Abstract
Limnoperna fortunei, a problematic freshwater invasive mussel in South America, was first detected in 1991 at Bagliardi Beach, Río de la Plata (Argentina). Since then, there has been a high increase in population density, reaching up to 150,000 ind. m−2. The distribution, density, individual sizes, and associated mollusk assemblages of L. fortunei were evaluated 30 years after its first detection. Seven sites along Río de la Plata River were sampled between 2018 and 2020. The highest density was recorded in the La Balandra Beach (above the stabilization value) and the lowest density in the Martín García Island (IMG—by its acronym in Spanish—, below the stabilization value). Two reproductive events were observed: late spring and late summer. Our results showed different sets of species associated with Limnoperna fortunei, with protected areas such as the IMG standing out, showing greater species richness, including first records, versus other coastal environments. We recommend increasing conservation efforts given the constant advance of urbanization in the coastal sites of the province of Buenos Aires, with environmental impact studies prior to coastal reforms, and implementation of density control strategies for Limnoperna fortunei in protected areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Limnoperna fortunei (Dunker, 1857), or golden mussel, is a problematic invasive freshwater bivalve, which was detected in the Río de la Plata River in 1991 originating from Southeast Asia (Pastorino et al. 1993). It presents an epifaunal way of life, atypical for freshwater bivalves of the Neotropical Region, with the presence of a byssus, and a high reproductive and adaptive power (Darrigran and Pastorino 1993, 2004). Since its first record for America, at Bagliardi Beach (Río de la Plata River, Buenos Aires, Argentina), this bivalve has significantly dispersed towards the north of the continent, towards a more tropical climate, even in unconnected basins (Guaiba and San Francisco basins—Brazil—, Córdoba and Tucumán provinces—Argentina—) (Darrigran et al. 2020). When it was first detected in the Río de la Plata River, the density of L. fortunei was 4–5 ind. m−2 (Darrigran and Pastorino 1993; Darrigran 2000). Density peaked at the same location in 1995 at ~ 150,000 ind. m−2, later stabilizing at ~ 40,000 ind. m−2 (Darrigran et al. 2003).
Limnoperna fortunei is considered an ecosystem engineer due to the changes it produces in the environment (Darrigran and Damborenea 2011). The economic impacts include macrofouling; easy invasion of water transfer tunnels where they adhere to tunnel walls and structures with high density, resulting in biofouling, pipe clogging, and structure corrosion (Darrigran and Damborenea 2005, 2011; Darrigran et al. 2007; Xu et al. 2015). In Argentina, it is estimated that the control cost for water treatment systems for domestic use and thermoelectric plants caused by L. fortunei annually amounts to US$1,600,000 and US$89,200. respectively (Zilio 2019). The economic impacts in Brazil were estimated at approximately US$10,000,000 per year (Adelino et al. 2021). For this reason, many studies (laboratory and field) have been carried out on this species. Among these, such asgrowth (Maroñas et al. 2003), densities (Darrigran et al. 1998), population size (Bonel et al. 2013; Musin et al. 2015), reproductive pattern (Giglio et al. 2016), ecosystem impacts (e.g., on phytoplankton, associated macroinvertebrates, nutrient cycling) (Boltovskoy et al. 2009; Darrigran and Damborenea 2011; Cataldo et al. 2012), predation by fishes (Penchaszadeh et al. 2000; Cataldo et al. 2002; Silva et al. 2020), gene flow (Ludwig et al. 2020) or microplastics ingestion (Pazos et al. 2020) have been previously published.
Reproductive events in Bagliardi beach since introduction has shown different patterns. After 2 years, five spawning events per year were detected (September–October, December–January, May–July, April–June, and October–November, Darrigran et al. 1999); at 5 years, three annual cohorts are detected (corresponding to three spawning events: November, January, and June; Maroñas et al., 2003); at 10 years, four reproductive events (September–October, February–March, longer and more intense, July–August of lesser importance, and October–December; Darrigran et al. 2003). In other basins where it has invaded, Guaiba Lake, Brazil, Limnoperna fortunei, 2–3 years after its detection at that site, obtained the same spawning pattern as those recorded at Bagliardi beach for the same five spawning periods with different intensities (January–March longer; May–June and July–August more intense spawning; April–May and October–November less intense).
