Abstract
Nitrification is a vital biogeochemical process during which ammonium (NH4+) is oxidized to nitrite (NO2−) and nitrate (NO3−) using oxygen. Nitrification is particularly important in estuaries, where nitrification rates have significant implications for ecosystem functioning. Our study investigated seasonal and spatial variations of nitrification rates in both water and sediment phases of mangrove ecosystems in the Indian Sundarbans, integrating the three major estuaries (Saptamukhi, Thakuran, and Matla). We used on board incubation to measure nitrifying activity [i.e., ammonium oxidation rate (Ra) and nitrite oxidation rate (Rn)] in the water column. We found that average Ra and Rn rates were similar, ranging from 31.1 to 31.8 nmol N L−1 h−1, respectively. Water column nitrification rates showed an inverse relationship with salinity (r = − 0.51, p < 0.001), while substrate concentrations of ammonium had a direct impact on nitrification rates. Annual dissolved inorganic nitrogen (DIN) concentrations were 11.7 ± 7.65 µM, with an average nitrate (NO3−) concentration of 4.5–16.3 µM and average ammonium (NH4+) concentrations from 2.1 to 5 µM. Suspended particulate matter and chlorophyll showed positive (r = 0.38, p = 0.009) and negative (r = − 0.31, p = 0.034) correlations, respectively, with water column nitrification rates. For water, both Ra and Rn occurred at similar rates in our study area. In sediment, nitrifying activity (SEDn) rates ranged from 0.34 to 8.69 nmol N g wet wt−1 h−1. SEDn showed positive and negative correlations with sediment temperature (temp) and pH, respectively. Based on our observations, the average water column nitrification potential ranged from 43 to 420 nmol N L−1 h−1 considering three estuaries, while the sediment nitrification potential estimated at Saptamukhi estuary showed a value of 69 nmol N g wet wt−1 h−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove ecosystems situated in semi-enclosed water bodies, where seawater is diluted by freshwater due to their location in an intermediate position between land and sea (Bianchi 2007). These ecosystems cover almost 1% of the world’s surface area (Singh et al. 2012).The major biogeochemical functions of mangrove ecosystems include primary production, nutrient cycling, organic matter dynamics (Alongi et al. 1992; Kristensen et al. 2008). The Sundarbans is one of the largest mangroves in both the ecological and economic perspectives and the pristine ecosystem was declared as a Ramsar site (Rahaman et al. 2013). They are dynamic systems that are very important and receive significant load of bio-available nitrogen (N) and carbon (C) through outwelling and runoff (Mukherjee et al. 2020).The Sundarbans estuarine system (SES) plays an important role in nutrient cycling. Nitrogen availability is particularly important for maintaining the productivity of the system (Dham et al. 2002; Majumder et al. 2011) and actively regulates the structure and functioning of both terrestrial and aquatic ecosystems (Elser et al. 2007; Chapin et al. 2011). Nitrogen fluxes in mangrove ecosystems are partitioned among the atmosphere, terrestrial, benthic, and aquatic compartments. Atmospheric deposition of both wet, dry riverine and oceanic supply along with the di-nitrogen fixation, which may be balanced later by losses through denitrification/anammox, export, and burial (Wang et al. 2019; Blackburn et al. 1996; Voss et al. 2013).
Total P (77%) and N (95%) of the nutrients in mangrove forests is stored in sediments. The degradation and remineralization of litter fall contribute to nitrogen concentrations in mangrove sediments (Ramos e Silva et al. 2007; Ray et al. 2014). These factors may result in recycling of nitrogen, which can be released to overlying estuarine waters by diffusion, bioturbation, or sediment resuspension (Xing et al. 2018). Several studies indicate that the remineralization of nitrogen, mediated by microbes, play an important role in supporting primary production in phytoplankton-dominated estuarine systems (Nixon 1981; Bebout et al. 1994; Herbert 1999). Therefore, in our estuarine system, understanding nitrogen fluxes, transformations, and removal mechanisms is crucial.
Biological nitrogen fixation (BNF) in the mangrove roots and microbial mats attached to the mangrove reduces atmospheric N2 to ammonia (NH3), which is one of the main ways that nitrogen is entered by some microorganisms (named diazotrophs) (Reis et al. 2017). Therefore, mangroves fix atmospheric nitrogen through nitrogen fixation (Alongi 2002, 2009). Benthic microbes play an essential role in estuarine nitrogen transformation, as the availability of N in mangrove ecosystems depends, in part, on complex patterns of bacterial activity within sediments. Within the oxic sediment layer, nitrifying bacteria carry out nitrification, converting ammonium (NH4+) to nitrite (NO2−) and nitrate (NO3−). In anoxic layers, denitrifying bacteria convert NO3− or NO2− into gaseous N2O and N2. The combination of these microbe-mediated processes may help mitigate land-derived N loading in coastal waters (Jensen 1996; Seitzinger et al. 2000).
A recent study in the Indian Sundarbans mangrove ecosystem (Ray et al. 2014) indicates that this ecosystem acts as a sink for atmospheric N2 via biosphere–atmosphere exchange (Biswas et al. 2005; Ganguly et al. 2009) and may retain some N, which can be recycled within the system. No such detailed study of N cycling has included all three estuaries of the mangrove-dominated Indian Sundarbans. Most studies were focused on carbon biogeochemistry (Dutta et al. 2019), and no clear reports describe the interrelationship between nitrification and other environmental parameters. Therefore, our present study conducted temporal and spatial research on dissolved N pools for water and sediment and measured nitrification rates for both water and subtidal bottom sediments in all three of these important estuaries (Saptamukhi, Thakuran, and Matla).
Nitrification process is complicated and very sensitive to several biogeochemical factors of the environment (temperature, salinity, dissolved oxygen (DO), pH, chlorophyll, and nutrients) (Miranda et al. 2008), which are less studied for our system. To understand the effects of these factors over the regional scale on the Sundarbans mangrove ecosystem in the sediment and water column, we conducted a comprehensive study of the spatiotemporal variation of nitrification rates, which has a significant impact on the microbiological process of nitrification, i.e., one of the objectives of the study. Short-term incubation of both water and subtidal bottom sediments were used for measuring nitrifying activity. It is envisaged that subtidal sediment in the mangroves is deposited as a major part of organic material carried by the flowing estuaries, and being compact by nature, it could act as an intense zone for microbial activities such as nitrification in comparison with the aquatic part. We hypothesized that the nitrification rates of the water column and sediments are interlinked. For this, we have taken some considerations here: denitrification rates in mangrove habitats are considered to be negligible part of the nitrogen budget (Rivera-Monroy et al. 1995; Kristensen et al. 1998). Another thing is that no other process is participating in these experiments. To test the hypothesis, our study enumerates the following objectives: (1) to measure the nitrification rates of surface water and sediments in the subtidal bottom zone at the same point; and (2) to measure the influence of physicochemical parameters and nutrient concentration on the nitrification rates of both water and sediment in the Indian Sundarbans mangrove system.
