Abstract
In the present work, the succession of consuming organisms and the dynamics of nutrients in flooded soils were studied in the laboratory. We collected soil samples in a sector of the Salado River middle basin from three topographies (upper, middle, lower) with different land uses (agricultural and mixed, involving both agriculture and cattle) that were flooded at dissimilar times. We measured the physical and chemical parameters of the water, nutrients, and chlorophyll a and realized an analysis of the organisms. The reactive fractions of phosphorus and nitrogen increased at the beginning of the flooding, mainly through the agricultural use of the upper topography. Chlorophyll a increased in the intermediate and final stages of the flooding in all treatments, with the maximum values being found in the lower agricultural topography. In the 195 taxa recorded, the species richness increased towards the intermediate stage, being slightly higher with agricultural land use. The upper topography exhibited the lowest richness, but the highest densities, due to protist contributions. Rotifers and microcrustaceans were recorded mainly in middle and lower topographies. Trophic-group diversity and complexity increased with decreasing topography and with increased flooding time. We conclude that the residence time of the water in the soil, and the adaptation strategies of the organisms, are key elements in edaphic development and composition during the stages of flooding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In floodable river systems, the hydrologic connectivity triggered by a flood pulse exerts a significant influence on the exchange of materials between the main river channel and the floodplain (Junk et al. 1989). The movement of water within the river system influences the transport of suspended sediments, the concentration of nutrients, and the exchange of organisms between the main channel and its floodplain, both during periods of flooding and isolation (Junk et al. 1989; Neiff 1990). Floodplains are low-slope areas exposed to periodic flooding due to rising river levels or associated lentic environments. The exchanges between these environments and the river are consequential, producing an enrichment of the lotic plankton and the changes that this circumstance represents (Amoros and Bornette 2002; Vázquez et al. 2007).
The Salado River basin is one of the most economically essential basins within the Buenos Aires province, being responsible for a third of the national agricultural production. The Salado is a typical lowland river with an average slope of 0.107 m/km and a length of 571 km, covering an area of ca. 150,000 km2. The regime of the river is quite variable; with the flow not exceeding 100 m3/s in dry periods and reaching up to 1500 m3/s in times of flooding, along with the consequent variations in conductivity and transport of dissolved and particulate materials (Gabellone et al. 2008; Solari et al. 2002).
The flooding of large areas is one of the characteristics of the basin (Gabellone et al. 2005). The floodplain includes many shallow lakes with different degrees of connectivity to the river (Bazzuri et al. 2018). The Salado and its associated wetlands constitute an ecosystem performing many functions that are a service—i.e., modulating floods, receiving and transporting effluent discharges from the towns located on the banks as well as from the agrochemicals that arrive through surface runoff. Certain types of these agrochemicals can be metabolized in the extensive wetlands, before flowing into the Samborombón Bay via the Río-de-La-Plata Estuary (Gabellone et al. 2013). During a flood, the water bodies of the plain—e.g., shallow lakes, paleochannels, and oxbow lakes—are connected with the course of the river, which receives different materials such as organic matter and minerals from the soil. These interactions and relationships demonstrate the significance of the interconnectivity of water bodies to the transport of inocula of organisms. In addition, the occurrence of floods has an effect on the quality of the soil, on the surface and underground water, and on the distribution of both the terrestrial and aquatic native flora and fauna (Gabellone et al. 2003).
Studies carried out in the selected area have verified a great similarity between the organisms of the zooplankton from flood water and those observed in the Salado River (Claps et al. 2009; Quaíni 2011). Many species arise from resistance structures that remain in the soil after a flooding or originate from edaphic communities that are maintained by soil moisture, ambient moisture, or sporadic rains (Cohen and Shurin 2003). Organisms exhibit different mechanisms of adaptation to desiccation—among which is dormancy, a state of reduced metabolic activity adopted by many organisms in reaction to environmentally stressful conditions. Dormancy implies a temporary suspension of cell division in the example of unicellular organisms such as bacteria (Van Vliet 2015), algae, and protists (Corliss and Esser 1974; Rengefors et al. 1998), or a suspension in cell-cycle progression, growth, development, and reproduction in multicellular organisms such as plants and metazoans (Keilin 1959; Alekseev et al. 2007; Hairston and Fox 2009). Various studies have demonstrated that diapause provides the advantage of promoting the colonization of new habitats, expanding the distribution area of the species, and facilitating the colonization of other environments (Alekseev et al. 2007).
An example of adaptation to unfavorable conditions is that of the bdelloid rotifers, which are a group that can secrete a cyst with a protective gel layer to survive desiccation (Williams 2006; Alekseev et al. 2007). Copepods can enter dormancy as eggs, juveniles, or adults, and as such survive desiccation at any life stage (Datry et al. 2012). Gooderham and Tsyrlin (2002) have recorded that cladoceran-crustacean ephippia from dry pond sediments have successfully hatched after 200 years and have been able to survive freezing and desiccation (Mellors 1975). Strachan et al. (2014), in six species of Cyprididae (ostracods), observed strategies to survive desiccation in an intermittent wetland. Typical soil species of nematodes can live in a film of soil water and also survive periodic dehydration in a state of cryptobiosis (Ruppert and Barnes 1995), while ciliate protists can develop resistance structures, some of which may remain viable for many years (Noland and Gojdics 1967). The presence of organisms in flooded soils is related to their ability to survive periods of drought, for example, through such resistance structures. In addition, the appearance of those structures at different times of flooding depends on the life cycle and the resources available to the species (Zaplara et al. 2018). The frequency of soil flooding is a determining condition for the type of communities that develop there. A soil with a higher frequency of flooding maintains a different community than another nearby site exposed to a different frequency and intensity of soil moisture (Quaíni 2011). Each of the different groups of zooplankton may prefer a certain topographical position for the laying of the inocula, resulting in the subsequent observation of a diverse succession of organisms in each instance (Quaíni 2011). Empirical knowledge of such successions enables the consideration that more complex ecosystems are more mature—that is, composed of many organisms, involving long food chains, and with well-defined, or more specialized, interspecific relationships (Margalef 1963). Regarding the previously stated information, the objective of the work reported here was to study, in the laboratory, through the use of microcosms, the effect of different soil-flooding times on the succession of consuming organisms and the effect on the dynamics of environmental variables through comparing different topographies and land uses (involving agriculture alone and agriculture plus livestock). We examined this under the hypothesis that the time of soil flooding, in relation to the different topographic positions, determines the availability of nutrients, the emergence of organisms, their development, the succession of communities, the observation of the development of organisms with longer life cycles and trophic webs, and more complex successions in those soils with longer exposure to flood water.
According to what was previously stated, the objective of this work was to study the effect of different soil-flooding times on the succession of consuming organisms and on the dynamics of environmental variables in laboratory microcosms, while comparing different topographies and land uses, with the hypothesis that the flooding time of the soil in different topographic positions determines the availability of nutrients, the emergence and growth of organisms, and the succession of communities, while noticing a development of organisms with longer life cycles, more complex trophic networks, and successions in those soils with a longer flood time.