Despite causing damage to human facilities by macrofouling as mentioned above, impacts on other mollusks in natural environments have shown different results. The presence of L. fortunei on native and non-native bivalves (e.g., Anodontites spp., Diplodon spp., Leila sp., and Corbicula spp.) and gastropods (e.g., Pomacea canaliculata) impede the closure of their valves or operculum, burial, and leaving them exposed to predation (Darrigran and Escurra de Drago 2000; Mansur et al. 2003). Studies carried out on macroinvertebrates associated with this invasive bivalve demonstrated the displacement of native mollusks (Uncancylus concentricus and Chilina fluminea) in favor of other native invertebrates such as annelids, crustaceans, insect larvae and nematodes (Darrigran et al. 1998). Experiments carried out in the field (Darrigran et al. 1998) and in the laboratory (Sylvester et al. 2007) showed that L. fortunei has a positive effect on the abundance of the gastropod Heleobia piscium (d’Orbigny 1835), showing an increase of 80%. On the other hand, Duchini et al. (2018) indicated that areas with L. fortunei favor the proliferation of other invertebrates (even gastropods), but without concluding whether this result increases or decreases their diversity. Instead, Ferraz et al. (2021) indicated for some lakes in southern Brazil that the presence of L. fortunei has been positive in non-native species, while negative in native species.
The objective of this work was to evaluate, in different sites of the Río de la Plata (Argentina), the distribution, density and possible dates of recruitment by season of Limnoperna fortunei after 30 years of introduction. Likewise, to evaluate if there is evidence that Limnoperna fortunei negatively affects the presence and density of native gastropods.
Materials and methods
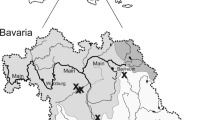
Seven sites on the Argentine coast of the Río de la Plata River were analyzed, from the source to the mouth (Fig. 1):
-
a.
Martín García Island (IMG) (34°10′57″S, 58°15′00″W): located in the internal area of the Río de la Plata, at the mouth of the Uruguay and Paraná rivers. It was declared a Multiple Use Nature Reserve in 1998. The north-east-south coast of the island is characterized by having a substrate made up of alternating spaces of rocks, reeds and small sandy beaches. The north-west-south coast of the island has a greater influence from the Paraná River and is characterized by alternate substrates of rushes and sandy-silty areas.
-
b.
Anchorena Beach (ANC) (34°29′10″S, 58°28′49″W): located in the internal area of the Río de la Plata. Urban area, with the natural substrate of the river, is “caliche” (compacted silt), which is being replaced by unnatural sandy beaches. Presents artificial substrates such as debris retained in a metal mesh.
-
c.
Natural Reserve Punta Lara (RNPL) (34°46′49″S, 58°00′56″W): located in the middle zone of the Río de la Plata. It has the most important protected area for the region. The sampling site was close to the mouth of the Boca Cerrada stream, on an artificial substrate (rubble and wall remain).
-
d.
Bagliardi Beach (BAG) (34°55′36″S, 57°43′23″W): located in the middle zone of the Río de la Plata. It is the site where Limnoperna fortunei was first detected. The area is characterized by an extensive beach, which has an elevated area with artificial rocks (which were part of a wall), where pools are formed that are submerged with high tides. It is located 1 km south of sewerage.
-
e.
La Balandra Beach (BAL) (34°52′29″S, 57°48′26″W): located within the middle zone of the Río de la Plata. It is the widest beach in the region, and it has a large sandy substratum and an area with a collapsed retaining wall where the specimens were collected. It is located about 10 km south of the previous site (BAG), with less sewage pollution.
-
f.
“El Destino” Private Nature Reserve (RNPED) (35°07′27″S, 57°22′55″W): located in the middle zone of the Río de la Plata. The substratum of the coast is sandy, with reed beds, ceiba trees and willow trees. Sampling was carried out on the river coast, branches and other hard substrates.
-
g.
Punta Piedras Beach (PP) (35°21′19″S, 57°10′26″W): located in the middle zone of the Río de la Plata, but with great influence from the external zone; that is, it receives marine influence and salinity is higher than in the other sampled sites. The coast is made up of silt to clay substrate at the surface, becoming sandier at depth. Sampling was carried out on a transect perpendicular to the coastline.
Sampling was carried out from autumn 2018 to summer 2020 (one per season, at each sampling site), during the downspout of the river (approximately 0.30 m or less), considering the winds (www.windguru.cz) and the tidal predictions (www.hidro.gov.ar). Due to the heights of the tides during the IMG campaigns, sampling was carried out only in the autumn and winter seasons. The samples were taken on a natural hard substrate (basalt in the IMG, and heterogeneous substratum called “caliche”) or artificial hard substrate (e.g., walls, groynes) with a 10 × 10 cm square with the help of a spatula (three squares per site per sampling, those values were extrapolated to ind. m−2). The squares were located randomly in easily accessible areas and where the invasive bivalve was found. The PP site was not considered in the analysis because the presence of individuals was too low to carry out the sampling, but it was considered as the most external presence of this bivalve in the Río de la Plata.