Materials and methods
Study area
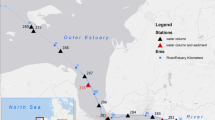
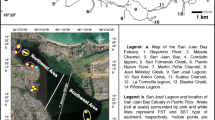
The study area focused on mangrove-dominated areas in the central part of the Sundarbans estuarine system (SES), West Bengal, India. The shallow and dynamic SES (~6 m depth) is India’s largest monsoonal, mesotidal [3.5–4 m, Chatterjee et al. (2013)] delta-front estuarine system, with a total surface area over 10,200 km2 (Acharya et al. 2022b), of which 2106 km2 is located in India (FSI 2015) and encompassing 1800 km2 of estuarine water surface area, drained into the Bay of Bengal. The area receives considerable rainfall from cyclones in premonsoon (March–June) and postmonsoon (December–February) months. Inner estuaries in the SES are influenced by semidiurnal tides at their mouths and receive freshwater from local runoff, most of which comes from monsoon rainfall [1500–2500 mm per year (Attri and Tyagi 2010)] and flooding from upstream parts of the Ganga during the monsoon. The dense mangrove cover contributes to heavy litter fall and is likely a major source of N of these estuaries (Ray et al. 2011). The present study included intertidal flats and mangrove-adjacent waters of three inner estuaries: Saptamukhi, Thakuran, and Matla (21.70–22.00° N, 88.30–88.72° E). A complex network of west–east flowing channels and “khals” (cuts) interconnects these north–south flowing estuaries. Among the three estuaries, the Saptamukhi estuary flows from Sultanpur to the Bay of Bengal and carries agricultural runoff. The Thakuran estuary, which originates near Jaynagar, is full of creeks and channels, and has interconnected khals to the Saptamukhi estuary. Bonnie Camp and Kalash islands are core parts of the Matla estuary.
Field sampling occurred in 2018–2020, covering the premonsoon (March through May), monsoon (July through October), and postmonsoon (November through February) seasons (Fig. 1). A total of 9 cruises were conducted for the Saptamukhi, and 12 for both the Thakuran and Matla. Surface water samples and subtidal sediment samples were collected from all three estuaries (Fig. 1S1–S30). Nitrification rates [both aquatic and sedimentary (Fig. 1S28, S21 and S15)] were measured on board.
Collection of water and sediment samples
Water samples (just below the surface at ~0.5 m) and estuarine bed sediment or subtidal bottom sediment (SS) samples were collected using Niskin water sampler (capacity 5 L, Ocean Test Equipment, USA) as well as a ponar grab sampler (with a bite area of 0.024 m2), respectively (Fig. 1: S1–S30), using a mechanized boat from 2018–2020 study period.
On board measurements of water and sediment samples
Water temperature (WT) and pH were collected in 60-mL polypropylene bottles and measured on board using a Ross combination electrode pH meter that was equipped with a temperature sensor (Orion Star A211) within 30 min of collecting the samples. The coefficient of variation (CV) ranged from 1% to 4% for both temperature and pH. Two glass bottles (125 mL) were filled from the Niskin bottle, without any air bubble using Tygon tubing, to measure DO on board by Winkler’s method with a CV of 1%. ~1000 mL of water from the Niskin bottle was filtered (0.7 µm, GF/F filter paper) using a hand-operated vacuum pump (Tarsons Products Ltd.) soon after collection (Bardhan et al. 2015). The residue was preserved in petri plates (Tarsons) for measuring suspended particulate matter (SPM) in laboratory and the filtrate was collected in four acid-cleaned HDPE bottles (~250 mL) and immediately stored in an icebox for transportation to the laboratory. Sediment samples were collected by gently removing the sediment-filled grab and collected in a container before that on board measurements included redox potential (Eh), temperature, and pH by lowering electrodes into the sediment section (Ray et al. 2014; Ghosh and Bhadury 2018). The coefficient of variation (CV) for Eh, temperature (temp), and pH was 1.22%, 0.13%, and 0.05%, respectively. The duplicate collected sediment columns were collected in air-sealed containers and stored in the icebox for analysis of the rest of the parameters to the laboratory.
Measurement of nitrification for both water and sediment
The incubation experiment was carried out on board for nitrification rate measurement immediate after taking a representative sample from each estuary (S28, Saptamukhi; S21, Thakuran; S15, Matla). For the incubation experiment, the samples were collected in January, February, March, June, July, August, November, and December. The rate of nitrification was measured in both water and sediment samples using two methods—inhibition of NH4+oxidation using N-Serve (Miranda et al. 2008), and inhibition of nitrite oxidation with sodium chlorate (Dai et al. 2008).
For the water nitrification incubation experiment, water samples were collected using a Niskin sampler. Thereafter, four sets of duplicate 250-mL amber-colored glass bottles (eight bottles) were filled from the sampler. The first set (means two bottles) (control) was left unamended, while among the other two sets (means four bottles), one set was treated with N-Serve (5 mg L−1, final concentration) and the other set was treated with sodium chlorate (10 mg L−1, final concentration), all of which were added separately, and the last set (remaining two bottles among eight) was amended with N-Serve and excess nitrite (at 10 µmol L−1) for measurement of potential nitrification. All bottles were incubated in the dark for 6–8 h, and from each bottle at 2-h intervals we withdrawn subsamples of 10 mL. Subsamples were preserved for nitrite analysis.
For the sediment nitrification incubation experiment, the same protocol was followed with three sets of 5 g of SS added to each bottle and filled with 250 mL of filtered seawater. The same procedures were followed for sediments as for the water column where three sets (six bottles) of 250 mL filtered bottom water with 5 g of subtidal sediments from the same place were incubated. The first set was left unamended as a control, while among the other two sets, one set was treated with N-Serve (5mg L−1, final concentration) and the last set was amended with N-Serve and excess nitrite (at 10 µmol L−1) for measurement of potential nitrification. All bottles were incubated in the dark for 6–8 h, and from each bottle at 2-h intervals we withdrawn subsamples of 10 mL. Subsamples were preserved for nitrite analysis. In all cases, loss of nitrite were taken as a measure of nitrification.
Laboratory measurement for water and sediment
Salinity was measured at laboratory using the Mohr-Knudsen argentometric method (Grasshoff et al. 1999) with a CV of 2%. For chlorophyll a (chl-a) analysis, 1000-mL HDPE dark bottles were used to collect samples, which were immediately filtered using 0.45 µm GF/C filter papers. The residue on the filter paper was stored in Cryochill vials and transported back to the laboratory in a liquid nitrogen can for further spectrophotometric analysis (Strickland and Parsons 1968), which exhibited a CV of 1%. After filtration the samples were brought to the laboratory in an ice box for measurements of dissolved inorganic N species (NO3− and NH4+). The laboratory measurements were done using standard spectrophotometric techniques followed by Grasshoff et al. (1999). The error in measurements for both NO3− and NH4+, measured against standard concentrations, were 2.2% and 1.1%, respectively.
