Abstract
The sea cucumber Holothuria (Mertensiothuria) leucospilota is a species native to the Persian Gulf that is commercially exported. Despite its high economic value, no study has been conducted on the reproductive biology of this species in this region. The aim of the present study was to describe the reproductive biology of H. leucospilota using gonad tissue indices and steroid sex hormones (for the first time in sea cucumbers). Spawning was observed to occur only once per year in this species. The annual reproductive cycle of H. leucospilota can be divided into six stages that include resting (recovery), growth, advanced growth, mature, spawning and spent. These stages were observed to occur simultaneously among the study population. The beginning of gametogenesis coincided with the end of January and continued until the end of July. From May to July, the majority of individuals in the population were mature and ready to spawn. Spawning finally took place in August when the water temperature reached its maximum value. However, spawning continued until the beginning of October. In general, the reproduction season of H. leucospilota from the Persian Gulf is the summer, when the temperature reaches its maximum. Most individuals were in the resting phase between late October and early January, during which it was impossible to distinguish the sex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea cucumbers belong to phylum Echinodermata phylum, class Holothuroidea. So far, about 1400 sea cucumber (Holothuroid) species have been reported worldwide, most of which are native to the Asia Pacific region (Pawson 2007). These benthic animals mainly live among coral reefs, although they are also found in sandy and muddy bed sediments (Castro and Huber 2000). Most species of holothuroids are found in the intertidal zones, but some species occur in deep ocean waters (Smirnov et al. 2000). Sea cucumbers play a beneficial role in the marine ecosystem by facilitating nutrient recycling: they, break down detritus and other organic matter, thereby facilitating the decomposition process by bacteria which continue the process (Brukner et al. 2003).
Holothuroids are typically dioecious, with separate male and female individuals, but some species are protandric. They are mostly gonochoric and have a single gonad consisting of one or two elongated tubular tufts attached to the dorsal mesentery (Ramon et al. 2022). These organisms are mostly broadcast spawners. The mature male and female spawners release their sperms and eggs widely in the water column, and fertilization takes place in the water (Ramon et al. 2022). It is almost impossible to distinguish male and female sea cucumbers using morphological characteristics of the body or gonads. However, in some species, the color of male and female gonads is different in adults (Navarro et al. 2012).
Holothuria (Mertensiothuria) leucospilota (Brandt, 1835) (black sea cucumber or black tarzan) belongs to the family Holothuriidae. It is an opportunistic species with a wide distribution in most tropical areas of the Indian Ocean, western central Pacific, Africa, and Asia. Holothuria leucospilota is the dominant species in the Persian Gulf (Afkhami et al. 2012) where it lives in calm and slightly deep areas on the sandy bottom or on coral rubble (Yang et al. 2020). This sea cucumber species is a medium-sized sea cucumber, reaching a length of up to 40 cm when relaxed and stretching to about 1 m when extended.
So far, parameters such as temperature (e.g., Chao et al. 1995), photoperiod (e.g., Muthiga 2006), and the salinity (e.g., Li et al. 2011) have been proposed as potential spawning inducers for temperate and tropical holothurian species. However, it can be a difficult task to determine which of these environmental factors is the main driver of spawning (Olive 1995). Yang et al. (2020) showed that this species is relatively resistant to changes in salinity and temperature and that it continues to grow even when these parameters are changed in the laboratory. In contrast, these authors observed that changes in temperature and salinity led to shrinking and evisceration in the Japanese sea cucumber (Apostichopus japonicus) and finally its death within 3 days (Yang et al. 2020).
The Persian Gulf, with an average depth of 35 m, is a very shallow basin compared to other gulfs in the world, such as the Gulf of Mexico (average depth 1650 m). The geographical location of the Persian Gulf and its shallow depth and limited water exchange lead to significant changes in the sea surface temperature. Due to the climate of the region, the amount of evaporation in the Persian Gulf is tenfold higher than that of the fresh water entering it (average 1.4–5 m/year), leading to the high salinity of the Persian Gulf (40–50 ppt). Wide temperature fluctuations and extreme salinity have limited the presence of many shallow-water species common at similar latitudes elsewhere in the Indo-Pacific region. On the other hand, this situation favors those species that are adapted to these harsh and unique conditions. Despite these environmental conditions, the Persian Gulf has a diverse range of animal and plant life with a number of species unique to the region.
The Persian Gulf H. leucospilota is an edible sea cucumber with a high content of bioactive compounds; as such, it is considered to be a valuable fishery resource in many regions (Han et al. 2010). Despite the ecological and economic importance of H. leucospilota, little information is available on the reproductive biology of this species in the Persian Gulf, and the information which is available is mainly related to its basic biology, food selection, and genetics (Dabbagh and Keshavarz 2011; Mashjoor and Yousefzadi 2019; Shushizadeh et al. 2019). If the reproductive cycle of this sea cucumber species is to be better understood, it is important to have data on its gonadal histomorphology given that life history information is a prerequisite for efficient natural resource management. Therefore, the aim of the present study was to elucidate the reproductive cycle of H. leucospilota in the Persian Gulf based on analysis of the steroid hormones and histology of the gonads, with a focus on the timing of the spawning period, and to provide documents on the environmental conditions involved in the release of gametes.
Materials and methods
Study areas and sampling
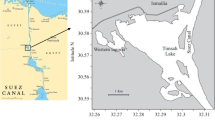
Specimens of H. leucospilota were collected along the intertidal zone during the lowest low tide between April 2020 and August 2021 along the Bushehr coast, situated in the northwest of the Persian Gulf (28°59′N, 50°50′E) (Figs. 1, 2). A total of 262 H. leucospilota sea cucumbers (125 females, 105 males, and 32 undifferentiated individuals based on the histological study of gonads) were randomly hand-picked monthly. Prior to dissection, the collected sea cucumbers were anesthetized for 12 h by releasing them in seawater at 3 °C (Thongbuakaew et al. 2021). After measuring the total body weight and length, sea cucumbers were then dissected and whole gonadal tubules were removed from the body and weighed. The gonads (10 specimens/month) were then fixed in 10% formalin buffer solution for 48 h and preserved in 50% ethanol until histological analysis. In addition, the gonads from ten other specimens were stored monthly at − 80 °C for further analysis of sex steroids. The care and use of experimental animals complied with the guidelines, animal welfare laws, and regulations of the Iran Fisheries Organization and the Iranian Department of Environment (IFO; program number 32886-1).
Using a Universal Datalogger (IRIS-R3; Saen Co. Tehran, Iran), environmental parameters, including water salinity and temperature, were measured monthly (at the time of sampling).