Materials and methods
Study area
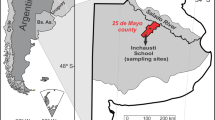
The study area (Fig. 1) is located in the middle basin of the Salado River, located in the ecoregion of the Argentine pampas plain (Viglizzo et al. 2006) and in the neotropical phytogeographic region of the Chaco Domain (La Pampa province; Cabrera 1971). The site selected for the collection of the samples was in the agrotechnical school M. C. and M. L. Inchausti, belonging to the National University of La Plata, located near the town of Valdés, (County of 25 de Mayo, Buenos-Aires province). The particular characteristics of this locale, such as climate, rainfall, and slope, among others, are detailed in Solari et al. (2018).
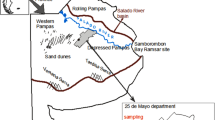
The plots selected for the extraction of the samples had different previous uses (Fig. 2): agricultural use (-a), and mixed use involving agriculture and livestock (-m). Likewise, the plots are located at different topographic levels: upper (U), middle (M), and lower (L). The recurrence of flooding in these areas is different, the area of the upper topography is the one that undergoes a lower recurrence of flooding and, because of the characteristics of that topography’s sandy soil, less water is retained. The intermediate zone, denoted middle, has a low recurrence of flooding, but the water remains longer since the soils contain a higher concentration of fine particles such as silt and clay. The lower topography, with frequent flooding and greater retention of water, is an inappropriate site for land use, and therefore remains without such use.
Schema illustrating the location of sampling points in each topography and land use. The difference in height totaling 1.4 m among the topographies is indicated to the right of the schema, whereas the distances between the sampling points, with respect to upper, are 22 m (M) and 40 m (L) and are denoted in each topography. The distances between the topographies, with respect to the hill and the extraction points of the soil samples, are specified for each land use: agricultural use (black pentagons) and mixed use (grey circles)
Extraction and treatment of samples: methodology applied in the field and in the laboratory
The extraction of the soil samples was carried out with metallic corers with an area of 28.3 cm2 and a soil height profile of 5 cm. The soil samples were placed in plastic bottles, covered, and transferred to the laboratory. For the microcosms, samples of soil plus water without chlorine were placed in glass jars with a volume of 0.75 L. In this water, the physical and chemical parameters had been previously measured, with those characteristics constituting the initial condition. The flooding was simulated by raising the water level vertically with ascending Pasteur pipettes. Subsequently, the units were covered and taken to breeding chambers programmed for temperature and photoperiod. The post-flooding study was carried out in two phases, since the capacity of the incubators was limited for the total number of samples: phase I with intensive daily sampling for 5 days, and phase II with weekly sampling, every 14 days and every 20 days. The duration of the flood was different for each topography, with the upper being flooded for 26 days, the middle for 54 days, and the lower for 96 days. Each sampling time contained three replicates per treatment, along with three control units without soil (Table 1).
The sampling sequence in each experimental unit at each time was as follows (Fig. 3): (1) the experimental units were removed from the incubators, (2) environmental variables in the water column were measured [pH, conductivity (µS/cm), temperature (°C), and dissolved oxygen (% saturation)] with a HORIBA U10 multiple sensor, the oxidation–reduction potential was measured with a Dr. Lange ECM multi-plus multiparameter sensor, and turbidity (in nephelometric turbidity units, NTUs) was measured with a HACH 2100p turbidimeter, (3) sampling for the chemical and biological analysis of each experimental unit was performed—extractions were made for the measurements as follows—(a) 100 mL of water was removed for analysis of ammonium, soluble reactive phosphorus, total phosphorus, nitrates and nitrites, and chlorophyll a (APHA 2012) and (b) 10 mL of water was removed with a syringe and hose system for the counting of protozoa by the Utermöhl method and the protozoa fixed with 1% (v/v) Lugol’s acetic acid, and (4) the remaining volume was filtered through a plankton net with a 35-µm pore mesh and fixed with 4% (v/v) aqueous formaldehyde for counting metazoans in Sedgewick-Rafter chambers. Subsequently, the experimental units were discarded.
Schema illustrating the sequence of protocols performed in the laboratory sampling. This diagramatic representation summarizes steps 1–4 outlined in the Materials and Methods. PZ Protozoa, MZ Metazoa, C Chemistry, Cf Filtered Chemistry, Cf. The red X indicates that the experimental units were not returned to the incubators, but were discarded
We estimated the density of the organisms (ind/cm2) in each of the groups present, obtained the accumulated specific richness via a presence-absence index, calculated the specific diversity by the Shannon index (H´), and determined the percentage of the predominant trophic groups in each stage of the flooding, as well as at each sampling time. We identified the organisms according to the following authors: Bick 1972 (ciliates), Foissner et al. 1999 (ciliates), Siemensma 2017 (amoebas), Koste and Shiel 1987 (rotifers), Segers 1995 (rotifers), Reid 1985 (copepods), Smirnov 1996 (cladocerans), Heyns 1971 (nematodes), Brinkhurst and Marchese 1992 (oligochaetes), and Claps and Rossi 1984 (tardigrades). Different sources were used to study the trophic groups: Baltañaz and Joanes (2015), Bick (1972), Coûteaux and Pussard (1983), Chardez (1967), Ferrando (2015), Gilbert et al. (2000, 2003), Ibáñez (2011), Karanovic (2012), Maly and Maly (1974), Obertegger et al. (2011), Oksana et al. (2006; Appendix 1), Pave and Marchese (2005), Ruppert and Barnes (1995), Torres (1996), Williamson and Reid (2001), and Williams (2006).
Statistical analysis
To analyze the interaction of the environmental variables, the nutrients, and chlorophyll a, with respect to topographic positions and land uses in each stage of the experiment, a factorial analysis of variance (ANOVA) was performed. To analyze the biological samples among treatments, the data were normalized and transformed by the square root, and a Bray–Curtis similarity matrix was constructed. An adjusted non-metric multidimensional (MDS) analysis representing samples as points in a two-dimensional space was performed. The relative distances that separate the points are in the same rank order as the relative dissimilarities of the samples (calculated based on the Bray–Curtis coefficients). The interpretation of the MDS is simple: the points that are closest represent samples that are more similar in species composition, and the points that are more distant represent samples of different associations. To carry out these analyses, the Primer V.5 program (Clarke and Gorley 2001) was used.
Results
Physical and chemical analysis of the water
The pH was acidic to slightly alkaline and did not undergo significant changes throughout the flooding, thus maintaining average values between 6.6 and 7. The statistical analysis, however, revealed significant differences between the topographies studied (ANOVA, p = 0.013). The temperature varied according to the ramp-mode programming of the brood chambers, with a maximum value of 20.8 °C in the first stage of the flooding and a minimum value of 13.8 °C in the final stage. The conductivity was maximum in the initial stage at average values of 600 µS/cm, and then decreased in the advanced stages of the flooding down to an average value of 410 µS/cm. This variable displayed significant differences within the corresponding values for topography and land use (ANOVA, p = 0.000012), with the minimum values in the L agriculture samples (L-a). The turbidity increased progressively with the stages of flooding and evidenced significant differences with respect to the topography and land uses (ANOVA, p = 0.00011–0.044, respectively). The minimum values occurred in the M mixed land-use samples (M-m) at 2.69 NTUs, with the maxima of 60–95 NTUs measured in the intermediate and final stages in L-a. The dynamics of the dissolved oxygen during the time of flooding involved a decrease from the pre-flooding condition, from 76.0% saturation at the beginning of the flooding down to 54.4%. In the intermediate stage, the oxygen in the water increased back to 76.3%, but later decreased again to 57.9% as a result of biologic activities (i.e., photosynthesis and respiration). This variable also exhibited significant differences between topographies (ANOVA, p = 0.000003), with a lower percent saturation in the U topography (45.7%) than in the M or the L, which values, on the average, were greater than 65%. The oxidation–reduction potential evidenced a pattern similar to that of oxygen in the initial stage and then increased during the later stages (Fig. 4).