After bringing samples to the laboratory, sediment porosity, salinity, and chlorophyll a (Sedchl-a) were analyzed in the laboratory (Ray et al. 2014; Ghosh and Bhadury 2018). The coefficient of variation (CV) for porosity, salinity, and Sedchl-a had a CV of 0.3%, 0.12%, and 0.5%, respectively. Nitrite (NO2−–N), Nitrate (NO3−–N), and ammonia–nitrogen (NH4+–N) concentrations of sediment were measured up to 5 cm from the surface. For sediment nutrients analysis, 30 g of wet sediments were extracted in 75 mL of 2 M potassium chloride solution and shaken in a micro shaker (Spinix-vortex shaker, Tarsons, cat. 3020) until well mixed. The slurries were allowed to stand overnight, then NO2−–N, NO3−–N, and NH4+–N parameters were measured using spectrophotometric methods (Riley and Vitousek 1995; Grasshoff et al. 1999; APHA 1995). The mean relative error in measurements of NO2−–N, NO3−–N, and NH4+–N, measured against the standard concentrations, were 0.7%, 0.4%, and 0.4%, respectively.
Statistical analysis
Minitab v. 18 was used to perform statistical analyses on the studied variables. A two-way analysis of variance (ANOVA) was implemented to assess inter-estuarine and seasonal variation of nitrification rates with water column and sediment N concentrations. Spearman correlation and regression were performed on the parameters to understand the relationships among nitrification rates, the nutrients, and the physicochemical parameters (Acharya et al. 2022b). variance inflation factor (VIF) for multiregression collinearity was also measured.
Results
Physicochemical characteristics of the environment
Water column
The distribution of some of the physicochemical parameters covering seasonal variation in the tropical estuaries of the Indian Sundarbans is presented in Table 1. For the period of our study, surface water temperature (WT) showed lowest during postmonsoon (mean, 22.5 ± 1.5 °C) and highest during premonsoon (mean, 29.8 ± 1.4 °C), which was followed by monsoon temperature (mean, 28.9 ± 0.8 °C). Salinity was lowest during monsoon (mean, 17.7 ± 4.5), highest during (mean, 30 ± 1.8), and intermediate during postmonsoon (mean, 24.2 ± 2.2). The average pH varied from 7.8 ± 2.9 during the monsoon to 8.2 ± 0.05 in the premonsoon, with an intermediate value observed for the post-monsoon (8.1 ± 3.0). The seasonal variation of DO was comparatively low, where the monsoon had the lowest value (mean, 6.42 ± 0.5 mg L−1) and the postmonsoon was the highest value (mean, 7.06 ± 0.8 mg L−1). However, suspended particulate matter (SPM) load annually varied widely from 23.3 to 740 mg L−1 and, unlike the other parameters, recorded its highest levels during the monsoon (226 ± 37 mg L−1) and its lowest levels during postmonsoon (56.6 ± 14 mg L−1), with intermediate values during premonsoon (118 ± 11 mg L−1). Annually, the average chl-a was recorded at its lowest during the monsoon (2.50 ± 0.5 µg L−1) and at its highest during postmonsoon (4.26 ± 0.88 µg L−1). SPM showed significant inter-estuarine and seasonal variation (p < 0.01 to p = 0.000).
Sediment characteristics
The physicochemical parameters of the bottom sediment have been summarized (Table 2), where Eh varied between −1.5 to −174 mV with an average of −66 mV (Table 2: − 8.77 ± 1.5 to − 94.6 ± 10.4) . The porosity of subtidal bottom sediment ranged from 0.49 to 0.76, with higher values during the premonsoon period, except for the Thakuran estuary (monsoon: 0.69). The average annual temperature of sediment varied from 21.4 ± 0.56°C to 29.2 ± 1.76 °C and reached a maximum during the monsoon. The pore water salinity varied from 22.5 ± 0.00 to 33.5 ± 1.53, showing the highest values during the premonsoon period. Sediment pH in the mangrove environment varied over a range of 6.59 ± 0.01 to 7.61 ± 0.21. The concentration of Sedchl-a varied from 1.30 ± 0.01 to 4.90 ± 0.07 µg g−1, with the highest concentration observed during the postmonsoon period. Sedchl-a (µg g−1), salinity, temperature (°C), and redox potential showed seasonal variation (p < 0.05 to p = 0.000) but no significant spatial distribution.
Dissolved inorganic nitrogen concentrations and nitrification rates in the water column and the sediment
Water column
The nitrifying activity changes with dissolved NO3− and NH4+ concentrations (Fig. 2). The distribution of dissolved inorganic nitrogenous nutrients (NO3− and NH4+) and nitrification rate in the water column represents in Table 3. Among the dissolved inorganic nitrogenous nutrients, the average NO3− concentration exhibited a higher range (4.50 ± 1.27 µM to 16.3 ± 1.49 µM) over NH4+concentration (2.1 ± 0.01 µM to 5.00 ± 0.52 µM). NO3− concentration was the highest during monsoon (15.4 ± 3.63 µM to 16.3 ± 1.19 µM) and the lowest during postmonsoon (4.50 ± 1.27 to 7.90 ± 4.48 µM). NH4+ concentration ranged from an average of 3.60 ± 0.55 to 5.00 ± 0.52 µM and was recorded to be the highest during monsoon for all estuaries. However, the lowest concentration of NH4+ was observed during premonsoon in the Saptamukhi and Matla and during postmonsoon at Thakuran.
The nitrification rate or nitrifying activity was expressed in two steps: oxidation of ammonia and oxidation of nitrite. First step, ammonium oxidation (Ra) (Table 3), which ranged from an average of 10.9 ± 2.80 to 49.7 ± 4.00 nmol N L−1 h−1, and the second step, nitrite oxidation (Rn) (Table 3), which ranged from an average of 7.6 ± 0.70 to 43.9 ± 3.20 nmol N L−1 h−1. Ra was the highest in monsoon at Saptamukhi (average 49 ± 5.30 nmol N L−1 h−1) and Thakuran (average 49.7 ± 4.00 nmol N L−1 h−1), while the same was observed for Matla during the postmonsoon (average 28.9 ± 2.90 nmol N L−1 h−1). The highest Rn was estimated during monsoon for all three estuaries (Saptamukhi: 43.9 ± 3.20 nmol N L−1 h−1, Thakuran: 36.1 ± 1.10 nmol N L−1 h−1, and Matla: 38 ± 2.20 nmol N L−1 h−1).