For identification of mollusks, they were relaxed with menthol crystals for 10 h, and then fixed with 70% ethanol. All mollusks in each square were counted and the L. fortunei specimens were also separated in size intervals of 5 mm (S1: 0.0–0.5 cm; S2: 0.5–1.0 cm; S3: 1.0–1.5 cm; S4: 1.5–2.0 cm; S5: 2.0–2.5 cm; S6: 2.5–3.0 cm), then the density was calculated (D = number of ind. m−2). We performed generalized linear models (GLM) for analysis of the data. All models were first fitted with a Gaussian error distribution and the residuals were tested for normality using a Shapiro–Wilk test. In all models, the data used were the density of the specimens recorded.
-
a
Density of L. fortunei: We used GLM with Gamma error distribution (link: inverse) for the mean density of L. fortunei recorded (response variable) and the predictor variables were Sites with five levels (IMG, ANC, RNPL, BAG, and BAL) and Dates with four levels (autumn, winter, spring, and summer).
-
b
Limnoperna fortunei sizes: in this case, we used GLM with Negative Binomial error distribution (link: log) for the analysis of the mean density in each size class (response variable). We performed one model for each size class separately. In all models, the predictor variables were Sites and Dates like in the previous analysis.
-
c
Associated mollusks: in these analyses, we used GLM and for each species, the distribution used was the most suited to each response variable.
Models with all possible combinations of predictor variables were considered in all three analyses (a, b, and c), including the null model. We evaluated the models using the Akaike Information Criterion corrected for small sample size (AICc) and model comparisons were made with ΔQAICc, which is the difference between the lowest QAICc value (i.e., best of suitable models) and QAICc from all other models (Burnham and Anderson 2002). Once the best model was selected for each response variable, a multiple comparison test (Tukey’s test) was performed to determine differences between different levels of the predictor variables. Statistical analyses were carried out with R (version 4.0.2, R Core Team, 2020) and RStudio (version 1.3.1056, R Core Team, 2020) software. The following R packages were used: lme4 (Bates et al. 2015); MASS (Venables and Ripley 2002); MuMIn (Barton 2020); multicmp (Sellers et al. 2018) and multcomp (Hothorn et al. 2008).
Results
Limnoperna fortunei was found in six of the seven sites (RNPE was the only site without L. fortunei, and in PP the density was very low). Since all model residuals were not normally distributed (Shapiro test: P < 0.01), they were refitted using alternative distributions more suited to each response variable. The total mean density recorded was 40,175 ind. m−2. The highest density value found was in BAL, while the lowest density was recorded in IMG. The GLM results only showed differences between sampling sites (Tables 1, 2). Regarding different size classes, the size with the highest densities was S2, while the size with the lowest densities was S6. No individuals larger than 3 cm were found at any of the sites (Fig. 2; Tables 1, 2).
The GLM results showed that the densities of specimens belonging to S1 were significantly higher in autumn (Z = -3.67; P = 0.001) and summer (Z = 3.93; P < 0.001) than in spring (Fig. 2, Table 2). The S3 analysis showed that the densities of specimens of this size class were significantly higher in spring than in summer (Z = -2.63; P = 0.042). Regarding the S5 analysis, the best model was the null model, which showed that the variables Sites and Dates were poor descriptors for the density in S5. Finally, no significant differences were found between the climate seasons of the year in S2, S4 and S6.
During the study, at least five species of mollusks were found associated with specimens of L. fortunei. Heleobia piscium and Corbicula fluminea Müller, 1774 (the latter is a non-native species) were recorded at all sites except IMG (Table 3). The genera Potamolithus, Chilina and Anisancylus, associated with L. fortunei, were only recorded in the IMG, and for that reason, they were not included in the statistical analyses. The GLM showed that there were no significant differences in the densities of Corbicula fluminea in the different sampled sites (model with Gamma error distribution and function link identity). The densities of Heleobia piscium in BAL are significantly higher than in ANC (Z = 3.15; P = 0.010) and BAG (Z = 2.74; P = 0.037) (model with Negative Binomial error distribution and functional link log).
Discussion
The results of this work show that the total mean density of Limnoperna fortunei (including all the sampled sites), after 30 years of invasion in the Río de la Plata River, is 40,000 ind. m-2, stabilization value found by Darrigran et al. (2003) after 10 years of the introduction. However, there is variation in densities and absences in different sites considered, which must be governed by environmental patterns such as:
-
Lack of hard substrate to settle the population. The RNPED site was the only one where L. fortunei was not found, this could be because the density of the bivalve is too low due to the lack of available hard substrate to adhere to.