Variation in the physical and chemical parameters in the combination of topographies and land uses during the flooding stages. In the figure, the pH (a), the temperature in ºC (b), the conductivity in µS/cm (c), the turbidity in NTUs (d), the dissolved oxygen concentration (DO) as the percent of saturation (e), and the oxidation–reduction potential (ORP) in mV (f) is plotted on the ordinate at each of the post flooding stages—initial (0–26 days), intermediate (26–54 days), or final (54–96 days)—indicated on the abscissa. The ordinate scales were adjusted to the range of values for each parameter in order to more clearly represent the differences between the stages. As indicated in the square to the lower right of the figure, in each box plot, the small black squares represent the means, the larger gray rectangles represent the means ± the standard errors (SEs), the brackets represent the means ± 2SEs, and the circles represent the outliers
Nutrients and chlorophyll a
The phosphorus fractions [soluble reactive phosphorus (SRP) and total phosphorus (TP)] evidenced a similar dynamic over time during the flooding. The SRP increased in concentration in the water during the initial stage as a result of a release of the fraction by the soil, exhibiting significant differences among the topography and uses (ANOVA, p = 0.054). The upper topography had the highest average concentrations, mainly with agricultural use (548 µg/l), while the middle and lower topographies manifested concentrations lower than those of the upper topography, but higher in the U-a unit than in the U-m. As the days of flooding progressed, the SRP concentration decreased in conjunction with the increase in algal biomass, as estimated by the concentration of chlorophyll a. The TP followed a pattern similar to that of the SRP. The nitrogen fractions (nitrates + nitrites, and ammonium) evidenced significant differences within the topographies (ANOVA, p = 0.0001) and the land uses (ANOVA, p = 0.046). The initial stage contained the highest concentrations of both fractions, with ammonium being higher in topography U-a, and nitrates plus nitrites higher in L-m. As the stages of the flooding progressed, both fractions decreased. Chlorophyll a—increased gradually with the flooding stages to become maximum at the end—manifested significant differences with respect to the various topographies (ANOVA, p = 0.0055), having a minimum value in M-m at 9.70 mg/m3 in the initial stage, and reaching a maximum in the final stage in L-a at 84.8 mg/m3 (Fig. 5).
Variation of nutrients during the different stages of flooding in the combination of topographies and land uses during the flooding stages. In the figure the concentrations of soluble reactive phosphorus (SRP) in µg/L (a), total phosphorus (TP) in µg/L (b), chlorophyll a (Cl a) in mg/m3 (c), nitrates plus nitrites (N + N) in µg/L (d), and ammonium (NH4) in µg/L (e) are plotted on the ordinate at each of the post flooding stages—initial (0–26 days), intermediate (26–54 days), or final (54–96 days)—indicated on the abscissa. In each stage, the values of both the uses and the sampling days were averaged. As indicated in the square to the lower right of the figure, in each box plot, the small black squares represent the means, the larger gray rectangles represent the means ± the SEs, the brackets represent the means ± 2SEs, and the circles represent the outliers
Microconsumers: specific richness, diversity, density, and trophic groups
We recorded 195 taxa distributed among Ciliophora (43), Amebozoa (22), Cercozoa (6) and naked amoebas (2), Heliozoa (1), Rotifera (68), Nematoda (11), Annelida Oligochaeta (3), Copepoda (6), Cladocera (17), and Ostracoda (4). The final 12 taxa were detected in other groups such as Tardigrada, Gastrotricha, Hexapoda, Chelicerata, Onychophora, Collembola, and Mollusca, with one or two species each. The average specific richness was slightly higher in the agricultural use than in the mixed use and increased with the time of flooding in the three elevation topographies, with maximum values in the intermediate stage. The upper topography possessed a lower richness than either the middle or the lower topographies, with a predominance of protists (ciliates and amoebas), and contained a maximum richness of 13 taxa at time T6 in topography U-m, and 11 taxa at times T6 and T8 in U-a. In the M and L topographies, the rotifers were more abundant, mainly being observed in M-a, with a maximum of 15 taxa in the intermediate stage of the flooding (T6 and T7). In these topographies, certain species of adult copepods appeared in the early stages of the flooding, but, as the flooding time progressed, the crustaceans increased in richness with adult copepods, cladocerans, and ostracods registering averages of 7 or 8 taxa, and attaining a maximum of 10 in M-a (Fig. 6).
Average specific richness of each group of organisms at different times of flooding. In the figure, the groups in the left column of panels represent agricultural land use, and the right column mixed (agriculture and livestock) land use. The upper, middle, and lower rows of panels denote the respective upper (U), middle (M), and lower (L) topographical elevations. In each panel, the species richness is plotted on the ordinate for each of the times after flooding in days indicated on the abscissa. Key to the bar textures: middle gray—Ciliophora, black-white descending diagonal hatching—Amoebozoa, white—Rotifera, gray—Crustacea, black-and-white horizontal hatching—nematodes, black—other miscellaneous taxa, i.e., those with a scarce specific richness that appeared in certain samplings such as annelids, gastrotrichs, hexapods, tardigrades, chelicerates, molluscs, and onychophorans. T denotes the time after flooding in days, as summarized in the box to the lower right of the figure
Microconsumer density
In topography U, the microconsumer density was higher than in M or L, with increases in the advanced stages of the flood. The contributions to the density in both land uses were mainly by ciliates such as Cyclidium sp. and Frontonia sp. In topography U-a, the amoebas increased in density over time, mainly owing to contributions from Nebela sp., while the rotifers became evident in the intermediate stage, with contributions mainly from the genus Lecane. In topography U-m, the contributing taxa were mainly certain amoebas, such as Euglypha tuberculata, Trinema enchelys, and Difflugia acuminata, along with rotifers characterized by Lecane clara. In both land uses the gastrotrichs, chelicerates, onychophorans, tardigrades, and nematodes were recorded in low densities at certain times during the flooding (Fig. 7, upper panels a).