Microscopic examination
As a first step, sex was determined based on the color of the gonads (gonadal tubes are usually yellow or orange in females and whitish in males). Gonad specimens were dehydrated in a graded series of ethanol, cleared in xylene, and embedded in paraffin wax using a Tissue-Tek Rotary Tissue Processor (model RX11B; Sakura Corp., Osaka, Japan). Sections were cut at a thickness of 5–6 µm using a semi-digital rotary microtome (model RM2245; Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin (H&E) (Fischer et al. 2008). The stained specimens were then analyzed microscopically to evaluate the sex and developmental stages of gonads using a light microscope (model BX50; Olympus Corp., Tokyo, Japan), and digital images were taken using a Dino-Lite lens equipped with DinoCapture software (version FDP2; AnMo Electronics, New Taipei City, Taiwan). Different stages of gonadal development in each sex were determined based on histological characteristics used to characterize other holothurians (Toral-Granda and Martínez 2007; Navarro et al. 2012; Benitez-Villalobos et al. 2013) including: inactive or resting (stage I [SI]), growth (stage II [SII]), advanced growth (stage III [SIII]), mature stage (stage IV [SIV]), spawning (stage V [SV]), and spent (stage VI [SIV]). Ten tissue sections from each sea cucumber (at least 6 sea cucumbers per month) were studied to determine the degree of gonad maturity each month. The following three principals were used to define the different stages of gonadal development for each sex: (1) sets of processes of gamete biological development; (2) changes in gonadal tubules; and (3) mechanisms of gonadal regeneration or absorption after spawning (Benitez-Villalobos et al. 2013).
Quantitative Histology
Gonado-Somatic Index
The Gonado-Somatic Index (GSI) was estimated using the following equation (Navarro et al. 2012):
GSI = (gonad weight [g]/total body weight [g]) × 100
Mean gonadal index
Mean gonadal index (MI) was calculated monthly for both sexes alone or in combination based on the microscopic gonad developmental stages using the following formula (Machensen et al. 2011):
MI = ([NSI × 0] + [NSII × 0.33] + [NSIII × 0.66] + [NSIV × 1] + [NSV× 0.66] + [NSVI × 0.33])/(NSI+S1I+SIII+SIV+SV+SVI), where Nx is the number of sea cucumbers at the x-gonadal stage; NSI+S1I+SIII+SIV+SV+SVI is the total number of sea cucumbers evaluated per month.
The MI ranged between 0 (if all sea cucumbers specimens were in S0) and 1 (if all sea cucumbers specimens were in S5).
Frequency of ovarian follicles in different stages
For this parti of the study, five female sea cucumbers were randomly selected each month, and ten tissue sections from each specimen and three microscopic fields in each section were examined microscopically. After determining the developmental stage of the ovarian follicles, the percentage of follicles in the same developmental stage was calculated according to Drumm and Loneragan (2005).
Gonad coverage area
The gonad coverage area (GCA) is the area occupied by the gonad within an area of 7.9 mm2 at a magnification of ×4. To calculate GCA, three different sections in each specimen and at least five specimens per month were studied. The GCA was calculated using the following formula (Rodríguez-Jaramillo et al. 2008):
GCA = (gonad occupation area/total area) × 100
Oocyte diameter
The diameter of at least 100 oocytes (with a visible nucleus) per female per month were measured (Galley et al. 2008). In this regard five female specimens were haphazardly selected each month, and ten tissue sections from each specimen were examined microscopically. The diameter of oocytes was measured using DinoCapture software in digital images captured by the Dino-Lite lens (AnMo Electronics).
Analysis of sex hormones
The concentration of estradiol (E2) and testosterone was measured in male and female sea cucumbers using an enzyme-linked immunosorbent assay (ELISA) method following Gauthier-Clerc et al. (2006). First, 0.5 g of gonad tissue from each sex (5 sea cucumber/sex/month) was homogenized in distilled water (1:5, w:w). Then, 1200 µL of 25 mM HCl was added to 1500 µL of homogenate and incubated at 40 °C for 15 min. Before organic extraction, 3.75 mL of 0.07 M Na2HPO4 (pH 7.4) was added. The homogenates were then extracted twice using 42 mL of dichloromethane, and the organic extracts were evaporated under nitrogen flow at 21–23 °C until dry. The resulting pellet was dissolved in 300 mL enzyme immuno-assay buffer and used for the determination of sex hormones. The concentrations of E2 and testosterone were determined using commercial ELISA kits (96 T; Monobind Co., Tehran, Iran) according to the manufacturer’s instruction. The concentration of each hormone was then measured at 405 nm. Standard curves were obtained between 6.6 and 4000 pg/mL for E2 and between 3.9 and 500 pg/mL for testosterone, and specimen concentrations were assessed according to standard curves.
Statistical analysis
All data were presented as the mean ± standard error (SE). The normality of data was assessed by the Shapiro-Wilk test. Statistical differences in all parameters were tested using one-way analysis of variance (ANOVA) followed by the post hoc Duncan's multiple range test. Linear regression analysis was used to determine the relationship between salinity and temperature as independent variables, and the reproductive factors, including GSI and MI, as dependent variables. A significance level of P ≤ 0.05 was accepted.
Results
Environmental changes
As shown in Fig. 3, water salinity did not change significantly during the year. The highest and lowest salinity recorded for the study area was 40.2 and 39.52 Practical Salinity Units (PSU), respectively; these levels were not significantly different (P > 0.05). In contrast, significant changes in the water temperature were recorded throughout the year (P ≤ 0.05; Fig. 3). The highest and lowest temperatures were measured in August (33.9 °C) and February (19 °C), respectively. Temperatures exceeding 30 °C are common in the study area.
Gonad histomorphology
The histomorphologic changes in the gonads of male and female H. leucospilota throughout the annual reproductive cycle are shown in Figs. 4, 5, and 6 . The gonads of H. leucospilota collected from the northwest of the Persian Gulf (Bushehr) were divided into numerous gonadal tubules which were approximately of the same diameter (range [mean ± SE]: < 0.9 ± 0.025 mm in inactive or sexually resting gonads up to 2 ± 0.081 mm in mature gonads) and length (range: 2.5 ± 0.5 cm in inactive or sexually resting gonads up to 15 ± 1.2 cm in mature gonads). Almost all tubules in each gonad were at the same developmental stage and matured simultaneously (Fig. 4). Gametogenesis occurred uniformly along the germinal epithelium (the innermost surface of the gonadal tubules). The color of the tubules varied from yellowish cream in the inactive or sexually resting gonads to brown in the mature gonads (Fig. 4). All gonadal tubules were joined to a single gonoduct leading to a gonopore located between the tentacles.