The inter-seasonal and inter-estuarine variability of dissolved inorganic nitrogenous nutrients and rates of nitrification were tested statistically using two-way ANOVA as shown in Table 4. Except for the ammonium oxidation rate, all parameters such as oxidation of nitrite (Rn: F = 11.8, p = 0.041), concentration of NO3− (F = 11.8, p = 0.000) and NH4+ (F = 4.93, p = 0.022) showed significant seasonal variation.
Sediment
Table 5 presents the variability of sediment nitrification (nitrite oxidation) rate (SEDn), and average NO3−, and NH4+ concentration in sedimentary phases at a seasonal scale. Sediment NH4+ concentration (5.3 ± 2.5 to 14.3 ± 2.00 µg g−1) showed the highest values during the post-monsoon period. NO3− concentration (0.13 ± 0.06 to 2.96 ± 0.60 µg g−1), showed the highest values during the post-monsoon period, except for the Saptamukhi estuary, where the highest value was recorded during the monsoon (0.52 ± 0.07 µg g−1).
SEDn varied from 0.52 ± 0.11 to 4.60 ± 0.76 nmol N g wet wt−1 h−1 (average: 2.62 nmol N g wet wt−1 h−1). The average sediment nitrification rates for the Saptamukhi, Thakuran, and Matla estuaries were 3.01 ± 0.85, 2.80 ± 0.05, and 1.58 ± 0.25 nmol N g wet wt−1 h−1, respectively. Among the subtidal bottom sediment nutrients, NH4+ ion was more dominant than NO3− ion with an average of 8.40 ± 5.35 µg g−1 and 0.76 µg g−1, respectively.
The result of statistical analysis to evaluate Inter-seasonal and estuary-wise variation of all above parameters is presented in Table 6. SEDn showed a significant seasonal variation (p = 0.034) but the inter-estuarine (p = 0.249) variation was not significant. Furthermore, to derive the interlink between water column and bottom sediment nitrification, both rates were scatter plotted (Fig. 3), which showed an exponential fit with a “r” value of 0.63.
Relationship between physicochemical parameters and nitrifying activity in the water column and sediment
Water column
To understand the individual response of physicochemical parameters on nitrifying activity, the whole range of its variation was plotted with respect to parameters like salinity, SPM, and chlorophyll (Fig. 4A–C). Water temperature generally showed a positive significant correlation with nitrification rate but the same was not found significant (r = 0.05, p = 0.743, figure not shown). Water nitrifying activity showed a negative and significant correlation (r = −0.51, p < 0.001) with salinity. It can be observed that the nitrification rate was minimal in the premonsoon at a salinity range of 30–35, while maximal with the salinity range 15–20, which was recorded during the monsoon and post-monsoon period (Fig. 4A). Nitrifying activity had a positive and significant correlation (r = 0.38, p = 0.009) with SPM (Fig. 4B). Nitrifying activity and chl-a showed significant negative correlation (r = −0.31, p = 0.034) (Fig. 4C). Also, all the VIF data for salinity (1.20), SPM (1.0), and chl-a (1.25) were ranged less than 3, which indicated that the correlation among the independent variables were insignificant.
The pH versus salinity variation showed a bimodal nature over the observed pH range. Up to pH 8, nitrification rates increased with increasing pH (Fig. 5A) (r = 0.57, p = 0.018), but beyond pH 8, the nitrification rates were inhibited (Fig. 5B) (r = − 0.44, p = 0.019).
Sediment
To gain insight into the influence of sediment temperature and pH on the variability of sediment nitrification, the datasets were plotted in a scatter plot (Fig. 6). The plots were fitted in polynomial fit which exhibited a positive and negative correlation with a value of r = 0.82 and r = − 0.61, respectively, for sediment temperature and pH. Therefore, sediment temperature exhibited a significant positive correlation with sediment nitrification rate, unlike the water column.
Discussion
Characteristics of the mangrove-dominated estuarine environment
Recent studies have extensively examined the physicochemical parameters of the Indian Sundarbans (Dutta et al. 2019; Mukherjee et al. 2020; Sanyal et al. 2020; Acharya et al. 2022a, b). Situated at the head of the Bay of Bengal, the region experiences a predominantly tropical climate, which is reflected in the observed water temperature. Salinity did not exhibit a defined gradient from the estuary's head to its mouth. Surface water salinity exhibited seasonal variation but showed no variation between surface and bottom water layers as recorded by Dutta et al. (2015). Seasonal patterns were evident in salinity variation, with lower values during the monsoon season, reflecting increased on-site precipitation and freshwater input and polyhaline characteristics (Acharya et al. 2022b; Dutta et al. 2015). The salinity, pH, and dissolved oxygen (DO) concentration ranges observed in this study align with recently published literature on the Indian Sundarbans, which describe the estuaries as polyhaline, well-oxygenated, and with limited inter-estuarine variation in physicochemical parameters (Dutta et al. 2019; Acharya et al. 2022b.
During the monsoon season, an increase in suspended sediment load resulting from surface runoff from the catchment can render the water column turbid. This turbidity limits light penetration and hampers the performance and growth of phototrophic primary producers, leading to low concentrations of chlorophyll-a (Chaudhuri et al. 2012). Similar observations have been made in recent studies conducted in the region (Acharya et al. 2022b). Conversely, the post-monsoon season exhibits the highest concentration of chlorophyll in the water column, indicating increased productivity.
The subtidal sediments of the studied estuaries were observed to consist predominantly of soft sediment with a substantial water content ranging from 40% to 60%. The salinity of the pore water in these sediments is comparable to that of the overlying bottom water, suggesting efficient diffusive transport of materials across the sediment–water interface. Sediment Eh values indicate prevailing anaerobic conditions in the sediments throughout all seasons and in the bottom sediment of all estuaries. These moderately reducing conditions in the sediments result in higher ammonium levels compared to nitrate values. Sediment pH displays considerable variation, likely influenced by freshwater seepage into the sediments during monsoonal runoff. However, most sediment parameters exhibit no significant seasonal or spatial variation, indicating relatively uniform and stable sediment conditions. Nevertheless, the sediments may be susceptible to changes in temperature and pH, which could profoundly affect nitrifying activity within that zone.
As observed from the results, the monsoonal surge of nutrients dissolved in water can beprimarily attributed to surface runoff, which often contains anthropogenically derived nitrogenous nutrients. During the monsoon, the runoff events increased the levels of NO3− and NH4+ by approximately 2–2.5 times and 1.5–2.0 times, respectively. Due to the lack of perennial fluvial input these monsoonal estuarine system exhibits increased NO3− and NH4+concentrations only during monsoonal runoff (Acharya et al. 2022b). It should be noted that the overall concentrations of dissolved nitrogenous nutrients in these estuaries (11.7 ± 7.65 µM) is recorded as lower compared to adjacent fluvial Gangetic estuaries namely the Hooghly estuary (14.7 ± 1.77 to 27.2 ± 2.05 µM) (Mukhopadhyay et al. 2006; Dutta et al. 2019). In the Gangetic estuaries, contribution of DIN sources may be nutrient loading from riverine discharge, increasing human population density, and rapid economic growth of surrounding areas. In contrast, in the Indian Sundarbans, DIN sources are primarily natural, leaf litter decomposition and land runoff during the monsoon season (Ramos e Silva et al. 2007).