Average density of the groups of organisms in the flooding stages. In the figure, the groups in the left column of panels represent agricultural land use, and the right column represents mixed (agriculture and livestock) land use. The upper, middle, and lower rows of panels denote the respective upper, middle, and lower topographical elevations. In each panel, the microconsumer density in individuals per cm2 is plotted on the ordinate for each of the stages—initial (26 days), intermediate (54 days), and final (96 days)—after flooding indicated on the abscissa. Key to the bar textures: middle gray—Ciliophora, black-and-white descending hatching—Amoebozoa, light- and dark-gray vertical hatching—Rotifera, white—other groups, light gray—nematodes and annelids, black—Crustacea. The other groups include taxa with minimal contributions to density (e.g., chelicerates, hexapods, gastrotrichs, tardigrades, springtails, onychophorans, molluscs)
In the M and L topographies (Fig. 7, b and c), the density of the ciliates decreased with respect to the topography of the upper topography, while the amoebas became more prevalent. In the M-a topography, the ciliates contributed with Euplotes sp. and a species of the Oxytrichidae family. The amoebas contributed mainly through increases in Netzelia gramen, Centropyxis aculeata, and Difflugia pyriformis. Rotifers were present throughout the experiment, though in low densities, through contributions by Trichocerca cylindrica. Copepods were recorded from the beginning, mainly as a result of nauplii larvae and adults of Metacyclops mendocinus. Cladocerans appeared from the intermediate to the final stage, featuring Coronatella poppei, while the ostracods were represented by contributions from the genus Cypridopsis. Other groups were recorded at certain times in low densities, such as nematodes, annelids, tardigrades, springtails, gastrotrichs, gastropods, and chelicerates (Fig. 7b). In the M-m topography, the ciliates that contributed the most to the density were Vorticella sp. and Cyclidium sp. Amoebas were present in all the stages of the experiment, with contributions from Cyclopyxis sp. and Difflugia bidens. Furthermore, rotifers were recorded at low densities with contributions from Philodina sp. and Lecane lunaris. Copepods, featuring juveniles and adults of Metacyclops mendocinus, and cladocerans became evident in the intermediate and final stages through an input from a species of the Chydoridae family. Adult ostracods were present at the end of the flooding, represented by Cypridopsis sp. and Chlamydotheca incisa. Other groups such as nematodes, gastrotrichs, molluscs, and tardigrades were recorded at low densities (Fig. 7b).
In the L-a topography, after an initial drop, the ciliates increased by the final stage, with contributions from Colpoda inflata and a species of Oxytrichidae (Fig. 7c). The amoebas increased in density over time, mainly through input by Euglypha acanthophora. The rotifers contributed with Lecane elsa, while the copepods were recorded throughout the experiment—at the beginning with juveniles and toward the end with adults of Notodiaptomus incompositus. Cladocerans and ostracods increased in abundance in the final stage, through the presence of Coronatella poppei, Daphnia spinulata, and Chlamydotheca incisa. Nematodes were recorded at low densities, featuring Dorylaimus sp. along with a species from the Tylenchidae family, whereas other groups remained at minimum densities at certain times—such as oligochaetes, gastrotrichs, hexapods, springtails, and molluscs (Fig. 7c). In the L-m topography, the ciliates were present with Halteria sp. and Euplotes sp., while the amoebas Euglypha tuberculata, Cyclopyxis sp., and Pseudodifflugia fascicularis were also recorded. The rotifers exhibited low densities, with contributions from the genus Trichocerca. The copepods were detected throughout the experiment, at the beginning with nauplii larvae and adults of Metacyclops mendocinus and Microcyclops anceps. In the final stage, adults of Notodiaptomus incompositus became present. In that stage, the cladocerans reached a maximum density, featuring Corontella poppei, while the ostracods—represented by Cypridopsis sp., Chlamydotheca incisa, Hetorocypris sp., and juveniles—became more evident in the intermediate and final stages. Nematodes and other groups such as annelids, gastrotrichs, and chelicerates were recorded in low densities at certain times during the flooding (Fig. 7c).
Statistical results for the biological analysis of samples (MDS)
The analysis showed a well marked separation between the high topography (upper) with respect to the lowest (middle and lower). The initial and intermediate stages were grouped by the presence of groups such as protists (ciliates and amoebas), rotifers, nematodes, annelids, and gastrotrichos, mainly, and certain species of copepods, with strategies of dormant structures in adults, such as Metacyclops mendocinus and Acanthocyclops robustus. In the low areas, the presence of certain groups such as crustaceans (copepods, cladocerans, and ostracods) could be responsible for the spatial differentiation and in the time of flooding, due to the fact that these specimens, in the adult stage, became evident in the intermediate and final stages (Fig. 8).
MDS statistical analysis. Spatial and temporal distribution of treatments. The arrow indicates the advance of the succession in time and the numbers in each geometric figure represent the flood times. The green oval highlights the differentiation and grouping of the topographic position of the hill and the ovals with dotted lines highlight the different stages of the flood. References, time (days): T1–T5 1–5 days, T6 12 days, T7 19 days, T8 26 days, T9 40 days, T10 54 days, T11 75 days, T12 96 days
Specific diversity and trophic groups in the topographies at each stage of the flooding
The specific diversity and complexity of the trophic groups increased as a function of the topographies and the flooding time. The lowest diversity values were observed in the U topographies, with values between 0.321 and 1.487, and with no differences among the land uses. The highest diversity was in the M-m topography during the intermediate stage of the flooding at a value of 2.14. In the L topography, the minimum diversities occurred during the initial stage, with an average value of 1.21, whereas the maximum appeared in the intermediate stage of the flooding for the mixed land use (2.09) and in the final stage for the agricultural land use (1.96).
The trophic groups differed within the various topographies and during the flooding stages (Fig. 8). In the upper topography, small bacterivores and omnivores, such as ciliates and amoebas, predominated and were recorded throughout the experiment with an abundance of Euplotes sp., Cyclidium sp., Euglypha tuberculata, and two species of the genus Trinema. Small herbivores such as bdelloid rotifers and Lecane sp. were also recorded. In certain sampling events, other groups of omnivores were recorded with lower densities, such as gastrotrichs, nematodes, and tardigrades, as well as ciliates, rotifers, chelicerates, and predatory nematodes. In the middle and lower topographies, the density of bacterivores was low throughout the experiment, whereas small omnivores increased, namely amoebas of the genera Cyclopyxis, Euglypha, and Trinema, along with the ciliates Halteria sp., Euplotes sp., Frontonia sp., and Vorticella sp. Other small herbivores, such as bdelloid rotifers, Lecane sp., Lepadella patella, and Euchlanis sp., were also recorded. Larger herbivores (e.g., nauplii larvae and adult copepods) such as the calanoid Notodiaptomus incompositus, along with cladocerans as exemplified by Daphnia spinulata, Daphnia obtusa, Ceriodaphnia dubia, Alona yara, and Coronatella poppei, plus a species of detritivorous Chydoridae, were detected within the intermediate and final stages of the flooding. Small predators such as rotifers of the genera Cephalodella and Trichocerca were also present in different samplings, as well as larger predators represented by the copepods Metacyclops mendocinus and Acanthocyclops robustus, plus nematodes of the Mononchydae family. Other groups of omnivores, namely nematodes (Dorylaimus sp.), gastrotrichs (Chaetonotus sp.), and copepods (Paracyclops fimbriatus), annelids (Naididae and Aelosoma sp.), tardigrades, and molluscs, were present in low densities in certain samplings. Towards the end of the flooding, the detritivorous pathway increased with adult ostracods of the genera Cypridopsis, Heterocypris, and the species Chlamydotheca incisa (Fig. 9).