Photographs of gonads at different developmental stages in male and female Holothuria leucospilota collected from the northern part of the Persian Gulf (Bushehr). a Inactive or resting stage (SI), b growth stage (SII), c advanced growth stage (SIII), d mature stage (SIV), e spawning stage (SV), f spent stage (SVI). GT Gonadal tubule
Representative micrographs of gonad tubules illustrating the different stages of gonad development in female Holothuria leucospilota from the north of the Persian Gulf (Bushehr): a inactive or resting stage (SI) showing empty gonad tubules; b, c and d. growth stage (SII) showing oogonia (white arrow head) and primary oocytes (black arrow heads) inside the tubules; e and f. advanced growth stage (SIII) showing tubules contained oogonia (white arrow head), primary oocytes (black arrow heads) and some secondary oocytes (black arrows); g, h, i. mature stage (SIV) showing tubules filled with secondary oocytes (black arrows); j and k. spawning stage (SV) showing tubules with scattered secondary oocytes (black arrows); L. spent stage (SVI) showing tubules filled with atretic oocytes (AO) and tissue debris (TD); N nucleus, nu nucleolus, TW tubular wall, L lumen, T: a, b, e, j (H&E ×290); c, f, h, k, l (H&E ×725); d, i (H&E ×2900); g (H&E ×200). (Color figure online)
Representative micrographs of gonad tubules illustrating the different stages of gonad development in male Holothuria leucospilota collected from the northern part of the Persian Gulf (Bushehr). a Inactive or resting stage (SI) showing empty gonad tubules. b, c Growth stage (SII) showing spermatogonia (Sg) and primary spermatocytes (PS) inside the tubules. e, e, f Advanced growth stage (SIII) showing tubules contained primary spermatocytes (PS) and scattered spermatids (St). g, h, i Mature stage (SIV) showing tubules filled with spermatozoa (S) and other germ cells. j, k spawning stage (SV) showing tubules with scattered germ cells. l Spent stage (SVI) showing empty tubules (ET). CT Connective tissue, GW gonadal wall, L lumen. Magnification: a, b, e, g, j, k: ×725 (H&E); c, f, h: ×2900 (H&E); d, l: ×290 (H&E); i: ×7250 (H&E). (Color figure online)
Small hollow tubules with a thin wall consisting mainly of connective tissue were characteristic of gonads in inactive sea cucumbers (stage I) (Figs. 5a, 6a). In growing gonads (stage II), small oogonia and primary oocytes (contain nucleus [N] without obvious nucleolus) in female H. leucospilota and spermatogonia and primary spermatocytes in male H. leucospilota were visible in the developing tubules (Figs. 5b–d, d, 6b, c). Tubules were considerably expanded in advanced growth gonads (stage III). In this stage, female gonadal tubules contained many larger secondary oocytes (vitellogenic oocytes) with some oogonia and primary oocytes (Figs. 5e, f). Spermatogonia, primary and secondary spermatocytes, spermatids, and some spermatozoa were observed in male gonadal tubules in the advanced growth stage (Fig. 6d–f). In the mature gonads (stage IV), the expanded tubules were completely filled with vitellogenic oocytes (contain nucleus with obvious nucleolus and vacuolated cytoplasm) in females and the spermatozoa in males (Figs. 5g–i, 6g–i). In the spawning stage (stage V), gonadal tubules contained some free vitellogenic oocytes in females and scattered spermatozoa in males (Figs. 5j, k, 6j, k). The germ cells were completely released or absorbed in spent H. leucospilota. The male gonadal tubules were mostly empty at this stage. Also, gonadal tubules mainly contained tissue debris and some atretic eggs in spent females (Figs. 5l, 6l).
Annual reproductive cycle and frequency of gonad developmental stages
Based on the gonad histological study, only one reproductive cycle was identified for H. leucospilota from the Persian Gulf throughout the year, which included a relatively long period of gametogenic activity, a period of several months of spawning, followed by a relatively short period of sexual rest. Figure 7 shows the frequency distribution of the different reproductive stages observed in the present study.
As the water temperature dropped during November, 80–90% of the specimens examined during the period November to mid-January were in the inactive or sexual rest stages (Fig. 7a, b). Beginning around mid-January, the inactive H. leucospilota gradually entered the growth stage, and 60% of females and 50% of males were in the growth stage by mid-March (20.3 °C) (Fig. 7a, b). About 20% of male and female H. leucospilota entered the advanced growth stage by the end of March (Fig. 7a, b), and from the end of March to the end of May (30 °C), 75% of female H. leucospilota and 70% of male H. leucospilota were in the advanced growth stage (Fig. 7a, b). Mature H. leucospilota were mostly detected from the end of May through to July, with the highest number of mature sea cucumbers observed in July when the seawater temperature was > 30°C (mean 32.9 °C) (Fig. 7a, b): 80% of females and 70% of males were mature by the end of July (Fig. 7a, b). Many mature H. leucospilota simultaneously entered the spawning stage (80% of the females and 90% of the males) when the water temperature reached its peak (33.9 °C). However, a few mature H. leucospilota (5% of females and 10% of males) had already started spawning during July (Fig. 7a, b). Although the spawning peak of H. leucospilota from the Persian Gulf was in August, the spawning continued until mid-October (Fig. 7a, b).
Biometric data and GSI
Based on the gonad histological study, 125 specimens of the H. leucospilota collected were female (mean [± SE] body weight 62.15 ± 8 g; mean body length 9.1 ± 1.54 cm), and 104 specimens were male (mean body weight 58.41 ± 7.4 g; mean body length 8.5 ± 1.5 cm). Of these, 32 were sexually indistinguishable (mean body weight 43.5 ± 5 g; mean body length 5.36 ± 1 cm). In general, female H. leucospilota were larger than male H. leucospilota. Female H. leucospilota collected during mid-June to mid-August were significantly larger than those collected during the rest of the year (P ≤ 0.05; Fig. 8a), and those collected in July were the largest in size (mean body weight 77.3 ± 10 g; mean body length 12.2 ± 2.3 cm) (P ≤ 0.05; Fig. 8a). Sexually indistinguishable H. leucospilota collected during late-October to late-December were the smallest in size (mean body weight 43.5 ± 5 g; mean body length 5.36 ± 1 cm) (P ≤ 0.05; Fig. 8a). In total, the weight and length of H. leucospilota collected during the year followed the following pattern: mature H. leucospilota > spawning H. leucospilota > advanced growth H. leucospilota ≥ spent H. leucospilota > inactive or sexual resting H. leucospilota. The weight of the gonads followed the same pattern as total body weight and length. Mature male and female H. leucospilota collected in July had the largest gonads (mean gonad weight 17.05 ± 1.2 g) (P ≤ 0.05; Fig. 8a). The smallest gonads were observed in inactive or sexual resting H. leucospilota (mean gonad weight 1.47 ± 0.08 g) (P ≤ 0.05; Fig. 8a).