However, the influence of marine water on the organic matter in the mangrove environment, facilitated by tidal action, results in this organic matter becoming deficient in organic N content (Ray et al. 2014). The decomposition of such nitrogen-deficient organic matter in pore water and finally supply less of it to the estuaries.
Nitrifying activity and DIN
The nitrification rate in the studied estuaries was either consistent or lower than the values reported for other estuaries represented in Table 7 with different geographical setting. All nitrification rates show higher rate (reference for Table 7) because high nutrients concentrations and other estuaries with moderate nutrients also having moderate showing moderate that of our study which is the lowest among all (reference for Table 7) due to have low nutrients. The nitrification rate is representative of both Ra and Rn; these metabolic processes may often show similar rates. In our study, the ratio of Rn and Ra showed values close to 1 and were significantly correlated with each other (r = 0.61, p = 0.003) (Fig. 7), indicating that the average rates are quite similar to each other and prompt and continuous. The positive correlation between water column and sediment nitrification rates highlights the interconnected nature of nitrification processes in this estuarine ecosystem. Nitrification, a process where nitrate is produced as a substrate from the precursor ammonium for further transformations/utilization, which occurs in suitable sites such as the water column and oxic-sediment layers (Priya et al. 2017). The concentrations of precursor and products are crucial in determining nitrification rates (Berounsky and Nixon 1993). Nitrifying activity depends on substrate concentration (NH4+) as well as it helps in supply of NO3− as product with nitrite (NO2−) acting as an intermediate (Dai et al. 2008; Bianchi et al. 1997; Damashek et al. 2016). In the Sundarbans estuaries, both NO3− and NH4+concentrations exhibit a significant positive correlation with nitrifying activity (Fig. 2A: r = 0.61, p = 0.000; Fig. 2B: r = 0.51, p = 0.000, respectively). Thus, the consequence of lesser amounts of regenerated nitrogenous nutrients translated to a lesser amount of nitrification activity in the subtidal sediment as well as the water column in the Indian Sundarbans estuaries compared to other estuaries (Dai et al. 2008; Owens 1986; Saomville 1978; Abril et al. 2000). This indicates that the rise in substrate concentration during monsoon in these estuaries directly influences nitrification rates to reach their maximum during that season. Sediment nitrification rates are, on the other hand, primarily restricted to the subtidal surface sediment column, which is considered to be a major source of nitrate in the water column in other tropical estuaries (Eyre 1994; Eyre and Balls 1999). Overall, the results demonstrate significant seasonal variations in dissolved inorganic nitrogenous nutrients and nitrification rates in both the water column and sediment. The higher concentrations and rates observed during the monsoon season indicate the influence of increased material inputs and freshwater runoff. Furthermore, the absence of significant inter-estuarine differences suggests that these patterns are consistent across the studied estuaries.
Environmental controls on nitrifying activity
Nitrification being a microbial process, it is likely that the interplay of several other factors affects the nitrification rate. Tropical estuaries are likely to promote nitrification as water temperatures typically fall within the optimal range for Nitrosomonas sp. (25–35 °C) and Nitrobacter sp. (25–30 °C) (Helder and de Vries 1983). Dai et al. (2008) found a positive correlation between nitrification rates and increasing temperatures. However, salinity exerts considerable control over different nitrogen transformation processes (Dittmar and Lara 2001a, b). In the studied estuaries, increasing salinity restricted nitrification rate (Fig. 4A) and similar trends were found in Seitzinger (1988) and Rysgaard et al. (1999) where the nitrification rate decreased with increasing salinity. The decreasing trend of nitrification with increasing salinity can be attributed to the possible low activity of microbes caused by dehydration or plasmolysis in highly saline waters (Csonka 1989; Madigan et al. 2018; Uygur and Kargi 2004).
Moreover, the pH of the system has the potential to inhibit nitrification (Dai et al. 2008). The optimum pH range for nitrification varies among studies. Shammas (1986) described a pH range of 8–9, whereas Meyerhof (1917) found that the optimum pH range for Nitrosomonas sp. was between 8.5 and 8.8, and for Nitrobacter sp., it was between 8.3 and 9.3. According to Engel and Alexander (1958) and Wild et al. (1970), the optimal pH range for nitrification is between 7.5 and 8.5. In our study, when all oxidation rates are arranged in the order of increasing pH, the nitrification rate shows maximum value in around pH 8.10 ± 0.01. Our study had the highest nitrification rate at pH 8, which is supported by the study by Jones and Hood (1980).
Suspended sediment load or suspended particulate matter (SPM) (Owens 1986; Balls et al. 1996) also plays a crucial role in controlling the rate of water column nitrification. This suggests that the nitrifying bacteria and archaea adhere to suspended particles and absorb NH4+ required for ammonia oxidation. Dai et al. (2008) observed higher nitrifying activity in waters with high SPM. Dutta et al. (2019) suggested that particulate matter enriched with δ15NPN (8.71–14.75‰) supports the nitrification process, with a similar δ15NPN range observed in our study.
Similar to our observation, other studies have also shown a negative correlation between water nitrification and chlorophyll-a (Fig. 4C) (Small et al. 2013). The seasonal variation in our study showed higher nitrification rates in the monsoon season, which were likely due to a combination of reduced salinity, moderate dissolved oxygen, high suspended particulate matter (SPM) load, low chlorophyll-a, and pH between 7.96 and 8.09. The phytoplankton community was reduced in the monsoon season, as indicated by the low concentration of chlorophyll-a, which may have led to the proliferation of nitrifying bacteria and facilitated higher nitrification rates (Dai et al. 2008; Wang et al. 2020).
Annual estimate and nitrification potential of the Indian Sundarbans estuaries
Water column nitrification potential ranged from an average of 43 to 420 nmol N L−1 h−1, while the sediment nitrification potential (Saptamukhi) showed a value of 69 nmol N g wet wt−1 h−1. The highest values were obtained for all the estuaries during the monsoon season. In the Ems estuary, nitrification potential rates in the water column ranged from 0.7 to 6.7 µmol N L−1 day−1 (29.2–279.2 nmol N L−1 h−1) (Sanders et al. 2018). In the Kochi backwaters, the nitrification potential was measured at 800 nmol N L−1 h−1 in the water column and 40 nmol N g wet wt−1 h−1 in sediments. The Sundarbans estuaries, as tropical mangrove environments, hold great ecological and biogeochemical significance due to their significant contribution to the adjacent marine ecosystem. Hence, we estimated the annual nitrification rate from the average nitrification rates in both water and sediment and calculated the nitrification potential of the study area. On an annual scale, the average nitrification for water column is estimated as 41.6 Gg N/y, whereas nitrification contributed by sediment (top 5 cm layer) is 144 Gg N/y. These values were obtained upon extrapolation of the average nitrification rates in the water and the sediment over a total estuarine cover of 1800 km2.