Discussion
The first effect of a flooding is the displacement of air by the water, since the dissolved oxygen of the water decreases in the first stage from the initial levels present in the water. This occurrence was much more marked in the U topography. This diminution could be due to a rapid consumption of oxygen by aerobic organisms, chemical oxidation, and/or diffusion between the water and the soil (Tusneem and Patrick 1971; Ponnamperuma 1972, 1985; De Datta 1981; Roger 1996). In the intermediate period, and at the beginning of the final stage of the flooding, the percentage saturation of dissolved oxygen increased in agreement with an elevation in the concentration of chlorophyll a and the corresponding photosynthetic activity of the algae. In contrast, toward the end of the experiment, the dissolved oxygen manifested a decrease attributable to an increase in the respiration of larger organisms such as microcrustaceans. The values of oxidation–reduction potential recorded during the experiment, in all the experimental units, were lower than 326 mV. According to different authors (Ponnamperuma 1972; Reddy and DeLaune 2008), the range of the oxidation–reduction potential of a flooded soil that remains submerged for several weeks is between 0 and 200 mV, and can reach negative values depending on the characteristics of the soil and the time of the flooding. The pH of the water that floods soil depends on the pHs of the source water and soil, the activity of the algae, the nature and amount of oxidized compounds, the portion and kind of organic matter, and the temperature (Ponnamperuma 1972; Morales et al. 2001). In the first days of flooding, the pH increased slightly and then decreased somewhat, probably related to oxidation–reduction reactions of the reduced environments rather than related to algal activity, which was scarce at this initial stage since acidity dominates the system under reduced conditions (Quaíni (2011)). In the intermediate and final stages, the increases in pH were attributed to the elevation in photosynthetic activity. Changes in conductivity are related to the reactions that produce the ions and those that inactivate or replace them (Ponnamperuma 1972). The increases in turbidity, for its part, were attributable to increases in bacterial activity and to the chemical characteristics of the floodwater, which depend mainly on the source of the water flooding as well, but also depend on the properties of the soil (Watanabe and Roger 1985) (Fig. 10).
An increase in chlorophyll a was observed in all the systems studied, mainly in the intermediate and final stages of the flooding, and occurred simultaneously with elevations in the concentrations of phosphorus and nitrogen. In previous studies of flooded soils, Quaíni (2011) observed that, in sites with a longer flooding time than 5 days, the environment was more stable than in those flooded for only 5 days. This stability at longer times enabled the community to reach a higher level of complexity, with an increased algal development that was closely related to the SRP and nitrate concentrations in the water. In addition, in all the sites of the present study, an increase in the SRP was observed in the initial stage of the flooding. An increase in the concentration of available phosphorus in the water is characteristic of such flooded environments, where the water displaces the air from the soil pores and anoxia is generated. Consequently, other elements take the place of oxygen as electron acceptors, such as Fe and Mn. These electrons, in the oxidized state, form compounds with phosphorus that are then reduced and released when flooding occurs, and the redox potential decreases (De Datta 1981; Hossner and Baker 1988; Sharpley et al. 1995; Pant and Reddy 2003; Chacón et al. 2005). In the U topography, the concentrations of SRP and TP exceeded the concentration values of the M and L topographies of both land uses, although the latter two topographies displayed higher values in the agricultural use. The fertilization in these sites, and the composition of the soil particles, were conditions that could influence the concentrations of SRP and TP in each land-use site, as well as the sandy texture exerting an influence on the phosphorus concentrations— sand is inert and does not readily adsorb the complexes associated with phosphorus, thus those complexes were released more easily. The ability of a soil to provide phosphorus to crops depends on the rate and amount of desorption (Silva Rossi 2011). The incorporation of SRP from the soil into the water favors an increase in the algal biomass, along with the successions related to the grazing and herbivory systems. In the first days of flooding, a slight increase in the concentration of chlorophyll a was observed, as well as higher values of TP in almost all the treatments. In this instance, the relationship among SRP and TP indicated that a great part of the phosphorus in the system was in a labile form, available to algae, and another part was incorporated into organisms as intrabiotic phosphorus.
The biologic interactions in the water column, such as photosynthesis by the producers, generate oxygen in the environment that enhances the mineralization of the accumulated organic matter and likewise releases phosphorus-containing components. Microorganisms play an essential role in phosphorus retention in these flooded environments, resulting in a high primary productivity; once phosphorus enters the system of the detritus-microalgal community, the probability that the phosphorus will be retained and recycled in the water is high (Reddy and DeLaune 2008).
The ammonium concentration was different between the topographies U, M, and L and during the stages of flooding. The variation in the amount of this ion in the soil may be due to different processes. The use of ammonium compounds (e.g., ammonium phosphate) in fertilization, followed by the eventual decomposition of the resulting ammonium-containing organic matter in the soil, releases the ion, thus increasing its concentration in flood water (De Datta 1981; Reddy & DeLaune 2008). Likewise, ammonification reactions increase upon the conversion of organic nitrogen into ammonium (Conzonno 2009). Thereafter, the ammonium can be assimilated by algae for the synthesis of nitrogenous organic matter, or can be converted to nitrite by bacterial oxidation (Conzonno 2009). In the first stage of the flooding in both land uses of topographies U and M, and in the U-a, the average concentrations of nitrate were higher than in the initial condition of the water, but were higher still in the lower topographies. The increases in nitrates in the first stage of the flooding could be due to nitrification. In the intermediate and final stages, the concentrations of nitrates decreased as a result of consumption by algae and a denitrification in the anaerobic soils that transformed nitrates into atmospheric nitrogen (Simon 2002).
Effects of flooding on the succession of consuming organisms
We observed a certain relationship among the number of taxa, the topographic position, and the time of flooding. The values we recorded of the abundance and specific diversity of taxa did not indicate major differences between the land uses, whereas the richness of species was slightly higher in the U-a topography than in the U-m. In all the sites, we observed an increase in specific richness during the intermediate stage of the flooding, in which the value then decreased slightly towards the end of the extended flood. These data agree with studies of taxa succession in flooded soils, likewise carried out in the same area (Quaíni 2011; Solari et al. 2018). In soils with longer flooding times, such as those of M and L, an increase in the number of species and specific diversity occurred. The protists—mainly ciliates and amoebae—were the dominant group in the U topography, with the former manifesting high densities that increased with the flooding time. These organisms have wide ranges of tolerance to environmental variables and are highly sensitive to moisture levels, while certain species have the ability to produce resistant states under drought conditions (Foissner 1987).