Monthly variation in body weight and size, gonad weight, and Gonado-Somatic Index (GSI) values (a), mean gonadal index (MGI) (b), and gonad coverage area (c) of male and female Holothuria leucospilota collected from the Persian Gulf (Bushehr). Different lowercase letters indicate significant differences among different months and gonad developmental stages
The GSI in H. leucospilota collected during the year followed a similar trend. The highest and lowest GSI were recorded in both sexes collected in July (mean GSI 22.05 ± 3.4) and November (mean GSI 2.01 ± 0.05), respectively (P ≤ 0.05; Fig. 8a). The pattern of GSI changes was as follows (Fig. 8a): mature H. leucospilota > spawning H. leucospilota > advanced growth H. leucospilota ≥ spent H. leucospilota > inactive or sexual resting H. leucospilota.
Mean gonadal index
The monthly changes in MGI followed a similar pattern in male and female H. leucospilota. Moreover, a significant positive correlation (Rs = 0.7; P = 0.001) was observed between total MGI and temperature (Fig. 8b). The highest MGI values (total, male and female MGI) were recorded from mid-May to July, with a peak in July in both males and females (P ≤ 0.05; Fig. 8b). The lowest values for MGI (total, male and female MGI) were recorded during November to mid-January (P ≤ 0.05; Fig. 8b).
Gonad coverage area
Significant differences were observed in the GCA between different gonadal developmental stages (P ≤ 0.05). The highest GCA was recorded in mature male and female H. leucospilota (SIV) in July (females 90% ± 8% ; males 87% ± 5%) (P ≤ 0.05; Fig. 8c); the lowest GCA was recorded in male and female H. leucospilota in the inactive or sexual rest stage (SI) (females and males 4.3% ± .05%) during November and December (P ≤ 0.05; Fig. 8c). The rate of GCA in different male and female gonad developmental stages followed the following pattern: SIV > SV > SIII > SII > SIV ≥ SI.
Monthly changes in oocyte maturation stages and oocyte sizes
The gonadal tubules of inactive or sexually resting female H. leucospilota were mostly empty of germ cells from late October to mid-January. With the start of gonadal development beginning in mid-January, a large number of oogonia (22 ± 4.2 µm) and primary oocytes (mean ± SE: 45.2 ± 6.7 µm) were gradually observed on the inner surface of the gonadal tubules in female H. leucospilota (Figs. 9a, 10a). From mid-March, the number of oocytes in the female gonad tubules increased, and a significant number of secondary oocytes (68.42 ± 11.2 µm) were observed inside the tubules from April (Fig. 9a, 10a). During May and July, the number of oocytes and their size increased significantly so that the oocytes, which were mainly vitellogenic (93.71 ± 16.2 µm) completely filled the developed gonadal tubules in mature females (Figs. 9a, 10b). During August and September, female H. leucospilota were spawning and the oocytes observed in their gonadal tubules were mainly vitellogenic. From mid-September to mid-October, only tissue debris and a number of atretic oocytes (50 ± 7.12 µm) were observed in the gonadal tubules of spawned female H. leucospilota. From late October to mid-January, mainly no germ cells were found in the gonad tubules of inactive or resting sexual females (Figs. 9a, 10b). Overall, the size of oocytes increased with the maturation of gonads (Figs. 9b). The mean size of oocytes gradually increased during winter and early spring and then peaked in July (Fig. 9b). The largest oocytes were recorded in the late vitellogenic stage and were observed in mature females in July (P ≤ 0.05; Figs. 9b, 10b). Female H. leucospilota with the smallest oocytes were recorded in the early growth stage in January and February (P ≤ 0.05; Figs. 9b, 10b).
Annual frequency of oocyte maturation stages (a) and oocyte size (b) during different gonadal development stages in female Holothuria leucospilota collected from the Persian Gulf (Bushehr). Different lowercase letters indicate significant differences among different months and gonad developmental stages
The concentrations of E2 and T in male and female gonads
The annual pattern of changes in E2 and testosterone concentrations were consistent with the gonad development. Thus, the maximum levels of E2 and testosterone were measured in mature male and female H. leucospilota during mid-June to late-July (E2: 471.68 ± 32.8 pg/g wet weight in females and 95.06 ± 12.5 pg/g wet weight in males; testosterone: 28.14 ± 5.2 pg/g wet weight in females and 112.56 ± 16 pg/g wet weight in males). The minimum levels of E2 and testosterone were measured in the inactive or sexual resting H. leucospilota during November and December (E2: 68.9 ± 6.8 pg/g wet weight in females and 42.9 ± 3.4 pg/g wet weight in males; T: 18.5 ± 1.32 pg/g wet weight in females and 25.5 ± 2.8 pg/g wet weight in males) (P ≤ 0.05; Fig. 11a, b).
Discussion
A number of aspects of the reproductive biology of H. leucospilota from different regions have been studied (Purwati and Luongvan 2003; Drumm and Lonergan 2005). However, intra-species differences as well as differences in environmental conditions cause different behaviors of a same species in habitats with different conditions. Therefore, we decided to investigate the reproductive biology of H. leucospilota in the Persian Gulf in order to collect information that would contribute to the proper management of this resources of this species in this environment. According to Sewell (1990), reproductive biology may even differ among specimens of the same species collected at different latitudes.
Holothuria leucospilota is a species that is native to the Persian Gulf where it is commonly found. It is gonochoric (have separate sexes). Unlike many aspidochirotida holothurians, such as H. fuscocinerea (Benitez-Villalobos et al. 2013), H. poli (Slimane-Tamacha et al. 2019), H. spinifera (Asha and Muthiah 2007), and H. (Platyperona) sanctori (Mezali et al. 2014), H. leucospilota shows in the Persian Gulf no sexual dimorphism. Drumm and Loneragan (2005) also reported that H. leucospilota from Rarotonga did not show external sexual dimorphism. Consequently, in the present study, sex was determined based on the color of the mature gonad (testes are beige and the ripe ovaries are pink/red) (Purwati and Luongvan 2003; Drumm and Loneragan 2005) and gonad histology.
Holothurians often exhibit an annual reproductive cycle (Chao et al. 1995); however, semiannual or even continuous reproductive cycles throughout the year have been also reported, especially in tropical regions (Conand 1993). Similar to H. poli from Lesvos Island, Greece (Bardanis and Batjakas 2018), the lack of hermaphroditic individuals and existence of an annual reproductive cycle were noted in the collected H. leucospilota in the present study.
The main environmental stimuli that directly or indirectly affect the sexual cycles of organisms include changes in temperature, salinity, photoperiod, availability of food, and habitat, as well as recruitment and survival of juveniles (Costelloe 1985). In the present study, we investigated two of these factors, namely, salinity and temperature.