-
Pollution and alteration of the environment. According to Bonel et al. (2013), contamination affects the growth and weight of L. fortunei. Specifically, at the ANC site, a low density of Limnoperna fortunei was found. This site is one of the most impacted on the Argentine coast of the Río de la Plata, due to its proximity to the city with the highest density of inhabitants in Argentina, Buenos Aires. In ANC the natural hard substrate, compact silt-sand, (caliche) has been decreasing since the early 1990s due to works carried out to expand land the Río de la Plata River (Bryan, common. pers.).
-
Combination of physical–chemical factors of the water. At the IMG site, a low density of Limnoperna fortunei was found. This site has an abundant natural hard substrate of basaltic rocks, where L. fortunei sits on the lateral margins. However, here the density is the lowest, compared to the rest of the sampled sites. The IMG receives greater influence from the Uruguay River, and the dispersion of said bivalve by the Uruguay River has been slower than by the Paraná River (Darrigran and Pastorino2004; Boltovskoy et al. 2006; Oliveira et al. 2015). A combination of physicochemical factors in the Uruguay River water is likely limiting for L. fortunei. For example, the concentration of Ca++ is one of the most important factors for the growth of freshwater mollusk populations (Martins-Silva and Barros 2001). Likewise, to maintain a normal aquatic biota, the minimum concentration of calcium carbonate is 20 mg.L-1 (Bain and Stevenson 1999). The Uruguay River on the Argentine coast rarely reaches these minimum values (Darrigran et al. 2012). Although the minimum Ca++ concentration mentioned in the literature for the growth of L. fortunei populations ranges between 1 mg.L-1 (Oliveira et al. 2010) and 3.96 mg.L-1 (Darrigran 2002), it is probable that in combination with one or more other factors it is also limiting for this species.
-
Predation by fish. Recently, Silva et al. (2020) carried out field studies, with the presence and absence of predatory fish, where they suggest that in the face of predation by fish, L. fortunei is distributed in margins or cavities of rocks and hard substrates, coinciding with the distribution observed in IMG.
High densities of Limnoperna fortunei were found at the intermediate sites (RNPL, BAG, and BAL). These intermediate sites presented the same type of substrate (artificial hard substrate—wall—), which is restricted to the coastline in the form of isolated patches. This type of substrate is the most favorable for the settlement of L. fortunei and in which it was recorded for the first time in Argentina (Pastorino et al. 1993). However, in BAG, the density of L. fortunei recorded was lower than in the other two sites. This could be since this site presents greater contamination due to sewage drains that are discharged 900 m to the north by the Río de la Plata River (Pazos et al. 2020).
Regarding the reproductive pattern, a continuous reproduction has been reported for the Plata basin, but with marked gonadal evacuations in certain months: September–October (early spring), December–January (early summer), May–June (autumn), and April–May (early autumn) (Darrigran et al. 1999). Cataldo and Boltovskoy (2000) conclude that L. fortunei reproduces continuously between August and April. The fixation time of the larvae in warm waters of the Río de la Plata River ranges from 15 to 20 days (Cataldo 2015). On the Río de la Plata, the presence of more than one cohort has been recorded for L. fortunei throughout the year (Maroñas et al. 2003; Darrigran et al. 2011; Bonel et al. 2013; among others). In general, these cohorts occur in the same period that is recorded in the present work. It was found that the increase of the densities of sizes S1 and S2 in the sampling sizes could indicate two reproductive events: one at the end of spring and the other one at the end of summer. Although in the IMG it is not possible to take sampling in all the climatic stations, we can infer reproductive events like the coastal sites, due to the similarity in the length intervals and densities. Maroñas et al. (2003) for Bagliardi Beach, and with sampling close to the invasion date, 1994–1995, detected three cohorts in the year, two like those recorded in this work (end of spring and end of summer) and one in winter, although this last with low densities of individuals. After 30 years of invasion, and considering that the L. fortunei, like other invasive species, is opportunistic and therefore establishing a general reproductive pattern is difficult (McMahon 2002), the results indicate a tendency for there to be only two major reproductive seasons.