Rotifers vastly outnumbered the rest of the groups in the topographies M and L. Many rotifer species are adapted to living in diverse types of habitats (Fontaneto et al. 2011). Ciliates and rotifers are ubiquitous because of the ability of many of them to produce forms of resistance that enable them to withstand and survive periods of developmentally unfavorable environmental conditions, such as the complete absence of water (Cohen and Shurin 2003; Foissner 1987; Brock et al. 2003). Crustaceans,e.g., adult copepods with different feeding habits, filter-feeding cladocerans, and detritivorous ostracods, were present in topographies M and L, mainly in the intermediate and final stages of the flooding. During the first days of the flooding, however, we recorded certain species of adult cyclopoid copepods. Consistent with this observation, Dobrzykowski and Wyngaard (1993) and Naess and Nilssen (1991) had described that diapause could occur from the copepodite stage II to the adult, as well as in fertilized females. Copepods can develop from egg to adult in a few days or weeks depending on the species and environmental circumstances, mainly temperature and the food source (Wyngaard and Chinnappa 1982). Metacyclops mendocinus is a species that was recorded in the first stage of the flooding. Studies have documented that both ovigerous males and females of M. mendocinus emerged from a temporary environment 48 h after a flooding (Echaniz and Vignatti 2010). The most effective strategy in temporary aquatic environments consists of producing resistant eggs that can remain in a dormant state for months or even years (Baltanás and Joanes 2015). In these experiments, what was notable was that the emergence and development of crustaceans coincided with an increase in chlorophyll a, and furthermore, that temporal variations in algal quality and quantity are essential for the formation of community structure and species interactions (Kirk 2002).
Relevance of the inoculum bank living in the soil
The presence of resistance structures in the soil contributes to the resilience of the ecosystem in a flood after a drought, since these structures enable species to develop quickly once the dormant period ends (Brock et al. 2003). The conditions necessary for interrupting diapause and inducing hatching depend mostly on the species and can often vary among populations of the same species (Wyngaard 1988). In shallow and short-lived temporary environments, changes in temperature, osmotic pressure, and oxygen concentration during the flooding cycle are the indicators most relevant to growth and reproduction (Brendonck 1996). We can infer that, in a comparison the three sites studied, the initial soil inoculum bank before the flooding was different in U from the corresponding bank in the other sites. This difference was evident because after exposure to similar times of flooding (up to day 26 of the experiment), the emergence and final nature of the communities was quite dissimilar, with a simpler community developing in topography L that involved a less complex food web, characterized by the absence of microcrustaceans. One explanation may be the agriculture intensive carried out in the U zone since, according to Brendonck and Meester (2003), the degree of sediment disturbance has a serious impact on the vertical structure of the soil’s egg bank. Another possibility may be the low frequency of flooding in these higher areas, thus preventing the reversion of the dormancy to the active state in the soil through either senescence or loss of viability. In diametric contrast, zone L remained moist longer, even when not flooded, owing to its proximity to the water table and the morphology of the basin. The edaphic community that remained with such soil moisture most likely gave rise to the aquatic community during the flood, since in the Lower the community was maintained over time (Quaíni 2011).
Conclusions
The residence time of flood water exerts a key influence on the emergence and development of consuming organisms. In the sites exposed to a few days of flooding, as in U, the species richness and specific diversity were low, whereas in the more prolonged flooding of L, the two indices were considerably higher. In the systems with a short flooding, mainly opportunistic organisms characterized by simple and short life cycles were recorded, with wide ranges of tolerance to environmental changes and which more easily colonized the most unstable environments. In the prolonged flooding, although many of the taxa already registered were still present, other organisms with long life cycles appeared at intermediate stages of development, thus demonstrating the relevance of those more stable scenarios.
The bank of propagules present in each site was different: topographically contrasting sites such as U and L evidenced a different succession of organisms after the same time of exposure to water. This difference indicates a relevance of both the soil flooding time and the nature and degree of viability of the the inocula previously present. Adaptations and strategies to combat desiccation are very diverse among organisms, enabling those with a long life cycle that would otherwise be present in the most advanced stages of the succession—such as certain adult cyclopoid copepods—to be instead recorded in the first days of the flooding. These species have the ability to remain dormant as adults and as ovigerous females, and then to reactivate in a short time once water again becomes available.
In conclusion, the characteristic trophic groups at the beginning of the succession were mainly omnivorous, being both bacterivores and small herbivores. As time progressed, organisms with higher biomass such as copepods and cladocerans developed, with feeding habits such as predation and herbivory. Toward the end of the succession, detritivore feeding increased. Many of the taxa recorded in the experiment were common to the organisms previously identified in the Salado River, thus demonstrating the relevance of connectivity during flooding and the active and passive dispersal agencies responsible for the movements of organisms between an aquatic environment and their habitats within the flood plain. The potential to exchange materials, nutrients, and organisms promotes the renewal, continuity, and prosperity of biologic communities over time in the aquatic environments of the basin, as well as in the main channel of the Salado River.
In a regional flooding with interconnectivity to associated water bodies, the soils in short-term episodes can provide nutrients, mainly in an inorganic form (e.g., phosphorus and nitrates),which may have implications for the trophic level of water bodies. In soils with prolonged flooding, however, these flooded terrestrial environments will contribute a complex and diverse variety of organisms to the water bodies, which can then generate changes in the assemblages of the organisms present in those water bodies (Fig. 9). The incorporation of those organisms can be used as a food source for other aquatic invertebrates and for fish, as well as other aquatic fauna, since most fish feed on plankton at some stage in their ontogeny (Lazzaro 1987).
Data availability
Data are available from the authors upon reasonable request.
References
Alekseev VR, De Stasio BT, Gilbert JJ (eds) (2007). Springer Science & Business Media, Dordrecht
Amoros C, Bornette G (2002) Connectivity and biocomplexity in waterbodies of riverine foodplains. Freshw Biol 47:761–776
APHA (2012) Standard Methods for the examination of waters and wastewaters, 22nd edn. APHA/AWWA/WPCF, Washington DC
Baltanás A and Joanes FM (2015). Class ostracoda, order podocopida. Magazine Ide@ SEA 74: 1–10.
Bazzuri ME, Gabellone NA, Solari LC (2018) The effects of hydraulic works and wetlands function in the Salado-River basin (Buenos Aires, Argentina). Environ Monit Assess 190:99
Bick H (1972) Ciliated protozoa: an illustrated guide to the species used as biological indicators in freshwater biology. World Health Organization, Ginebra, Suiza
Brendonck L (1996) Diapause, quiescence, hatching requirements: what we can learn from large freshwater branchiopods (Crustacea: Branchiopoda: Anostraca, Notostraca, Conchostraca). Hydrobiologia 320:85–97
Brendonck L, De Meester L (2003) Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491:65–84
Brinkhurst RO, Marchese MR (1992) Guide for the identification of continental aquatic oligochaetes of South and Central America. São Tomé, Association of Natural Sciences of the Coast: Climax Collection Climax Nº 6:1–207
Brock MA, Nielsen DL, Russell JS, Green JD, Langley JD (2003) Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshw Biol 48:1207–1218
Cabrera A. (1971). Phytogeography of the Argentine Republic. Bulletin of the Argentine Society of Botany. Volume XIV: 1–42.