Conclusion
Our study provides information on the dynamics of nitrogenous species and the factors that control nitrification rates in the estuaries and the sediments in the Indian Sundarbans system. The estuaries experience significant sediment and nutrient loads from surface runoff during the monsoon season, leading to high turbidity and low chlorophyll concentration, which affects the growth of primary producers. Despite this, estuaries maintain high dissolved oxygen levels and relatively constant pH due to the constant input of organic matter from intertidal mangrove sediments. The concentration of dissolved nitrogenous nutrients is comparatively lower in these estuaries than in fluvial mesotidal estuaries, primarily due to more tidal intrusion of seawater and limited mixing. The nitrification rate is effected by several factors, i.e., water temperature, salinity, particulate matter concentration, substrate concentration, and oxygen saturation. While environmental controls such as salinity, suspended particulate matter, chlorophyll-a influence water column nitrification. Temperature and pH have the influence over sediment nitrification rates. Potential nitrification rates have shown that it must have the potential to maintain the pristine nature of the estuaries. Although, sediment nitrifying activity is mostly restricted to the bottom sediments due to lack of aerobic condition in compare to other estuaries. The sediment zone of the Indian Sundarbans is thus more capable of mitigating excess ammonium concentrations in the system due to its higher nitrification potential than the Sundarbans waters.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abril G, Riou SA, Etcheber H, Frankignoulle M, Wit RD, Middelburg JJ (2000) Transient, tidal time-scale, nitrogen transformations in an estuarine turbidity maximum—fluid mud system (The Gironde, South-west France). Estuar Coast Shelf Sci 50(5):703–715. https://doi.org/10.1006/ecss.1999.0598
Acharya A, Sanyal P, Paul M, Gupta VK, Bakshi S, Mukhopadhyay SK (2022a) Distribution and dynamics of radiatively active gas (RAG) emissions from major estuaries of the Sundarbans Mangrove, India. Front Earth Sci 10:1. https://doi.org/10.3389/feart.2022.806897
Acharya A, Sanyal P, Paul M, Gupta VK, Bakshi S, Bhattacharyya P, Mukhopadhyay SK (2022b) Seasonal quantification of carbonate dissolution and CO2 emission dynamics in the Indian Sundarbans estuaries. Region Stud Mar Scie. https://doi.org/10.1016/j.rsma.2022.102413
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29(3):331–349. https://doi.org/10.1017/S0376892902000231
Alongi DM (2009) The energetics of mangrove forest. Springer, Berlin, pp 11, 24
Alongi DM, Boto KG, Robertson AI (1992) Nitrogen and phosphorus cycles. Coast Estuar Stud. https://doi.org/10.1029/CE041p0251
APHA 20005 (1995) Standard methods for the examination of water and waste water. Washington. p 5–15
Attri SD, Tyagi A (2010) Climate profile of India. Contribution to the Indian Network of Climate Change Assessment (NATIONAL COMMUNICATION-II). 1:1–129
Balls PW, Brockie N, Dobson J, Johnston W (1996) Dissolved oxygen and nitrification in the Upper Forth Estuary during summer patterns and trends. Estuar Coast Shelf Sci 42(1):117–134. https://doi.org/10.1006/ecss.1996.0009
Bardhan P, Karapurkar SG, Shenoy DM, Kurian S, Sarkar A, Maya MV, Naik H, Varik S, Naqvi SWA (2015) Carbon and nitrogen isotopic composition of suspended particulate organic matter in Zuari Estuary, west coast of India. J Mar Syst 141:90–97. https://doi.org/10.1016/j.jmarsys.2014.07.009
Bebout BM, Paerl HW, Bauer JE, Canfield DE, Des Marais DJ (1994) Nitrogen cycling in microbial mat communities: the quantitative importance of N-fixation and other sources of N for primary productivity. In: Stal LJ, Caumette P (eds) Microbial mats. NATO ASI series, vol 35. Springer, Berlin. https://doi.org/10.1007/978-3-642-78991-5_27
Berounsky VM, Nixon SW (1993) Rates of nitrification along an estuarine gradient in Narragansett Bay. Estuaries 16:718–730. https://doi.org/10.2307/1352430
Bianchi T (2007) Biogeochemistry of. Estuaries. https://doi.org/10.1093/oso/9780195160826.001.0001
Bianchi M, Feliatra F, Tréguer P, Vincendeau MA, Morvan J (1997) Nitrification rates, ammonium and nitrate distribution in upper layers of the water column and in sediments of the Indian sector of the Southern Ocean. Deep Sea Res Part II 44(5):1017–1032. https://doi.org/10.1016/S0967-0645(96)00109-9
Biswas H, Chatterjee A, Mukhopadhyay SK, De TK, Sen S, Jana TK (2005) Estimation of ammonia exchange at the land–ocean boundary condition of Sundarban mangrove, northeast coast of Bay of Bengal, India. Atmos Environ 39(25):4489–4499. https://doi.org/10.1016/j.atmosenv.2005.03.041
Blackburn TH, Hall POJ, Hulth S, Landen A (1996) Organic-N loss by efflux and burial associated with a low efflux of inorganic N and with nitrate assimilation in Arctic sediments (Svalbard, Norway). Mar Ecol Prog Ser 141:283–293
Burgos M, Sierra A, Ortega T, Forja JM (2015) Anthropogenic effects on greenhouse 466 gas (CH4 and N2O) emissions in the Guadalete River Estuary (SW Spain). Sci Total Environ 503–504:179–189. https://doi.org/10.1016/j.scitotenv.2014.06.038
Chapin FS, Pickett ST, Power ME, Jackson RB, Carter DM, Duke C (2011) Earth stewardship: a strategy for social–ecological transformation to reverse planetary degradation. J Environ Stud Sci 1:44–53
Chatterjee M, Shankar D, Sen GK, Sanyal P, Sundar D, Michael GS et al (2013) Tidal variations in the Sundarbans Estuarine System, India. J Earth Syst Sci 122:899–933. https://doi.org/10.1007/s12040-013-0314-y
Chaudhuri K, Manna S, Sarma KS et al (2012) Physicochemical and biological factors controlling water column metabolism in Sundarbans estuary, India. Aquat Biosyst 8:26. https://doi.org/10.1186/2046-9063-8-26
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147
Dai M, Wang L, Guo X, Zhai W, Li Q, He B, Kao SJ (2008) Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: the Pearl River Estuary, China. Biogeosciences 5:1227–1244. https://doi.org/10.5194/bg-5-1227-2008