The relationship between the reproductive cycle and natural environmental conditions has been investigated for a number of holothurians (Costelloe 1985; Sewell and Bergquist 1990; Hamel et al. 1993; Hopper et al. 1998; Asha and Muthiga 2007; Benitez-Villalobos et al. 2013; Kazanidis et al. 2014; Panola-Madrigal et al. 2017). Similar to Holothuria tubulosa and H. fuscocinerea, H. leucospilota reproduces in warmer months when the days are longer (Benitez-Villalobos et al. 2013; Kazanidis et al. 2014). In the H. leucospilota population of the Persian Gulf, gamete release coincides with a significant increase in temperature (33.9 °C) in July-August. Similarly, H. tubulosa spawns during the warm months (from July to September) in the Adriatic Sea, when the surface water temperature is at its warmest (Despalatovic et al. 2004). The main reason for spawning at relatively high water temperatures is that the larvae have more food available during this time (warm months) due to the phytoplankton proliferation (Kazanidis et al. 2014; Panola-Madrigal et al. 2017). According to Conand (1993), the seasonal reproductive cycle in holothurians is characteristic of most temperate species, whose spawning season is associated with increasing temperature and increasing phytoplankton biomass, with an increased food source for the sea cucumber. Unlike these species, the spawning period of some holothurians, such as Aslia lefevrei (Costelloe 1985), Holothuria forskali (Ballesteros et al. 2021) and Holothuria whitmaei (Shiell and Uthicke 2006), occur in colder months. However, taking the stimulation of spawning by thermal changes into account, it would appear that temperature is probably the most important factor in the release of gametes in many holothurian species. Therefore, temperature changes have been largely used to stimulate spawning in holothurian cultivation systems (Rakaj et al. 2018; Ballesteros et al. 2021). On the other hand, Vargas-Yanez et al. (2017) stated that temperature might not affect the gonadal cycle of Parastichopus regalis collected from depths of 60 to 150 m in the northwestern Mediterranean Sea. One possible explanation is that the water temperature at these depths is quite constant at about 13–14 °C in winter, spring and summer when the reproductive cycle develops (Vargas-Yanez et al. 2017). In autumn, when the temperature decreases with depth (from 18 °C at 50 m to 13 °C at 150 m), no gonads were found in the studied P. regalis (Vargas-Yanez et al. 2017).
Salinity could be another factor affecting the reproductive cycle in holothurians. However, in the present study, we found no correlation between gonadal development in H. leucospilota and salinity. Analysis of salinity fluctuations in the Persian Gulf based on 10-year reports revealed that changes in salinity in the region have a constant trend. The average salinity is 39.12 PSU across any 1 year, with a decrease to a minimum of 38.68 PSU in the summer and an increase to a maximum of 39.34 PSU in the winter (Omidi and Norinezhad 2008). Therefore, salinity would seem not to have a significant effect on the reproductive cycle of H. leucospilota.
We noted that H. leucospilota did not show the characteristic pattern of a continuous reproductive cycle. This finding was expected, given that the reproductive cycles of this tropical species are timed so that the production of larvae or fry coincides with periods favorable for feeding or survival (Giese and Pearse 1974). Slimane-Tamacha et al. (2019) also reported a single annual reproductive cycle for Holothuria poli. On the other hand, Gaudron et al. (2008) reported a biennial cycle consisting of a dominant warm-season spawning and a smaller and variable secondary peak in H. leucospilota in the western Indian Ocean.
We also noted that the gonad morphology and gametogenesis stages were similar to those recorded for holothurians such as H. leucospilota in the western Indian Ocean (Gaudron et al. 2008). The gonad of H. leucospilota from the Persian Gulf was similar to that of H. fuscocinerea (Benitez-Villalobos et al. 2013) and H. poli (Slimane-Tamacha et al. 2019): it consisted of two bundles of long tubules, one on each side of the dorsal mesentery. However, the results of the present study differed in some aspects from the results obtained by other researchers even on the same species in other regions. The gonad tubules were absorbed after spawning in Stichopus californicus (Smiley 1988). However, similar to H. leucospilota in the western Indian Ocean (Gaudron et al. 2008) and H. fuscocinerea (Kazanidis et al. 2014), in H. leucospilota from the Persian Gulf, the tubules with progenitor cells along the germinal epithelium were visible at the end of the following January.
The annual reproductive cycle of H. leucospilota culminated in spawning in mid-summer. Similar to Cucumaria frondosa (Hamel and Mercier 1996), gametogenesis in H. leucospilota started in late January, after a short period of recovery. Intense gamete synthesis and storage continued until spawning (late July to mid-October), when gametes were released from mature gonad tubules that had reached their maximum size. This growth period (on average 6–8 months) seems to be necessary for the maturation of the gonad tubules. Such results are quite different from those reported by Gaudron et al. (2008) for the same species in the western Indian Ocean. In the present study, male and female H. leucospilota were mainly in developmental stages 4 and 5 from July to mid-October, while at the same time of year, the western Indian Ocean H. leucospilota was reported to be in stage 3 (Gaudron et al. 2008). A higher percentage of H. leucospilota were in post-spawning stage by the end of October, while the post-spawning stage of western Indian Ocean H. leucospilota was at the end of February (Gaudron et al. 2008). Some western Indian Ocean H. leucospilota remained at this stage until April, and then a second spawning event occurred in May. According to Gaudron et al. (2008), females that failed to spawn in February were able to spawn during the wet season of the Australian summer.
Similar to the western Indian Ocean H. leucospilota (Gaudron et al. 2008), the existence of different stages of developing gametes within one tubule was usual in male and female H. leucospilota from the Persian Gulf. In the present study, about 75–90% of male and female H. leucospilota had gonads every month of the year. However, the gonads were very small in < 25% of specimens, especially between December and mid-January. Christophersen et al. (2020) revealed that 67–100% of Parastichopus tremulus specimens had gonads every month of the year. Based on the microscopic observations, gonad development in male and female H. leucospilota corresponded to a synchronous "tubule recruitment pattern" with all gonad tubules being at the same stage of development in this species. It would appear that this synchrony may be due to changes in water temperature and photoperiod (Drumm and Loneragan 2005). There are many other holothurian species that follow the same trend, such as Holothuria fuscocinerea (Benitez-Villalobos et al. 2013). The same result was also reported by Gaudron et al. (2008) for H. leucospilota in the western Indian Ocean. On the other hand, the asynchronous model is usual in some other holothurians, such as H. atra (Chao et al. 1994), H. fuscogilva and H. mauritiana (Ramofafia and Byrne 2001).
Body size is an important factor that explains both interspecies and intraspecies changes in reproductive function. With increasing body size and weight, the weight of the gonads and thus the reproductive index increase in the most holothurian species (Conand 1993).