The presence of L. fortunei in invaded environments causes impacts on the natural environment such as alterations to artificial environments. For example, in the caliche, also the substrate available for L. fortunei is limited to the lateral sectors and crevices of this, as mentioned by Silva et al. (2020), it could coincide with the predation of fish. In this environment, before the presence of L. fortunei, in 1987 and 1988, three species of bivalves and nine of gastropods were recorded (Darrigran and López Armengol 1998; López Armengol and Darrigran 1998). Of these gastropods, those with the highest densities were the representatives of the genus Potamolithus (with four species), which were not found in the present sampling (neither associated with L. fortunei, nor on other substrates such as caliche, beach, artificial substrate). Of the other five species of gastropods that were present in ANC in 1987 and 1988, only specimens of Heleobia piscium were found on L. fortunei; while isolated individuals of Chilina fluminea were found on the beach and caliche; and specimens of Uncancylus concentricus (Ancylinae) were found on an artificial substrate, both at low densities (de Lucía, pers. obs.), but never associated with Limnoperna fortunei. It should be noted that studies conducted at the BAG site carried out between 1992 and 1995 indicated a displacement of these gastropod species (Chilina fluminea, Uncancylus concentricus) (Darrigran, et al. 1998). For its part, Spaccesi and Rodrigues Capitulo (2012), 10 years after the invasion of L. fortunei, found Heleobia piscium and U. concentricus as companion mollusks. In our results, only H. piscium was found associated with L. fortunei. Regarding the aforementioned, a potential displacement can be raised of species of L. fortunei on the native mollusks (Darrigran and Damborenea 2011).
Likewise, the presence of L. fortunei creates a homogeneous microenvironment (Darrigran and Damborenea 2005) favorable for the development of invertebrates and unifies their accompanying fauna, which led us to think that there would be no significant differences between the species with similar environmental characteristics. However, since there are significant differences between the densities of L. fortunei between the five sampled sites, we observed that our results agree with those proposed by Sylvester et al. (2007) and Darrigran et al. (1998) where the higher the density of L. fortunei, the higher the density of H. piscium (BAL) and the lower the density of L. fortunei, the higher the richness of species is recorded (IMG). Silva et al. (2020), in experiments on the Uruguay River, and an environment like IMG, highlight that the presence of predatory fish reduces the densities of L. fortunei, leading to an increase in the densities of native species of benthic macroinvertebrates, such as the genera Potamolithus and Chilina. The same occurs with the presence of the genus Anisancylus, which is present in places where the density of L. fortunei is low or absent. It is noteworthy that the record of the genus Anisancylus is the first for the IMG (Martín García Island) and the province of Buenos Aires. This genus is found in the provinces of Córdoba, Río Negro, and Neuquén in Argentina; and it is also found in the Republic of Uruguay, in places close to the IMG (Ovando et al. 2017). On this site, IMG, 27 species of mollusks were already recorded, the product of years of campaigns by various researchers (Martín and Negrete 2006; Cesar et al. 2012). These results reaffirm the need to carry out control programs for invasive species and the non-modification of natural substrates by artificial substrates, thus protecting a greater diversity, in this case, of mollusks.
The bio-invasion of Limnoperna fortunei does not favor the biodiversity of the invaded environment. Its presence creates similar environments where it invades (e.g., Plata, Guaiba and San Francisco basins) favoring the presence of the same species throughout a basin and thus reducing diversity. What it does is a local scale homogenization of the environment where it invades (Darrigran and Damborenea 2011). Invasion by L. fortunei may result in the elimination of regional differences in epifaunal species (Darrigran 2002); i.e., it causes not only a loss of species but also of heterogeneous pre-invasion environments. Something similar occurs with Dreissena polymorpha (Pallas 1771), another invasive bivalve with similar adaptive/reproductive characteristics to L. fortunei, but in the northern hemisphere. Ward and Ricciardi (2007) point out an increase in the number of species, but ecologically similar, in the environment invaded by D. polymorpha; however total species richness (ecologically similar and non-similar species) tended to decrease in large bodies of water (on a regional scale).
In short, the presence of a bio-invasion such as that of Limnoperna fortunei or golden mussel causes alterations in native biodiversity. Our results showed different sets of species associated with Limnoperna fortunei, with protected areas such as the IMG standing out, showing greater species richness, including first records, versus other coastal environments. We recommend increasing conservation efforts given the constant advance of urbanization in the coastal sites of the province of Buenos Aires, with environmental impact studies before coastal reforms, and implementation of density control strategies for Limnoperna fortunei in protected areas, and thus protect the native fauna.
Although the present work is a valuable spatiotemporal description of the dispersion of Limnoperna fortunei in the Río de la Plata basin, an extensive study of the golden mussel in the invaded basins is advisable. These studies will allow order to identify the status of invasive populations and short- and long-term trends and, to assess the relationships between the introduction of invasive species, biodiversity, and changes in ecosystem services.
Data availability
The data and material analyzed during the current study are available from the corresponding author on reasonable request.
References
Adelino JRP, Heringer G, Diagne C, Courchamp F, Faria LDB, Zenni RD (2021) The economic costs of biological invasions in Brazil: a first assessment. In: Zenni RD, McDermott S, García-Berthou E, Essl F (Eds) The economic costs of biological invasions around the world. NeoBiota 67: 349–374.
