% always wear boots, and 25% wash their hands with soap after work. Only 8.9% participants always used all of these four protective measures when using biogas wastewater for irrigation. Similarly, the percentages of people practicing drain-unblocking activities indicating that they always use these protective measures were from 17 to 35%. Around 15% people saying that they always used all of these protective measures when unblocking the drains.
Biogas characteristics and operation
Each household in the survey had one biogas unit. 90.2% of household used pig manure in their biogas, whereas 29.7% used poultry manure. 80.3% of household also discharged human excreta into the biogas due to the proximity of livestock and human areas. The age of biogas was 5.2 ± 3.5 year (0.2–28 years) and the volume of the digester was 11.3 ± 3.1 m3 (3.0–22.0 m3). Most of the biogas digesters were built by brick and cement (87%) and plastic (13%).
Risks of infection and diarrhea diseases
Single infection risks of E. coli O157:H7, G. lamblia, and C. parvum in different activities are presented in Fig. 2. The mean single infection risk of these pathogens per individual in all irrigation activities was 0.63, 0.0037, and 0.0008, respectively, and the mean infection risks of these pathogens per individual in unblocking drains was 0.6031, 0.0007, and 0.0005.
With regard to the annual risk of diarrhea, we assume that exposure to biogas wastewater through the selected activities in this study were the only cause of diarrhea. The annual risks of diarrhea caused by the chosen pathogens are presented in Fig. 3. G. lamblia caused the highest risk of diarrhea when irrigating vegetables (0.0023) and lowest when unblocking drains (0.007). The annual diarrhea risk of E. coli O157:H7 was highest when people were exposed to wastewater through unblocking drains (0.22), and lowest when they were irrigating crops (0.174). Similarly, people who participated in unblocking drains or irrigating vegetables had the highest risk of diarrhea by C. parvum (0.005), whereas irrigating crops posed the lowest risk of diarrhea by C. parvum (0.002).
Discussion
Results in our study revealed high concentrations of E. coli, G. lamblia, and C. parvum in biogas wastewater. In particular, E. coli concentration in the effluent tanks was 14.7–147 times higher than the recommended standard (104–105 E. coli/100 mL) of the WHO guidelines for the safe use of wastewater in agriculture (WHO 2006a). The prevalence of E. coli in biogas effluent has also been shown in previous studies conducted in Vietnam. A study of the hygienic aspects of small-scale biogas plants showed that the mean concentration of E. coli in biogas effluent from pig farms was 5000 CFU/mL (Huong et al. 2014), which, however, is almost three times lower than the results from our study. This might be explained by the difference in sampling points in the two studies. Huong et al. (2014) took samples in the second outlet tanks, whereas our study analyzed samples from the first outlet tank (effluent tank) of the biogas plants. Some settling process in the first outlet tank might reduce pathogens concentration in the second outlet tank. Another study in Can Tho Province—in the South of Vietnam—showed a high prevalence and high concentration of E. coli in the outflow of biogas plants of pig farms. The concentration of E. coli in 4/5 of the samples was greater than 105 CFU/mL (Kobayashi et al. 2003). The result is ten times higher than in our studies. However, the Kobayashi’s study only collected five samples at each sampling points without repetitions and the study dates back 12 years. During the last 12 years, Vietnamese farmers have improved their knowledge on how to best operate biogas plants which has probably also improved the quality of biogas effluent thanks to several biogas promotions by SNV and the Ministry of Agriculture and Rural Development of Vietnam.
Vögeli and colleagues conducted some case studies to evaluate the quality and the use of biogas effluent in other developing countries, namely India, Tanzania, Nepal and Lesotho (Vögeli et al. 2014). Results showed that these countries present a similar context of applying and using biogas plants. Most of the case study results concluded that quality of the effluent did not meet the requirements for use as fertilizer or discharge into receiving water bodies, although people still use the effluent for irrigation and/or discharge into the surface waters.
Regarding the hygienic conditions of drains, the microbial quality of wastewater in household drains was mentioned in the study of wastewater and excreta use in agriculture in Ha Nam Province during the period of 2009–2010 (Phuc 2012). The wastewater samples were taken not only from households with biogas plants, but also from household drains,. The mean concentration of E. coli (78.75 × 106 CFU/100 mL) was much greater than in our study that was 9.3 × 105 CUF/mL. However, the mean concentration of G. lamblia (28 cysts/100 mL) and C. parvum (28 oocysts/100 mL) in this study was comparable.
Some studies were conducted in other developing countries where local people were exposed to different sources of wastewater to determine the contamination level of the water. For example, wastewater from canals and lagoons in Abidjan, Côte d’Ivoire, were shown to be heavily contaminated with G. lamblia and E. coli (Yapo et al. 2014), whereas wastewater from canals and sewers at household level in the Klong Luang municipality of Thailand even showed higher contaminations by G. lamblia (Ferrer et al. 2012).