In the present study, female and male H. leucospilota with a body weight between 61 and 70 g and a GSI > 12% had gonads at stage III (growing stage), close to sexual maturity. In comparison, in H. leucospilota with a body weight between 72 and 85 g and a GSI > 19%, the gonads were in stage VI of sexual maturity and fully mature. However, these values are higher than the average body weight reported for growing and mature H. leucospilota in southern Vietnam (45 and 67 g, respectively) (Nguyen and Britaev 1993).
Drumm and Loneragan (2005) reported female and male H. leucospilota with a body weight exceeding 37.5 and 55 g were in the growing and mature gonad development stages, respectively. In addition, the lowest GSI values (between 1.2% and 2.7%) belonged to ‘sexually undifferentiated’ H. leucospilota in the resting months (late October to early January). Benitez-Villalobos et al. (2013) also recorded the lowest values of GSI (≤ 2%) for H. fuscocinerea between October and January. The MGI has been used to determine the pattern of gonadal growth in many holothurian species (Conand et al. 2002; Foglietta et al. 2004; Christophersen et al. 2020). In the current study, the MGI was used to document the development pattern of gonads. Due to the relatively low dispersion of MGI between male and female H. leucospilota individuals, its use is recommended to obtain adequate information on the reproductive cycle.
However, in some studies on holothurian reproduction, the use of this index has not been successful. For example, Ramon et al. (2022) did not suggest using this index due to the high dispersion of data in Parastichopus regalis, especially in the summer when the gonad reached its maximum size. During the H. leucospilota reproductive cycle (except from August to the end of November), the MGI was higher in females than males. Tuwo and Conand (1992) also stated that female H. forskali had a higher gonadal index than their male counterparts. Inversely, the mean gonadal index was reported to be higher in male Cucumaria frondosa (Hamel and Mercier 1996) and Psolus fabricii (Hamel et al. 1993) than in their female counterparts, before the spawning event. On the other hand, there was no inequality between male and female gonadal indices in holothurians such as Parastichopus californicus (Cameron and Fankboner 1986) and Stichopus mollis (Sewell and Bergquist 1990). In the present study, it seems that the higher MGI values in females were due to the presence of larger gonads than males and the need for more energy to produce an equivalent number of gametes compared to males. In particular, considering that there was no significant difference in the body size of males and females during the reproductive cycle. Lawrence and Lane (1982) showed that ovaries are richer than testes even though they are of similar size. Synthesis of female gametes probably initially needs more energy than the synthesis of male gametes (Hamel and Mercier 1996).
The oocyte size is another important factor in the reproductive cycle. In the holothurians studied, the size of the mature oocytes varied between 65 and 150 µm (Conand 1993; Hamel and Mercier 1996). In the present study, the average diameter of the mature oocytes reached a maximum size (86.57 ± 18 µm) in H. leucospilota in stage VI of maturity (May to July). This result is comparable with the mature oocyte diameter reported in Holothuria atra (88 µm; Harriott 1982); Holothuria parvula (93 µm; Emson and Mladenov 1987).
The findings of the present study revealed the existence of E2 and testosterone in the gonad tissues of H. leucospilota. This helps confirm the presence of steroids in invertebrates, including holothurians. The presence of steroids has been proven in a limited number of holothurian species, such as H. scabra (Thongbuakaew et al. 2021), but the amount of these hormones has not been measured in these species. The results presented here are consistent with reports on testosterone and E2 in other echinoderms, such as the sea urchin Paracentrotus lividus (Barbaglio et al. 2007) and the echinoderm Paracentrotus lividus (Janer et al. 2005). Like other species, there was a close relationship between testosterone or E2 levels and the degree of gonad growth in H. leucospilota. A significant difference in the concentration of testosterone and E2 was found across months of the year as well as between males and females in H. leucospilota. However, Barbaglio et al. (2007) found no clear correlation between testosterone and E2 levels and the distribution of reproductive stages across a year in sea urchin P. lividus.
In agreement with the results of the present study, Janer et al. (2005) reported that the testosterone level was significantly lower in the testis of P. lividus during early and advanced spermatogenesis than in the mature stage (the end of gametogenesis). This result shows the role of testosterone in regulating spermatogenesis and spawning. Similar to the sea urchin Lytechinus variegatus (Wasson et al. 2000), testosterone levels increased immediately in H. leucospilota after the resting (recovery) phase (when the gonads were preparing to reorganize for the next reproductive cycle), indicating the involvement of testosterone in the initiation of a new spermatogenesis. Hines et al. (1992) stated that testosterone level also increased during vitellogenesis in ovaries in the sea star Asterias vulgaris, indicating the involvement of testosterone also in oogenesis. In the present study, the higher concentration of E2 compared to testosterone in females indicated the more important role of this hormone in females. Similar to L. variegatus (Wasson et al. 2000) and A. vulgaris (Hines et al. 1992), the mean concentration of E2 increased in ovaries of H. leucospilota immediately after the recovery stages and was high throughout vitellogenesis, indicating the special role of this hormone in the regulation of oogenesis. According to the data, the significant role of testosterone and E2 in the reproductive biology of echinoderms, especially sea cucumber, seems evident.
Conclusion
The present study provides important information on the reproduction of H. leucospilota in the Persian Gulf. For a long time, H. leucospilota has been hunted indiscriminately in this area and commercially exported to other countries while the Iranian Fisheries Organization has been trying to culture this species. In the present study, the indices MGI and GSI were shown to be reliable tools for assessing reproductive cycle timing based on similar results between microscopic examination and these indices. The present study showed an annual and synchronogamic pattern characterized by a spawning period in the summer months. It seems that high values of sea water temperature play a role in the release of gametes (Kazanidis et al. 2014). In terms of the general reproductive pattern of H. leucospilota in the Persian Gulf, the maturation period was characterized by an increase in the percentage of matures (stages III and IV) from March to July, followed by spawning from August to mid-October, while the resting period lasted from late October to early January.
Data availability
All data generated or analyzed during this study are included in this article.
References
Afkhami M, Ehsanpour M, Dabbagh AR, Sarhadizadeh N, Mirzadeh G (2012) New observation of two sea cucumber species from Abu Musa Island (Persian Gulf, Iran). Eur J Exp Biol 2(3):611–615
Asha PS, Muthiah P (2007) Reproductive biology of the commercial sea cucumber Holothuria spinifera (Echinodermata: Holothuroidea) from Tuticorin, Tamil Nadu, India. Aquac Int 16:231–242
Ballesteros T, Tubío A, Rodríguez R, Hernández A, Costas D, Troncoso J (2021) Reproductive cycle of the sea cucumber Holothuria forskali (Holothuriida: Holothuriidae) in the Ría de Vigo (NW of Spain). Rev Biol Trop 69:101–117
Barbaglio A, Sugni M, Benedetto CD, Bonasoro F, Schnell S, Lavado R, Porte C, Carnevali DM (2007) Gametogenesis correlated with steroid levels during the gonadal cycle of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea). Comp Biochem Physiol A 147:466–474
Bardanis E, Batjakas I (2018) Reproductive cycle of Holothuria poli in Gera Gulf, Lesvos Island, Greece. Volos: 3rd International Congress on Applied Ichthyology and Aquatic Environment, 8–11 November 2018. https://hydromedit.gr/.