Bain M, Stevenson N (1999) Aquatic habitat assessment. Common methods. American Fisheries Society, Maryland, p 136
Barton K (2020) MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn. Accessed Aug 2020
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Boltovskoy D, Correa N, Cataldo D, Sylvester F (2006) Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata watershed and beyond. Biol Invasions 8:947–963
Boltovskoy D, Karatayev A, Burlakova L, Cataldo D, Karatayev V, Sylvester F, Mariñelarena A (2009) Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636:271–284
Bonel N, Solari CL, Lorda J (2013) Differences in density, shell allometry and growth between two populations of Limnoperna fortunei (Mytilidae) from the Río de la Plata Basin, Argentina. Malacologia 56:43–58
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A practical information-theoretic approach, 2nd edn. Springer, New York
Cataldo DH (2015) Larval development of Limnoperna fortunei. Limnoperna fortunei. Springer, Cham, pp 43–53
Cataldo DH, Boltovskoy D (2000) Yearly reproductive activity of Limnoperna fortunei (Bivalvia) as inferred from the occurrence of its larvae in the plankton of the lower Paraná river and the Río de la Plata estuary (Argentina). Aquat Ecol 34:307–317
Cataldo D, O’Farrell I, Paolucci E, Sylvester F, Boltovskoy D (2012) Impact of the invasive golden mussel (Limnoperna fortunei) on phytoplankton and nutrient cycling. Aquat Invasions 7:91–100
Cataldo D, Boltovskoy D, Marini V, Correa N (2002) Limitantes de Limnoperna fortunei en la cuenca del Plata: la predación por peces. Tercera jornada sobre conservación de la fauna íctica en el río Uruguay. Paysandu, Uruguay, La Comisión Administradora de Río Uruguay. 25-26/04/2002. pp. 1–5
César II, Martín SM, Rumi A, Tassara M (2012) Mollusks (Gastropoda and Bivalvia) of the multiple-use reserve Martín García Island, Río de la Plata river: biodiversity and ecology. Braz J Biol 72:121–130
Darrigran G (2000) Invasive freshwater bivalves of the Neotropical region. Dreissena 11:7–13
Darrigran G (2002) Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol Invasions 4:145–156
Darrigran G, Damborenea MC (2005) A bioinvasion history in South America. Limnoperna fortunei (Dunker, 1857), the golden mussel. Am Malacol Bull 20:105–112
Darrigran G, Damborenea C (2011) Ecosystem engineering impact of Limnoperna fortunei in South America. Zool Sci 28:1–7
Darrigran G, Escurra de Drago I (2000) Invasion of the exotic freshwater mussel Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae) in South America. The Nautilus 114(2):69–73
Darrigran G, López Armengol MF (1998) Distribución, estructura y composición de la malacofauna presentes en substrato duro de la costa argentina del Río de La Plata. Gayana Zoología 62:79–89
Darrigran G, Pastorino G (1993) Bivalvos invasores en el Río de la Plata. Argentina Comun Soc Malacol Urug 7(64–65):309–313
Darrigran G, Pastorino G (2004) Distribution of the golden mussel Limnoperna fortunei (Dunker, 1857), (Bivalvia: Mytlidae) after 10 years invading America. J Conchol, Spec Publ 3:95–102
Darrigran G, Martín SM, Gullo B, Armendariz L (1998) Macroinvertebrates associated with Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) in Río de la Plata, Argentina. Hydrobiologia 367:223–230
Darrigran G, Penchaszadeh P, Damborenea MC (1999) The reproductive cycle of Limnoperna fortunei (Dunker, 1857) (Mytilidae) from a neotropical temperate locality. J Shellfish Res 18:361–365
Darrigran G, Damborenea C, Penchaszadeh P, Taraborelli C (2003) Adjustments of Limnoperna fortunei (Bivalvia: Mytilidae) after ten years of invasion in the Americas. J Shellfish Res 22:141–146
Darrigran G, Damborenea C, Greco N (2007) Freshwater invasive bivalves in man-made environments: a case study of larvae biology of Limnoperna fortunei in a hydroelectric power plant in South America. Ambio 36(7):575–579
Darrigran G, Bonel N, Colautti D, Cazzaniga NJ (2011) An alternative method to assess individual growth of the golden mussel (Limnoperna fortunei) in the wild. J Freshwater Ecol 26:527–535
Darrigran G, Damborenea C, Drago EC, Ezcurra de Drago I, Paira A, Archuby F (2012) Invasion process of Limnoperna fortunei (Bivalvia: Mytilidae): the case of Uruguay River and emissaries of the Esteros del Iberá Wetland. Argentina Zoologia (curitiba) 29(6):531–539