Damashek J, Casciotti K, Francis C (2016) Variable Nitrification Rates Across Environmental Gradients in Turbid, Nutrient-Rich Estuary Waters of San Francisco Bay. Estuaries Coasts. https://doi.org/10.1007/s12237-016-0071-7
Dham VV, Heredia AM, Wafar S, Wafar M (2002) Seasonal variations in uptake and on-site regeneration of nitrogen in mangrove waters. Limnol Oceanogr. https://doi.org/10.4319/lo.2002.47.1.0241
Dittmar T, Lara RJ (2001a) Do mangroves rather than rivers provide nutrients to coastal environments south of the Amazon River? Evidence from long-term flux measurements. Mar Ecol Prog Ser 213:67–77
Dittmar T, Lara RJ (2001b) Driving forces behind nutrient and organic matter dynamics in a mangrove tidal creek in north Brazil. Estuar Coast Shelf Sci 52:249–259. https://doi.org/10.1006/ecss.2000.0743
Dutta MK, Mukherjee R, Jana TK, Mukhopadhyay SK (2015) Biogeochemical dynamics of exogenous methane in an estuary associated to a mangrove biosphere; the Sundarbans, NE coast of India. Mar Chem 170:1–10. https://doi.org/10.1016/j.marchem.2014.12.006
Dutta MK, Kumar S, Mukherjee R, Sanyal P, Mukhopadhyay SK (2019) The post-monsoon carbon biogeochemistry of the Hooghly-Sundarbans estuarine system under different levels of anthropogenic impacts. Biogeosciences 16:289–307. https://doi.org/10.5194/bg-16-289-2019
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x
Engel MS, Alexander M (1958) Growth and autotrophic me tabolism of nitrosomonas Europea. J Bacteriol 76:217
Eyre BD (1994) Nutrient biogeochemistry in the tropical Moresby River Estuary system North Queensland, Australia. Estuar Coast Shelf Sci 39:15–31
Eyre B, Balls P (1999) A comparative study of nutrient behavior along the salinity gradient of tropical and temperate estuaries. Estuaries 22:313–326. https://doi.org/10.2307/1352987
Forest Survey India (2015) State of forest report. http://www.fsi.nic.in
Ganguly D, Dey M, Sen S, Jana TJ (2009) Biosphere-atmosphere exchange of NOx in the tropical mangrove forest. J Geophys Res 114:G04014. https://doi.org/10.1029/2008JG000852
Ghosh A, Bhadury P (2018) Investigating monsoon and post-monsoon variabilities of bacterioplankton communities in a mangrove ecosystem. Environ Sci Pollut Res 25:5722–5739. https://doi.org/10.1007/s11356-017-0852-y
Grasshoff K, Wenck A (1972) A modern version of the Mohr-Knudsen titration for the chlorinity of sea water. ICES J Mar Sci 34(3):522–528. https://doi.org/10.1093/icesjms/34.3.522
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley-VCH, Weinheim, p 600
Grundle DS, Juniper SK (2011) Nitrification from the lower euphotic zone to the sub-oxic waters of a highly productive British Columbia fjord. Mar Chem 126(1–4):173–181. https://doi.org/10.1016/j.marchem.2011.06.001
Helder W, De VRTP (1983) Estuarine nitrite maxima and nitrifying bacteria (Ems-Dollard estuary). Neth J Sea Res 17(1):1–18. https://doi.org/10.1016/0077-7579(83)90002-9
Herbert RA (1999) Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev 23(5):563–590. https://doi.org/10.2307/1352923
Hofman T, Lees H (1953) The biochemistry of the nitrifying organisms. Biochem J 54:579
Jensen ES (1996) Grain yield, symbiotic N-2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 182:25–38
Jones RD, Hood MA (1980) Interaction between an ammonium-oxidizer, Nitrosomonas sp., and two heterotrophic bacteria, Nocardiaatlantica and Pseudomonas sp.: a note. Microb Ecol 6:271–275. https://doi.org/10.1007/BF02010392
Kristensen E, Jensen MH, Banta GT, Hansen K, Holmer M, King GM (1998) Transformation and transport of inorganic nitrogen in sediments of a southeast Asian mangrove forest. Aquat Microb Ecol 15:165–175
Kristensen E, Bouillon S, Dittmar T, Marchand C (2008) Organic carbon dynamics in mangrove ecosystems: a review. Aquat Bot 89(2):201–219. https://doi.org/10.1016/j.aquabot.2007.12.005
MacFarlane GR, Koller CE, Blomberg SP (2007) Accumulation and partitioning of heavy metals in mangroves: a synthesis of field-based studies. Chemosphere 69(9):1454–1464. https://doi.org/10.1016/j.chemosphere.2007.04.059
Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA (2018) Brock biology of microorganisms, 15th edn. Pearson Education Limited, San Francisco
Majumder N, Chowdhury C, Ray R, Jana T (2011) DNA base composition heterogeneity in two Avicennia species in response to nitrogen limitation in the Sundarban Mangrove Forest, India. Int J Biol Chem 5:238–247. https://doi.org/10.3923/ijbc.2011.238.247
Meyerhof O (1917) Untersuchungen Über Den Atmungsvorgang Nitri fizierenden Bakterien. Pflugers Arch Ges Physiol 166:240
Miranda J, Balachandran KK, Ramesh R, Wafar M (2008) Nitrification in Kochi backwaters. Estuar Coast Shelf Sci 78(2):291–300. https://doi.org/10.1016/j.ecss.2007.12.004
Mukherjee R, Acharya A, Gupta V, Bakshi S, Paul M, Sanyal P, Mukhopadhyay S (2020) Diurnal variation of abundance of bacterioplankton and high and low nucleic acid cells in a mangrove dominated estuary of Indian Sundarbans. Cont Shelf Res. https://doi.org/10.1016/j.csr.2020.104256
Mukhopadhyay SK, Biswas H, De TK, Jana TK (2006) Fluxes of nutrients from the tropical river hoghly at the land–ocean boundary of sundarbans, NE coast of bay of Bengal, India. J Mar Syst 62(1–2):9–21. https://doi.org/10.1016/j.jmarsys.2006.03.004
Nagelkerken I, Dorenbosch M, Verberk WCEP (2000) Importance of shallow-water biotopes of a Caribbean Bay for Juvenile Coral Reef fishes: patterns in biotope association, community structure and spatial distribution. Mar Ecol Progr Ser 202:175–192
Nixon SW (1981) Remineralization and nutrient cycling in coastal marine ecosystems. In: Neilson BJ, Cronin LE (eds) Estuaries and nutrients. Contemporary issues in science and society. Humana Press, Totowa. https://doi.org/10.1007/978-1-4612-5826-1_6
Owens NJP (1986) Estuarine nitrification: a naturally occurring fluidized bed reaction? Estuar Coast Shelf Sci 22(1):31–44. https://doi.org/10.1016/0272-7714(86)90022-3
Pratirupa B, Karapurkar SG, Shenoy DM, Kurian S, Sarkar A, Maya MV, Naik H, Varik S, Naqvi SWA (2015) Carbon and nitrogen isotopic composition of suspended particulate organic matter in Zuari Estuary, west coast of India. J Mar Syst 141:90–97. https://doi.org/10.1016/j.jmarsys.2014.07.009