Benitez-Villalobos F, Avila-Poveda OH, Gutierrez-Mendez IS (2013) Reproductive biology of Holothuria fuscocinerea (Echinodermata: Holothuroidea) from Oaxaca, Mexico. Sex Early Dev Aquat Org 1:13–24
Bruckner AW, Johnson KA, Field JD (2003) Conservation strategies for sea cucumbers. Can a CITES Appendix II listing promote sustainable international trade? SPC Beche-de-Mer Inf Bull 18:24–33
Cameron JL, Fankboner PV (1986) Reproductive biology of the commercial sea cucumber Parastichopus californicus (Stimpson) (Echinodermata: Holothuroidea). I. Reproductive periodicity and spawning behavior. Can J Zool 64:168–175
Castro P, Huber ME (2000) marine biology. 3rd edition. McGraw-Hill Higher Education, New York
Chao SM, Chen CP, Alexander PS (1994) Reproduction and growth of Holothuria atra (Echinodermata: Holothuroidae) at two contrasting sites in southern Taiwan. Mar Biol 119:565–570
Chao SM, Chen CP, Alexander PS (1995) Reproductive cycles of tropical sea cucumbers (Echinodermata: Holothuroidea) in southern Taiwan. Mar Biol 122:289–295
Christophersen G, Bjørkevoll I, Bakke S, Kjerstad M (2020) Reproductive cycle of the red sea cucumber, Parastichopus tremulus (Gunnerus, 1767), from western Norway. Mar Biol Res 16:423–430
Conand C (1993) Reproductive biology of the holothurians from the major communities of the New Caledonian Lagoon. Mar Biol 116:439–450
Conand C, Uthicke S, Hoareau T (2002) Sexual and asexual reproduction of the holothurian Stichopus chloronotus (Echinodermata): a comparison between La R´eunion (Indian Ocean) and east Australia (Pacific Ocean). Invertebr Reprod Dev 41:235–242
Costelloe J (1985) The annual reproductive cycle of the holothurian Aslia lefevrei (Dendrochirota: Echinodermata). Mar Biol 88(2):155–165
Dabbagh A, Keshavarz M (2011) Three sea cucumbers from the Bandare Bostaneh Coast (Persian Gulf, Iran). World Appl Sci J 13(8):1933–1937
Despalatovic M, Grubelic I, Simunovic A, Antolic B, Ante Z (2004) Reproductive biology of the holothurian Holothuria tubulosa (Echinodermata) in the Adriatic Sea. J Mar Biol Assoc UK 84:409–414
Foglietta LM, Camejo MI, Gallardo L, Herrera FC (2004) A maturity index for holothurians exhibiting asynchronous development of gonad tubules. J Exp Mar Biol Ecol 303:19–30
Drumm DJ, Loneragan NR (2005) Reproductive biology of Holothuria leucospilota in the Cook Islands and the implications of traditional fishing of gonads on the population. N Z J Mar Freshw Res 39:0028–8330 /05/3901 – 0141
Emson RH, Mladenov PV (1987) Studies of the fissiparous Holothurian Holothuria parvula (Selenka) (Echinodermata: Holothuroidea). J Exp Mar Biol Ecol 111:195–211
Fischer A, Jacobson A, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. https://doi.org/10.1101/pdb.prot4986
Galley EA, Tyler PA, Smith CR, Clarke A (2008) Reproductive biology of two species of holothurians from the deep- sea order Elasipoda, on the Antarctic continental shelf. Deep-Sea Res II 55:2515−2526.
Gaudron SM, Kohler SA, Conand C (2008) Reproduction of the sea cucumber Holothuria leucospilota in the Western Indian Ocean: biological and ecological aspects. Invertebr Reprod Dev 51:19–31
Gauthier-Clerc S, Pellerin J, Amiard JC (2006) Estradiol-17β and testosterone concentrations in male and female Mya arenaria (Mollusca bivalvia) during the reproductive cycle. Gen Comp Endocrinol 145:133–139
Giese AC, Pearse JS (1974) Reproduction: general principles. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates, vol 1. Academic Press, New York, pp 1–49
Hamel JF, Himmelman JH, Dufresne L (1993) Gametogenesis and spawning of the sea cucumber Psolus fabricii (Duben and Koren). Biol Bull 66(1):47–57
Hamel JF, Mercier A (1996) Gonad morphology and gametogenesis of the sea cucumber Cucumaria frondosa SPC Beche-de-Mer Inf Bull 8:21–33
Han H, Zhang W, Yi YH, Liu BS, Pan MX, Wang XH (2010) A novel sulphated holostane glycoside from sea cucumber Holothuria leucospilota. Chem Biodivers 7(7):1764–1769
Harriott V (1982) Papers from the Echinoderm Conference. Sexual and asexual reproduction of Holothuria atra Jaeger at Heron Island Reef, Great Barrier Reef. Aust Mus Mem 16:53–66
Hines GA, Watts SA, Sower SA, Walker CW (1992) Sex steroid levels in the testes, ovaries and pyloric caeca during gametogenesis in the sea star Asterias vulgaris. Gen Comp Endocrinol 87:451–460
Hopper D, Hunter CL, Richmond RH (1998) Sexual reproduction of the tropical sea cucumber, Actinopyga mauritiana (Echinodermata: Holothuroidea) in Guam. Bull Mar Sci 63(1):1–9
Janer G, Sternberg RM, LeBlanc GA, Porte C (2005) Testosterone conjugating activities in invertebrates: are they targets for endocrine disruptors? Aquat Toxicol 71:273–282
Kazanidis G, Lolas A, Vafidis D (2014) Reproductive cycle of the traditionally exploited sea cucumber Holothuria tubulosa (Holothuroidea: Aspidochirotida) in Pagasitikos Gulf, western Aegean Sea, Greece. Turk J Zool 38:306–315
Lawrence JM, Lane JM (1982) The utilization of nutrients by post-metamorphic echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema AA, Rotterdam, pp 31–371
Li L, Li Q, Sun X, Kong L (2011) Effects of temperature and salinity on larval growth, survival, and development of the sea cucumber Apostichopus japonicas. North Am J Aquac 73(3):296–303
Machensen AK, Brey T, Sonnenholzner S (2011) The rate of Spondylus stocks (Bivalvia: Spondylidae) in Ecuador: is recovery likely? J Shellfish Res 30:115–121
Mashjoor S, Yousefzadi M (2019) Cytotoxic effects of three Persian Gulf species of holothurians. Iran J Vet Res 20(1):19–26
Mezali K, Soualili DL, Neghli L, Conand C (2014) Reproductive cycle of the seacucumber Holothuria (Platyperona) sanctori (Holothuroidea: Echinodermata) in the southwestern Mediterranean Sea: Interpopulation variability. Invertebr Reprod Dev 58:179–189