Chacón N, Dezzeo N, Muñóz B, Rodriguez JM (2005) Implications of soil organic carbon and the biogeochemistry of iron and aluminum on soil phosphorus distribution in flooded forests of the lower Orinoco River, Venezuela. Biogeochemistry 73:555–566
Chardez D (1967) Histoire naturelle des protozoaires Thécamoebiens les naturalies. Bruxelles: Les Naturalistes Belges. 48:484–588
Claps MC, Rossi GC (1984) Contribution to the knowledge of the tardigrades of Argentina. II. J Argentine Entomol Soc 40:107–114
Claps MC, Gabellone NA, Neschuk NC (2009) Influence of regional factors on zooplankton structure in a saline lowland river: the Salado River (Buenos Aires province, Argentina). River Res Appl 25:453–471
Clarke KR, Gorley RN (2001). Book: manual/tutorial for the use of Primer V5.
Cohen GM, Shurin JB (2003) Scale-dependence and mechanisms of dispersal in freshwater zooplankton. Oikos 103:603–617
Conzonno VH (2009) Chemical limnology. Editorial of the University of La Plata, La Plata, Argentina, p 222
Corliss JO, Esser SC (1974) Comments on the role of the cyst in the life cycle and survival of free-living protozoa. Trans Am Microsc Soc 93:578–593
Coûteaux MM, Pussard M (1983) Nature du régime alimentaire des protozoaires du sol. En: international colloquium of soil biology. Louvain La Neuve Belgium 8:179–195
Datry T, Corti R, Philippe M (2012) Spatial and temporal aquatic–terrestrial transitions in the temporary Albarine River, France: responses of invertebrates to experimental rewetting. Freshw Biol 57:716–727
De Datta SK. (1981). Chemical changes in submerged rice soils. pp. 89-145. In: Principles and practices of rice production. John Wiley Sons, Singapore. pp 618.
DobrzykowskiWyngaard AEGA (1993) Phenology of dormancy in a Virginia population of Mesocyclops edax (Crustacea: Copepoda). Hydrobiologia 250:167–171
Echaniz SA, Vignatti AM (2010) Diversity and changes in the horizontal distribution of crustaceans and rotifers in an episodic wetland of the central region of Argentina. Biota Neotrop 3:133–141
Ferrando NS (2015) Doctoral thesis: Zooplankton of aquatic environments of the Salado river basin (Buenos Aires): study of interspecific relationships and main control factors through laboratory and microcosm experiences. UNLP, Faculty of Natural Sciences and Museum.
Foissner W (1987) Soil Protozoa: fundamental problems, ecological significance, adaptations in ciliates and tetaceans, bioindicators and guide to the literature. Prog Protistol 2:69–212
Foissner W, Berger H, Schaumburg J (1999) Identification and ecology of limnetic plankton ciliates. Informations berichte des Bayer. Landesamtes für Wasserwirtschaft, Heft 3/99: 1–799.
Fontaneto D, Westberg M, Hortal J (2011) Evidence of weak habitat specialization in microscopic animals. PLoS ONE 6(8):e23969. https://doi.org/10.1371/journal.Pone.0023969
Gabellone NA, Claps MC, Solari LC, Neschuk NC (2005) Nutrients conductivity and plankton in a landscape approach to a Pampean saline lowland river (Salado River, Argentina). Biogeochemistry 75:455–477
Gabellone NA, Solari LC, Claps MC, Neschuk NC (2008) Chemical classification of the water in a lowland river basin (Salado River, Buenos Aires, Argentina) affected by hydraulic modification. Environ Geol 53:1353–1363
Gabellone NA, Claps MC, Solari LC, Neschuk NC, Ardohain DM (2013) Spatial and temporal distribution pattern of phosphorus fractions in a saline lowland river with agricultural land use (Salado River, Buenos Aires, Argentina). Fundam Appl Limnol 183:271–286
Gabellone NA, Sarandón R, Claps MC (2003) Characterization and ecological zoning of the Salado River Basin. In: Floods in the Pampas región En: (Eds. Maiola OC. Gabellone NA, Hernández MA) Editorial of the National University of La Plata, pp. 266.
Gilbert D, Amblard C, Bourdier G, Shiel R (2000) Le régime aliementaire des Thécamoebiens (Protista, Sarcodina). L’année Biologique 39:57–68
Gilbert D, Mitchell E, Amblard C, Francez A (2003) Population dynamics and food preference of the testate Amoeba Nebela tincta major-bohemica-collaris complex (Protozoa) in a Sphagnum peatland. Acta Protozoologica 42:99–104
Gooderham J, Tsyrlin E (2002) The waterbug book: a guide to the freshwater macroinvertebrates of temperate Australia. CSIRO Publishing, Clayton., p 232
HairstonFox NGJA (2009) Egg banks. In: Likens GE (ed) Encyclopedia of inland waters. Elsevier, Oxford, pp 659–666
Heyns J (1971) A guide to the plant and soil nematodes of South Africa. AA Balkema. Cape Town pp. 223.
Hossner LR, Baker WH (1988) Phosphorus transformations in flooded soils. In: Hook DD et al (eds) The ecology of wetlands, vol 1. Timber Press, Portland, Oregon, USA, pp 293–306
Ibáñez JJ. (2011). Soil nematodes: trophic or functional groups. http://www.madrimasd.org/blogs/university/2011/04/12/137716.
Junk W, Bayley P, Sparks R (1989) The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci 106:110–127
Karanovic I. (2012). Recent freshwater ostracods of the world. Crustacean, Ostracoda, Podocopida. Department of Life Science Seoul 133–79, Korea and University of Tasmania. Institute of Marine and Antarctic Studies (IMAS).
Keilin D (1959) The problem of anabiosis or latent life: history and current concepts. Procedings of the royal society of London. Proc R Soc Lond B Biol Sci 150:149–191
Kirk KL (2002) Competition in variable environments: experiment with planktonic rotifers. Freshw Ecol 47:1089–1096
Koste W, Shiel RJ (1987) Rotifera from Australian inland waters. II. Epiphanidae and Brachionidae (Rotifera: Monogononta). Invertebr Syst 1:949–1021
Lazzaro X (1987) A review of planktivorous fishes: their evolution, feeding behaviours, selectivities, and impacts. Hydrobiologia 146:97–167
Maly EJ, Maly MP (1974) Dietary differences between two co-occurring calanoid copepod species. Oecologia 17:325–333
Margalef R (1963) On certain unifying principles in ecology. Am Nat 97:357–374
Mellors WK (1975) Selective predation of ephippal Daphnia and the resistance of ephippal eggs to digestion. Ecology 56:974–980
Morales LA, Vázquez S, Paz- GA (2001) Effects of scaling on physical-chemical and chemical changes in flooded soils. Cadernos Do Laboratorio Xeolóxico De Laxe, Coruña 26:255–265
Naess T, Nilssen JP (1991) Diapausing fertilized adults: a new pattern of copepod life cycle. Oecologia 86:368–371
Neiff JJ (1990) Ideas for the ecological interpretation of the Paraná. Interscience 15:424–441
Noland LE, Gojdics M (1967) Ecology of free-living protozoa. Res Protozool. 2:215–266
Obertegger U, Smith HA, Flaim G, Wallace RL (2011) Using the guild ratio to characterize pelagic rotifer communities. Hydrobiologia 662:157–162
Oksana PA, Magnusson AK, Williams DD (2006) Responses of intermittent pond ciliate populations and communities to in situ botton up and top-down manipulations. Aquat Microb Ecol 42:293–310
Pant HK, Reddy KR (2003) Potential internal loading of phosphorus in a wetland constructed in agricultural land. Water Res 37:965–972
Pavė PJ, Marchese M (2005) Benthic invertebrates as indicators of water quality in urban rivers (Paraná-Entre Ríos, Argentina). Southern Ecol 15:183–197
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agronomy 24:29–96
Ponnamperuma FN (1985). Chemical kinetics of wetland rice soils relative to soil fertility. In: Wetland soils: characterization, classification, and utilization: proceedings of a workshop, Banta SJ, United States. Soil Management Support Services, Bureau of Soils Filipinas, International Rice Research Institute (IRRI). Philippines. pp. 71- 90.