Comparing the mean concentration of the three pathogens in the effluent tanks and drains of the three communes, there was no significant difference in E. coli and C. parvum in these two sampling points (p > 0.05; Mann–Whitney test). However, the mean concentration of G. lamblia in the effluent tanks was eight times greater than in the drains. This difference is statistically significant (p < 0.05; Mann–Whitney test). It might be possible that when biogas effluent flows from the effluent tanks to the drains it is diluted with other wastewater in the drains which reduces the concentration of G. lamblia.
The mean infection risk by G. lamblia per single exposure in our study was found to be similar to that of the study in the Klong Luang municipality of Thailand which showed that 44 out of 100 people having contact with canal water would have infection by G. lamblia, or out 100 times contact with canal water, a person was 44 times at risk of infection by G. lamblia (Ferrer et al. 2012). In this study, Entamoeba histolytica, another protozoa associated with wastewater, was also shown to have a similar infection risk by a single exposure when having contact with canal water (46%).
Our results were comparable to those obtained from other studies in Vietnam for annual diarrhea risk due to E. coli O157:H7. A study that applied the QMRA method to assess diarrhea risks of the exposure activities associated with wastewater and excreta use in agriculture in Ha Nam province of Vietnam showed that the annual diarrhea risks due to E. coli O157:H7 was 0.25. This is similar to the overall incidence of diarrheal disease as presented in the same study area of 0.28 per person-year as a result of a cohort study covering over one year of the follow-up period (Phuc 2012). Another cohort study which investigated in the suburb of Hanoi in Vietnam with 18 months follow-up showed similar results in which the incidence rate of diarrheal diseases was 28.1 episodes per 100 person-year counted for the adult population exposed to wastewater in agricultural and aqua-cultural productions (Trang et al. 2007).
In comparison with our study, the study performed in Thailand by Mamadou and collaborators who assessed the diarrheal risks related to exposure to canal water through irrigation activities, showed comparable results in diarrhea risks caused by G. lamblia at 0.67 per person-year (Mamadou et al. 2008). Among the three selected pathogens in this study, the risk of diarrhea due to E. coli O157:H7 was also much higher than for G. lamblia and C. parvum.
The annual diarrhea risks in our study was below the diarrheal disease incidence per person per year (0.4–0.6) among those aged above 5 years in developing countries in 2000, as reported in the WHO guidelines for the safe use of wastewater, excreta and greywater. However, in comparison with the WHO reference level (10−3) of waterborne disease from drinking water, the annual diarrhea risks in our study were much greater (WHO 2006a).
Our study did not measure the association between the health risks and the use of personal protective measures while participating in activities exposed to wastewater. However, the study on the practices of farmers in some agriculture activities in the Ha Nam province of Vietnam showed that inadequate use of protective measures, and lack of washing hands with soap were associated with increased risks of diarrhea (Phuc 2012). While appropriate treatment methods of biogas effluent are not yet available in Vietnam, improving the use of personal protective measures might be a suitable solution for protecting the health of the exposed population. It was also unfortunate that our study did not measure the temperature of biogas digester to link with pathogen die-off, which needs to be considered in future studies.
In conclusion, E. coli, G. lamblia, and C. parvum remained at high concentrations in the biogas wastewater. The single and annual risks of diarrhea caused by these pathogens in the exposed activities were relatively high. These facts suggest that further actions to improve the biogas effluent quality are required to reduce health risk due to the exposure to biogas wastewater. This study provides evidences to enhance the awareness of people when handling biogas wastewater, and hence promoting practices of using personal protective measures. Results in this study could be taken into consideration to understand the health risks associated with exposure to biogas wastewater through different activities in other areas of Vietnam as well as in other developing countries with a similar context of applying biogas plants.