Darrigran G, Agudo-Padrón I, Baez P, Belz C, Cardoso F, Carranza A, Collado G, Correoso M, Cuezzo MG, Fabres A, Gutiérrez Gregoric DE, Letelier S, Ludwig S, Mansur MC, Pastorino G, Penchaszadeh P, Peralta C, Rebolledo A, Rumi A, Santos S, Thiengo S, Vidigal T, Damborenea C (2020) Non-native South American mollusks: emergent patterns in an understudied continent. Biol Invasions 22:853–871. https://doi.org/10.1007/s10530-019-02178-4
Duchini D, Boltovskoy D, Sylvester F (2018) The invasive freshwater bivalve Limnoperna fortunei in South America: multiannual changes in its predation and effects on associated benthic invertebrates. Hydrobiologia 817:431–446
Ferraz JD, Gois MV, Yabu MHS, Garcia DAZ, Marques ACV, Casimiro ACR, Vidotto-Magnoni AP, Orsi ML (2021) Malacofauna bentônica do Lago Igapó, Londrina (Paraná, Brasil), com ênfase na espécie invasora mexilhão-dourado Limnoperna fortunei (Dunker, 1857). Semin, Ciênc Biol Saúde 42:3–14
Giglio ML, Dreher Mansur MC, Damborenea C, Penchaszadeh PE, Darrigran G (2016) Reproductive pattern of the aggressively invasive species, Limnoperna fortunei (Bivalvia, Mytilidae) South America. Invertebr Reprod Dev 60(3):175–184
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363. https://doi.org/10.1002/bimj.200810425
López Armengol MF, Darrigran G (1998) Distribución del género neotropical Potamolithus Pilsbry y Rush, 1896 (Gastropoda: Hydrobiidae) en el estuario del Río de la Plata. Iberus 16(2):67–74
Ludwig S, Sari EHR, Paixão H, Montresor LC, Araújo J, Brito CFA, Darrigran G, Pepato AR, Vidigal THDA, Martinez CB (2020) High connectivity and migration potentiate the invasion of Limnoperna fortunei (Mollusca: Mytilidae) in South America. Hydrobiologia 848:499–513. https://doi.org/10.1007/s10750-020-04458-w
Mansur MCD, Santos CPD, Darrigran G, Heydrich I, Callil CT, Cardoso FR (2003) Primeiros dados quali-quantitativos do mexilhão-dourado, Limnoperna fortunei (Dunker), no Delta do Jacuí, no Lago Guaíba e na Laguna dos Patos, Rio Grande do Sul, Brasil e alguns aspectos de sua invasão no novo ambiente. Rev Bras Zool 20:75–84
Maroñas ME, Darrigran GA, Sendra ED, Breckon G (2003) Shell growth of the golden mussel, Limnoperna fortunei (Dunker, 1857) (Mytilidae), in the Río de la Plata, Argentina. Hydrobiologia 495:41–45
Martín SM, Negrete LHL (2006) Primer registro de Heleobia guaranitica (Doering, 1884) (Gastropoda: Cochliopidae) en la reserva natural de usos múltiples “Isla Martín García.” Comun Soc Malacol Urug 9(89):71–73
Martins-Silva MJ, Barros M (2001) Occurrence and distribution of fresh-water molluscs in the Riacho Fundo Creek Basin, Brasilia. Brazil Rev Biol Trop 49(3–4):865–870
McMahon RF (2002) Evolutionary and physiological adaptations of aquatic invasive animals: r selection versus resistance. Can J Fish Aquat Sci 59:1235–1244
Musin GE, Molina FR, Giri F, Williner V (2015) Structure and density population of the invasive mollusc Limnoperna fortunei associated with Eichhornia crassipes in lakes of the Middle Paraná floodplain. J Limnol 74(3):537–548
Oliveira M, Hamilton S, Jacobi C (2010) Forecasting the expansion of the invasive golden mussel Limnoperna fortunei in Brazilian and North American rivers based on its occurrence in the Paraguay River and Pantanal wetland of Brazil. Aquat Invasions 5:59–73
Oliveira MD, Campos MC, Paolucci EM, Mansur MC, Hamilton SK (2015) Colonization and spread of Limnoperna fortunei in South America. In: Boltovskoy D (ed) Limnoperna Fortunei. Invading Nature. Springer Series in Invasion Ecology, vol 10. Springer, Cham, pp 333–355. https://doi.org/10.1007/978-3-319-13494-9_19
Ovando XM, Richau MS, Santos SB (2017) The genus Anisancylus Pilsbry, 1924 (Planorboidea, Ancylinae) in South America: species distribution and new records. Check List 13:267
Pastorino G, Darrigran G, Martín SM, Lunaschi L (1993) Limnoperna fortunei (Dunker, 1875) (Mytilidae), nuevo bivalvo invasor en aguas del Río de la Plata. Neotrópica 39(101–102):34
Pazos RS, Suárez JC, Gómez N (2020) Study of the plastisphere: biofilm development and presence of faecal indicator bacteria on microplastics from the Río de la Plata estuary. Ecosistemas 29(3):2069. https://doi.org/10.7818/ECOS.2069