Muthiga NA (2006) The reproductive biology of a new species of a sea cucumber, Holothuria (Mertensiothuria) arenacava in a Kenyan marine protected area: the possible role of light and temperature on gametogenesis and spawning. Mar Biol 149:585–593
Navarro PG, García-Sanz S, Tuya F (2012) Reproductive biology of the sea cucumber Holothuria sanctori (Echinodermata: Holothuroidea). Sci Mar 76:741–752
Nguyen VN, Britaev TA (1993) Reproductive cycle of sea cucumber Holothuria leucospilota in Nha Trang Bay (southern Viet Nam). Russ J Mar Biol 18:185–191
Olive PJW (1995) Annual breeding cycles in marine invertebrates and environmental temperature: probing the proximate and ultimate causes of reproductive synchrony. J Therm Biol 20:79–90
Omidi S, Norinezhad M (2008) Investigation of salinity fluctuations in the waters of Bushehr province (Persian Gulf) In: Persian Gulf International Conference, Bushehr, Iran
Panola-Madrigal A, Calderón-Aguilera LE, Aguilar- Cruz CA, Reyes-Bonilla H, Herrero-Pérezrul MD (2017) Reproductive cycle of the sea cucumber (Isostichopus fuscus) and its relationship with oceanographic variables at its northern most distribution site. Rev Biol Trop 66(1):180–196
Pawson L (2007) Phylum Echinodermata. Zootaxa 1668:749–764
Purwati P, Luong-van JT (2003) Sexual reproduction in a fissiparous holothurian species, Holothuria leucospilota Clark 1920 (Echinodermata: Holothuroidea). SPC Beche-de-Mer Inf Bull 18:33–38
Rakaj A, Fianchini A, Boncagni P, Lovatelli A, Scardi M, Cataudella S (2018) Spawning and rearing of Holothuria tubulosa: A new candidate for aquaculture in the Mediterranean region. Aquac Res 49(1):557–568
Ramofafia C, Byrne M (2001) Assessment of the “tubule recruitment model” in three tropical aspidochirote holothurians. SPC Beche-de-Mer Inf Bull 15:13–16
Ramon M, Amor MJ, Galimany E (2022) Reproductive biology of the holothurian Parastichopus regalis in the Mediterranean Sea and its implications for fisheries management. Fish Res 247:106191
Rodríguez-Jaramillo C, Hurtado MA, Romero-Vivas E, Ramírez JL, Manzano M, Palacios E (2008) Gonadal development and histochemistry of the tropical oyster, Crassostrea corteziensis (Hertlein, 1951), during an annual reproductive cycle. J Shellfish Res 27(5):1129–1141
Sewell MA, Bergquist PR (1990) Variability in the reproductive cycle of Stichopus mollis (Echinodermata: Holothuroidea). Invertebr Reprod Dev 17(1):1–7
Shiell G, Uthicke S (2006) Reproduction of the commercial sea cucumber Holothuria whitmaei [Holothuroidea: Aspidochirotida] in the Indian and Pacific Ocean regions of Australia. Mar Biol 148(5):973–986
Shushizadeh MR, Mohammadi Pour P, Mahdieh M, Yegdaneh A (2019) Phytochemical analysis of Holothuria leucospilota, a sea cucumber from Persian Gulf. Res Pharm Sci 14(5):432–440
Slimane-Tamacha F, Soualili DL, Mezali K (2019) Reproductive biology of Holothuria (Roweothuria) poli (Holothuroidea: Echinodermata) from Oran Bay, Algeria. SPC Beche-de-mer Inf Bull 39:47–53
Smiley S (1988) The dynamics of oogenesis and annual ovarian cycle of Stichopus californicus (Echinodermata: Holothuroidea). Biol Bull 175:79–93
Smirnov AV, Gebruk AV, Galkin SV, Shank TM (2000) New species of holothurian (Echinodermata: Holothuroidea) from hydrothermal vent habitats. J Mar Biol Assoc UK 80:321–328
Thongbuakaew T, Suwansaard S, Chaiyamoon A, Cummins SF, Sobhon P (2021) Sex steroids and steroidogenesisrelated genes in the sea cucumber, Holothuria scabra and their potential role in gonad maturation. Sci Rep 11:2194
Toral-Granda MV, Martinez PC (2007) Reproductive biology and population structure of the sea cucumber Isostichopus fuscus (Ludwig, 1875) (Holothuroidea) in Caamaño, Galapagos Islands, Ecuador. Mar Biol 151:2091–2098
Tuwo A, Conand C (1992) Reproductive biology of the holothurian Holothuria forskali (Echinodermata). J Mar Biol Assoc UK 72:745–758
Vargas-Yanez M, García-Martíneza MC, Moya F, Balbín B, López-Jurado JL, Serra M, Zunino P, Pascual J, Salat J (2017) Updating temperature and salinity mean values and trends in the Western Mediterranean: The RADMED project. Prog Oceanogr 157:27–46
Wasson KM, Gower BA, Hines GA, Watts SA (2000) Levels of progesterone, testosterone and estradiol, and androstenedione metabolism in the gonads of Lytechinus variegatus (Echinodermata: Echinoidea). Comp Biochem Physiol C 126:153–165
Yang F, Zhou C, Tran NT, Sun Z, Wu J, Ge H, Lu Z, Zhong C, Zhu Z, Yang Q, Lin Q (2020) Comparison of the complete mitochondrial genome of Phyllophorus liuwutiensis (Echinodermata: Holothuroidea: Phyllophoridae) to that of other sea cucumbers. FEBS Open Biol 10(8):1587–1600
Acknowledgments
This work was practically supported by the Khorramshahr University of Marine Science and Technology.
Author information
Authors and Affiliations
Contributions
NS conceived the study and designed the methodology. FAF and AF collected the samples and data. All authors contributed in to analysis of the data. NS wrote the original draft of the manuscript. All authors contributed critically to subsequent drafts and approved the final version for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Ethical approval
The specimens were collected with a permission obtained from the Iran Fisheries Organization and Iranian Department of Environment.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faridani, F.A., Salamat, N., Doostshenas, B. et al. Timing of the reproductive cycle of Holothuria (Mertensiothuria) leucospilota (Brandt, 1835) from the Persian Gulf based on evaluation of gonad histomorphology and sex hormones. Aquat Sci 85, 99 (2023). https://doi.org/10.1007/s00027-023-00997-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-00997-1