References
Ferrer A, Viet HN, Zinsstag J (2012) Quantification of Diarrhea risk related to wastewater contact in Thailand. Ecohealth 9:49–59
Haas C, Eisenberg JNS (2001) Risk assessment. In: Fewtrell L, Bartram J (eds) Water Quality: Guidelines, Standards and Health: Assessment of risk and risk management for water-related infectious disease. IWA Publishing, London, pp 161–183
Haas CN, Rose JB, Gerba CP (1999) Quantitative microbial risk assessment. Wiley, New York
Haas C, Rose J, Gerba C (2014) Quantitative microbial risk assessment. Wiley, New York
Hoglund C, Stenstrom TA, Ashbolt N (2002) Microbial risk assessment of source-separated urine used in agriculture. Waste Manag Res 20:150–161
Howard G, Pedley S, Tibatemwa S (2006) Quantitative microbial risk assessment to estimate health risks attributable to water supply: can the technique be applied in developing countries with limited data? J Water Health 4:49–65
Huong LQ, Madsen H, Anh LX, Ngoc PT, Dalsgaard A (2014) Hygienic aspects of livestock manure management and biogas systems operated by small-scale pig farmers in Vietnam. Sci Total Environ 470:53–57
Kobayashi H, Khai LTL, Phan TT, Yamasaki S, Taniguchi T (2003) Prevalence of pathogenic Escherichia coli in swine breeding environment in Can Tho province. Vietnam JARQ 37:59–63
Lohri CR, Gauthier M, Oppliger A, Zurbrugg C (2014) Ensuring appropriateness of biogas sanitation system for prisons: Analysis form Rwanda, Nepal and the Philippines. In: Bolay J-C, Hostettler S, Hazboun E (eds) Technologies for sustainable development: a way to reduce poverty? Springer, Switzerland
Mamadou BCD, Anceno AJ, Tawatsupa B, Houpt ER, Wangsuphachart V, Shipina OV (2008) Infection risk assessment of diarrhea-related pathogens in a tropical canal network. Sci Total Environ 407:223–232
Mota A, Mena KD, Soto-Beltran M, Tarwater PM, Chaidez C (2009) Risk Assessment of Cryptosporidium and Giardia in Water Irrigating Fresh Produce in Mexico. J Food Protect 72:2184–2188
Ottoson J, Stenstrom TA (2003) Faecal contamination of greywater and associated microbial risks. Water Res 37:645–655
Phi VTY, Clemens J, Rechenburg A, Vinneras B, Lenßen C, Kistemann T (2009) Hygienic effects and gas production of plastic bio-digesters under tropical conditions. J Water Health 07:590–596
Phuc PD (2012) Wastewater and excreta use in agriculture in northern Vietnam: health risks and environmental impacts. University of Basel, Switzerland
REN21 (2011) Renewables 2011. Global status report. Renewable energy policy network for the 21st Century
Rose JB, Haas CN, Regli S (1991) Risk assessment and control of waterborne Giardiasis. Am J Public Health 81:709–713
Seadi TA, Rutz D, Prassl H (2008) Biogas handbook. University of Southern Denmark Esbjerg, Biogas for Eastern Europe
Tilley E, Ulrich E, Lüthi C, Reymond P, Zurbrügg C (2014) Compendium of sanitation systems and technologies, 2nd edn, Swiss Federal Institute of Aquatic Science and Technology (Eawag), Dübendorf, Switzerland
Teunis PF, Ogden ID, Strachan NJ (2008) Hierarchical dose response of E. coli O157:H7 from human outbreaks incorporating heterogeneity in exposure. Epidemiol Infect 136:761–770. doi:10.1017/s0950268807008771
Trang DT, Hien BTT, Cam PD, Dalsgaard A (2007) Epidemiology and aetiology of diarrhoeal diseases in adults engaged in wastewater-fed agriculture and aquaculture in Hanoi. Vietnam Trop Med Int Health 12:23–33
WHO, UN-Water (2010) UN-Water global annual assessment of sanitation and drinking-water (GLAAS) 2010: targeting resources for better results
Vögeli Y, Lohri CR, Gallardo A, Diener S, Zurbrügg C (2014) Anaerobic digestion of biowaste in developing countries. Eawag-Swiss Federal Institute of Aquatic Science and Technology, Switzerland
WHO (2006a) WHO guidelines for the safe use of wastewater, excreta and greywater, Volume II: wastewater use in agriculture. World Health Organization, Geneva
WHO (2006b) World Health Organization. Guidelines for drinking-water quality: incorporating first addendum. Vol. 1, Recommendations. 3rd edn
WHO (2016) Quantitative microbial risk assessment: application for water safety management. WHO, Geneva
Yapo RI, Kone B, Bonfoh B, Cisse G, Zinsstag J, Nguyen-Viet H (2014) Quantitative microbial risk assessment related to urban wastewater and lagoon water reuse in Abidjan, Cote d’Ivoire. J Water Health 12:301–309
Acknowledgements
The authors thank the numerous individuals, communities, and organizations for providing valuable information and assistance to accomplish this study. Especially, we thank Ms Nguyen Thi Hong for her great contribution to this study. We thank the National Institute of Hygiene and Epidemiology for analyzing the wastewater samples. This study was supported by the Canadian International Development Research Center (IDRC) through the project of Ecohealth Field Building Leadership Initiative (FBLI, Grant No. 106556). TL was supported by an Eawag Partnership Program (EPP). HNV has been supported by the CGIAR Program Agriculture for Nutrition and Health (A4NH) led by IFPRI and by the Chrono-environment laboratory of the Universite de Franche-Comte through a visiting professorship. This study received ethical clearance from the Institutional Review Board of Hanoi School of Public Health No. 051/2004/YTCC-HD3, Code: 014-051/DD-YTCC.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the supplement “Health and social determinants of health in Vietnam: local evidence and international implications”.
Rights and permissions
About this article
Cite this article
Le-Thi, T., Pham-Duc, P., Zurbrügg, C. et al. Diarrhea risks by exposure to livestock waste in Vietnam using quantitative microbial risk assessment. Int J Public Health 62 (Suppl 1), 83–91 (2017). https://doi.org/10.1007/s00038-016-0917-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-016-0917-6