Penchaszadeh PE, Darrigran G, Angulo C, Averbuj A, Brogger M, Dogliotti A, Pirez N (2000) Predation of the invasive freshwater mussel Limnoperna fortunei (Dunker, 1857) (Mytilidae) by the fish Leporinus obtusidens Valenciennes, 1846 (Anostomidae) in the Rio de la Plata, Argentina. J Shellfish Res 19:229–232
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Sellers K, Morris DS, Balakrishnan N, Davenport D (2018) multicmp: Flexible Modeling of Multivariate Count Data via the Multivariate Conway-Maxwell-Poisson Distribution. R package version 1.1. https://CRAN.R-project.org/package=multicmphttps://doi.org/10.1016/j.jmva.2016.04.007
Silva I, Naya D, de Mello FT, D’Anatro A, Tesitore G, Clavijo C, González-Bergonzoni I (2020) Fish vs. Aliens: predatory fish regulate populations of Limnoperna fortunei mitigating impacts on native macroinvertebrate communities. Hydrobiologia 848:2281–2301
Spaccesi FG, Rodrigues Capitulo A (2012) Benthic communities on hard substrates covered by Limnoperna fortunei Dunker (Bivalvia, Mytilidae) at an estuarine beach (Río de la Plata, Argentina). J Limnol 71:144–153
Sylvester F, Boltovskoy D, Cataldo D (2007) The invasive bivalve Limnoperna fortunei enhances benthic invertebrate densities in South American floodplain rivers. Hydrobiologia 589:15–27
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Ward JM, Ricciardi A (2007) Impacts of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Divers Distrib 13(2):155–165
Xu M, Darrigran G, Wang Z, Zhao N, Lin CC, Pan B (2015) Experimental study on control of Limnoperna fortunei biofouling in water transfer tunnels. J Hydro-Environ Res 9(2):248–258
Zilio, MI (2019) El impacto económico de las invasiones biológicas en Argentina: cuánto cuesta proteger la biodiversidad. LIV Reunión Anual de la Asociación Argentina de Economía Política. Bahía Blanca, Buenos Aires, Argentina. https://aaep.org.ar/anales/works/works2019/zilio.pdf
Acknowledgements
We thank ASAM (Asociación Argentina de Malacología) for awarding the 2018 “Juan José Parodiz” Prize to the first author, which covered part of the expenses necessary for the development of this research. We also thank Agustina Zivano, Marina Lenguas Francavilla and Nélida M. Viega for their collaboration in data collection. To Laura Estefanía Paz for her help in the statistical analyses and critical review of this work. We also thank María Fernanda Jaime, translator of the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC), for reviewing and editing the final version of the manuscript. We are also grateful to the organizations that granted the permits for the collection of the specimens (OPDS and Fishing and Aquaculture Activities—Ministerio de Agroindustria—) in protected and unprotected areas. To the park rangers of the IMG (María E. Cueto, Gloria Domínguez, and Nazareno Asin), to the director of School N ° 7 Cacique Pincén of the IMG (Claudio Galeno), and to the staff of the Hostel and Camping School of Grumetes for the logistics and support during the sampling. We also thank two anonymous reviewers who made useful comments on the original version of the manuscript.
Funding
Partial financial support was received from Agencia Nacional de Promoción Científica y Tecnológica (PICT-2019-01417), Universidad Nacional de La Plata (11/N927) and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 1966).
Author information
Authors and Affiliations
Contributions
MdL: conceived the main idea. MdL-DGG: helped with sampling; analyzed the data and wrote the manuscript. GD: helped with the revision of the drafts and contributed with the discussion of the results.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declares any conflict of interest.
Ethical approval
The study did not involve endangered or protected species.
Consent to participate
All the authors declare consent.
Consent for publication
All the authors declare consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Lucía, M., Darrigran, G. & Gutiérrez Gregoric, D.E. The most problematic freshwater invasive species in South America, Limnoperna fortunei (Dunker, 1857), and its status after 30 years of invasion. Aquat Sci 85, 5 (2023). https://doi.org/10.1007/s00027-022-00907-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00907-x