Quaíni KP (2011) Study of the succession of aquatic microorganisms in soils with different uses in the Salado river basin (province of Buenos Aires). Doctoral Thesis N° 1154, UNLP Faculty of Natural Sciences and Museum, pp. 259
Reddy KR, DeLaune RD (2008) Biogeochemistry of Wetlands: Science and applications. Florida, USA, pp. 800
Reid J (1985) Clave de identificaçaõ e lista de referências bibliográficas para as especies continentais sulamericanas de vida livre da ordem Cyclopoidea (Crustacea, Copepoda). Boletim De Zoologia Universidade Sao Paulo 9:17–143
Rengefors K, Karlsson I, Hansson LA (1998) Algal cyst dormancy: a temporal escape from herbivory. Proc Biol Sci 265:1353–1358
Roger PA (1996) Biology and management of the floodwater ecosystem in ricefields. IRRI (International rice research institute), Los Baños, Philippines, pp. 250.
Ruppert EE, Barnes RD (1995). Invertebrate zoology. McGraw-Hill Interamericana (ed.) Sixth edition. Mexico, pp. 1114.
Segers H (1995) Rotifera 2: The Lecanidae (Monogononta). In: Dumont HJ, Nogrady T (eds) Guides to the identification of the microinvertebrates of the continental waters of the world 6. SPB Academic Publishing the Netherlands, The Hague, p 226
Sharpley AN, Hedley MJ, Sibbesen E, Hillbricht-Ilkowska A, House A, Ryszkowski L (1995) Phosphorus transfers from terrestrial to aquatic ecosystem. In: Phosphorus in the Global Environment-transfers, Cycles and Management, Scope 54. John Wiley, pp. 171–200.
Siemensma FJ (2017) Microworld, world of amoeboid organisms. World- wide electronic publication, Kortenhoef, the Netherlands. https://www.arcella.nl.
Silva Rossi MM (2011) Doctoral thesis: phosphorus adsorption and desorption in soils of the central area of the Pampean region. National University of Cordoba. Faculty of Agricultural Sciences, pp: 179
Simon J (2002) Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol Rev 26:285–309
Smirnov NN (1996) Cladocera: the chydorinae and sayciinae (Chydoridae) of the world. Guides to the Identification of the microinvertebrates of the continental waters of the world 11. SPB Academic Publishing, Amsterdam., p 197
Solari LC, Claps MC, Gabellone NA (2002) River-backwater pond interactions in the lower basin of the Salado River (Buenos Aires, Argentina). Arch Hydrobiol 13:99–119
Solari LC, Quaíni KP, Gabellone NA (2018) Succession of microconsumers in waterlogged pampean soils (Buenos Aires, Argentina) and its significance for nearby wetlands. Aquat Sci 80:42
Strachan SR, Chester ET, Robson BJ (2014) Microrefuges from drying for invertebrates in a seasonal wetland. Freshw Biol 59:2528–2538
Torres VS (1996) Estudios sobre Difflugia lobostoma Leidy (Protista, Rhizopoda, Testacea lobostosea). Revista Brasileira De Zoologia 13:475–487
Tusneem ME, Patrick WH Jr (1971) Nitrogen transformations in waterlogged soil. LSU Agric Exp Stn Rep 657:3–75
Van Vliet S (2015) Bacterial dormancy: how to decide when to wake up. Curr Biol 25:753–755
Vázquez P, Masuelli S, Platzeck G, Boolsen O (2007) Recurrence of flooding in the Salado river depression: sub-basin B4. Remote Sens Magazine Spanish Remote Sens Assoc 30:47–59
Viglizzo E. F., Frank F. C. & Carreño L. (2006). Environmental situation in the Pampa and Campos y Malezales ecoregions. In: The Argentine Environmental Argentina situation 2005 (eds. Brown A., Martínez Ortiz U., Acerbi M. y Corcuera J.), Editorial Fundación Vida Silvestre Argentina, Buenos Aires, pp. 263–269.
Watanabe I. & Roger P. A. (1985). Ecology of flooded rice fields. In: Wetland soils: Characterization, classification and utilization. International Rice Research Institute (IRRI). Los Baños, Philippines pp. 229–243.
Williams DD (2006) The biology of temporary waters. Oxford University Pres, United States
Williamson C. E. & Reid J. W. (2001). Ecology and classification of North American Freshwater invertebrates. 2nd edition.
Wyngaard GA (1988) Geographical variation in dormancy in a copepod: evidence from population crosses. Hydrobiologia 167(168):367–374
Wyngaard GA, Chinnappa CC (1982) General biology and cytology of cyclopoids. In: Harrison RW, Cowden RC (Eds.), Developmental biology of freshwater invertebrates A. R. Liss, New York. pp. 485-533
Zaplara VS, Solari LC, Benítez HH, Gabellone NA (2018) Consumer microorganisms in soils of the flood plain of El Pescado stream (Bs. As). Experiences in microcosms. Magazine Argentine Museum Natural Sci 20:311–322
Acknowledgements
We thank the staff of the M. C. and M. L. Inchausti School for allowing us to use the facilities on sampling days and for providing us with the information for this work. We are grateful to Dr. Gabriela Küppers, Dr. Cristina Claps, Dr. Laura Armendáriz, Dr. Analía Diaz, and Dr. Augusto Salas, for their contributions and availability in the determination of the different groups of organisms. We want to thank the Institute of Limnology and Dr. Raúl A. Ringuelet for the use of the facilities and vehicles for campaign trips. We also extend our thanks to CONICET for financing the doctoral thesis from which this research arose. This work was partially funded by the Argentine Agency for Science and Technology promotion (ANPCyT; PICT 0228 ), National Council of Sciences and Technology (CONICET), and the La Plata National University (grant N849). We would also like to thank the Cuenca del Salado laboratory for their unconditional support in carrying out these experiments. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
Funding
Agencia Nacional de Promoción Científica y Tecnológica,0228, Virginia Zaplara, Consejo Nacional para Investigaciones Científicas y Tecnológicas, Universidad Nacional de La Plata, N849, Virginia Zaplara.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaplara, V.S., Solari, L.C., Gabellone, N.A. et al. Succession of microconsumers in waterlogged pampean soils (Buenos Aires, Argentina): a microcosm experiment. Aquat Sci 85, 62 (2023). https://doi.org/10.1007/s00027-023-00961-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-00961-z