Priya N, Ranjan P, Sappal SM, Ramanathan AL (2017) Reactive nitrogen dynamics in the mangroves of India. The Indian nitrogen assessment. Elsevier, Amsterdam, pp 335–359. https://doi.org/10.1016/B978-0-12-811836-8.00022-7
Rahaman SM, Sarder L, Rahaman MS, Ghosh AK, Biswas SK, Siraj SS, Huq KA, Hasanuzzaman AF, Islam SS (2013) Nutrient dynamics in the Sundarbans mangrove estuarine system of Bangladesh under different weather and tidal cycles. Ecol Process 2:1–13. https://doi.org/10.1186/2192-1709-2-29
Ramos e Silva CAR, Oliveira SR, Rêgo RD, Mozeto AA (2007) Dynamics of phosphorus and nitrogen through litter fall and decomposition in a tropical mangrove forest. Mar Environ Res 64(4):524–534. https://doi.org/10.1016/j.marenvres.2007.04.007
Ray R, Ganguly D, Chowdhury C, Dey M, Das S, Dutta MK, Mandal SK, Majumder N, De TK, Mukhopadhyay SK, Jana TK (2011) Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmos Environ 45(28):5016–5024. https://doi.org/10.1016/j.atmosenv.2011.04.074
Ray R, Majumder N, Das S, Chowdhury C, Jana T (2014) Biogeochemical cycle of nitrogen in a tropical mangrove ecosystem, east coast of India. Mar Chem. https://doi.org/10.1016/j.marchem.2014.04.007
Reis CRG, Nardoto GB, Oliveira RS (2017) Global overview on nitrogen dynamics in mangroves and consequences of increasing nitrogen availability for these systems. Plant Soil 410:1–19. https://doi.org/10.1007/s11104-016-3123-7
Riley RH, Vitousek PM (1995) Nutrient dynamics and nitrogen trace gas flux during ecosystem development in Montane Rain Forest. Ecology 76(1):292–304. https://doi.org/10.2307/1940650
Rivera-Monroy VH, Twilley R, Boustany RG, Day JW (1995) Direct denitrification in mangrove sediments in Terminos Lagoon, Mexico. Mar Ecol Progr Ser. https://doi.org/10.3354/meps126097
Rysgaard S, Thastum P, Dalsgaard T (1999) Effects of salinity on NH4 + adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22:21–30
Sanyal P, Ray R, Paul M, Gupta VK, Acharya A, Bakshi S, Jana TK, Mukhopadhyay SK (2020) Assessing the dynamics of dissolved organic matter (DOM) in the coastal environments dominated by mangroves, Indian Sundarbans. Front Earth Sci 8:218. https://doi.org/10.3389/feart.2020.00218
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnol Oceangr 33:702–724
Seitzinger SP, Kroeze C, Styles RV (2000) Global distribution of N2O emissions from aquatic systems: natural emissions and anthropogenic effects. Chemosphere Glob Change Sci 2(3–4):267–279. https://doi.org/10.1016/S1465-9972(00)00015-5
Shammas NKh (1986) Interactions of temperature, pH, and biomass on the nitrification process. J Water Pollut Control Feder 58(1):52–59
Singh AK, Ansari A, Kumar D, Sarkar UK (2012) Status, biodiversity and distribution of mangroves in India: an overview. Marine biodiversity one ocean, many worlds life. Uttar Pradesh State Biodiversity Board, Lucknow, pp 59–67
Small G, Bullerjahn G, Sterner R et al (2013) Rates and controls of nitrification in a large oligotrophic lake. Limnol Oceanogr 58:276–286. https://doi.org/10.4319/lo.2013.58.1.0276
Saomville M (1978) A method for the measurement of nitrification rates in water. Water Res 12(10):843–848. https://doi.org/10.1016/0043-1354(78)90036-2
Strickland JDH, Parsons TR (1968) A practical handbook of seawater analysis. Bull Fisher Res Board Can 167:1–311
Uygur A, Kargi F (2004) Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor. Enzyme Microbial Technol 34:313–318. https://doi.org/10.1016/j.enzmictec.2003.11.010
Voss M, Bange HW, Dippner JW, Middelburg JJ, Montoya JP, Ward B (2013) The marine nitrogen cycle: recent discoveries, uncertainties and the potential relevance of climate change. Philos Trans R Soc Lond B Biol Sci 368(1621):20130121. https://doi.org/10.1098/rstb.2013.0121
Wang J, Kan J, Qian G, Chen J, Xia Z, Zhang X et al (2019) Denitrification and anammox: understanding nitrogen loss from Yangtze Estuary to the east China sea (ECS). Environ Pollut. https://doi.org/10.1016/j.envpol.2019.06.025
Wang JP, Zhang R, Li SM, Zhu ML (2020) Nitrification process of Lianhua Stream in Xiamen, Fujian Province. IOP Conf Ser Earth Environ Sci 435(1):012021. https://doi.org/10.1088/1755-1315/435/1/012021
Wild HE et al (1970) Factors affecting nitrification kinetics. 43rd Ann. Conf., Water Pollut. Control Fed., Boston, Mass
Xia X, Zhang S, Li S, Zhang L, Wang G et al (2018) The cycle of nitrogen in river systems: sources, transformation, and flux. Environ Sci Process Impacts. https://doi.org/10.1039/C8EM00042E
Xing X, Liu L, Yan W, Wu T, Zhao L, Wang X (2018) Bioturbation effects of Chironomid larvae on nitrogen release and ammonia-oxidizing bacteria abundance in sediments. Water 10(4):512. https://doi.org/10.3390/w10040512
Acknowledgements
The authors express their gratitude to the Department of Science and Technology, Ministry of Science and Technology (DST) for providing financial support to carry out this study (sanction no. DST/INSPIRE Fellowship/2017/IF170932, dated 30.08.2018). We would also like to thank the Sundarbans Biosphere Reserve (SBR) and Divisional Forest Office (DFO), South 24 Parganas, Govt. of West Bengal for their assistance and support during the field works. We extend special thanks to Dr. Rishmita Mukherjee for her help in proofreading and editing this manuscript. Lastly, we acknowledge the Department of Marine Science (University of Calcutta) for allowing us to conduct this research.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by S.B., A.A., P.S., V.K.G., M.P., and N.M. S.K.M. contributed to the study of conception and design. The first draft of the manuscript was written by S.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakshi, S., Acharya, A., Sanyal, P. et al. Distribution of inorganic nitrogenous species and nitrification in the mangrove environment of the Indian Sundarbans. Aquat Sci 86, 56 (2024). https://doi.org/10.1007/s00027-024-01066-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01066-x