Abstract
Carbon dioxide (CO2) emission from fluvial systems represents a substantial flux in the global carbon cycle. However, variation in fluvial CO2 fluxes at the air–water interface as well as its drivers are poorly understood, especially in non-forested headwaters. Here, we measured CO2 concentration and fluxes in 14 lowland open-canopy streams (Pampean region, Argentina) that cover a wide range of land use and water quality. We also analyzed drivers of CO2 concentration and fluxes, including factors related to metabolism, gas solubility, alkalinity, and gas transfer. Metabolic rates varied considerably among the study sites, but most streams (i.e., 8 out of the 11 where we were able to estimate ecosystem metabolism) were net heterotrophic. Metabolic differences among sites were mostly driven by the aromatic carbon content and the percent of the stream reach covered by primary producers. All streams, even those that were not net heterotrophic were CO2 supersaturated. Alkalinity combined with in-stream primary production explained 52.3% of the variance in the partial pressure of CO2 (pCO2), but our observations suggest that pCO2 might be controlled by external groundwater inputs of dissolved inorganic carbon rather than by internal metabolism. All streams were net emitters of CO2 to the atmosphere. Significantly more variance in CO2 flux was explained by gas transfer velocity (63.7%) than by pCO2 (21.9%). We also observed high spatial heterogeneity in CO2 fluxes within each stream, which was associated with flow variation and the presence of different macrophyte and algae patches. Overall, our results indicate that CO2 emission in these extremely low turbulence streams is controlled by a combination of external and internal biogeochemical processes and limited atmospheric exchange.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluvial ecosystems play a central role in the global carbon cycle (Cole et al. 2007; Aufdenkampe et al. 2011; Raymond et al. 2013; Regnier et al. 2013). Despite occupying < 1% of the terrestrial surface (Allen and Pavelsky 2018), streams and rivers can release ~ 1.8 Pg C y−1 in the form of carbon dioxide (CO2) to the atmosphere, exceeding the CO2 emissions from lakes and reservoirs (Raymond et al. 2013). In particular, the contribution of small streams to CO2 emission is not completely understood, but conservative estimates suggest that 36% of total fluvial CO2 outgassing may be accounted for by these systems (Marx et al. 2017).
The exchange of CO2 across the air–water interface depends on the gas transfer velocity and the partial pressure of CO2 (pCO2) at the water surface (Alin et al. 2011; Raymond et al. 2013). The gas transfer velocity is controlled by surface water turbulence that, in turn, is strongly related to geomorphic and hydraulic variables, such as stream channel slope and stream flow velocity (Raymond et al 2012; Hall and Ulseth 2020). The magnitude of pCO2 relies on both external and internal dissolved inorganic carbon (DIC) sources (Hotchkiss et al. 2015). External DIC sources include soil respiration and weathering of minerals in the catchment that enter streams via groundwater inputs, whereas internal DIC sources include in-stream photochemical mineralization and ecosystem respiration. In general, external sources contribute more than internal sources to CO2 emissions from low-order streams, which tend to be supersaturated with CO2 compared to the overlying atmosphere (Hotchkiss et al. 2015; Marx et al. 2017; Demars 2019). Net ecosystem production (NEP) may increase pCO2 in the stream water when ecosystem respiration exceeds gross primary production, and thus, NEP is negative (Jones and Mulholland 1998; Duarte and Prairie 2005; Hotchkiss et al. 2015). However, internal metabolism typically accounts for < 20% of total CO2 evasion from streams to the atmosphere, and this contribution may increase with stream order (Butman and Raymond 2011; Hotchkiss et al. 2015; Gómez-Gener et al. 2016; Hill et al. 2017; Demars 2019).
Knowledge on CO2 dynamics in streams is based mostly on data from forested headwaters of the northern hemisphere, whereas other stream types and regions remain understudied (Lauerwald et al. 2015). In South America, for instance, there are few studies on CO2 dynamics from lowland streams (except for Amazonian fluvial systems). These prevalent freshwater ecosystems are characterized by slow-flowing waters, lack of riparian forest, high irradiance, dense assemblages of macrophytes, and high primary production, especially in summer (Feijoó and Lombardo 2007; García et al. 2017). These features, which resemble those of nutrient-rich, lowland streams elsewhere in the world, may strongly affect the dynamics of CO2. Low water turbulence strongly reduces CO2 exchange by decreasing the gas transfer velocity between water and atmosphere (Hall and Ulseth 2020). This effect is enhanced in the presence of floating macrophytes, which further diminish physical gas exchange (Attermeyer et al. 2016; Peixoto et al. 2016). In addition, primary production and respiration of macrophytes strongly influence stream metabolism in lowland streams, leading to high ecosystem metabolic rates (García et al. 2017; Alnoee et al. 2021). Thus, the physical and metabolic consequences of macrophyte presence are expected to significantly influence pCO2 and CO2 exchange (Attermeyer et al. 2016; Peixoto et al. 2016; Pu et al. 2017). Finally, high irradiance can also influence CO2 dynamics by enhancing the photochemical mineralization of organic matter and primary production (Lu et al. 2013).
In this study, we examined the magnitude and drivers of pCO2 and CO2 exchange at the air–water interface in lowland Pampean streams covering a wide range of catchment land uses, water physicochemical characteristics, and rates of ecosystem metabolism. We predicted that the study streams would be saturated with CO2 compared to the overlying atmosphere, and that metabolism would contribute significantly to pCO2. We further predicted that, despite high pCO2, slow current velocities and low water turbulence would strongly restrict CO2 exchange between water and atmosphere. Finally, we predicted that low current velocities and high abundance and diversity of emergent, submerged, and floating macrophytes would enhance the spatial heterogeneity of CO2 exchange within stream reaches.
Materials and methods
Study sites
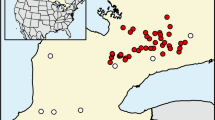
We sampled 14 streams of the Pampean region (Buenos Aires province, Argentina) (Fig. 1). The region is covered by a sequence of sands and loess deposited during the Tertiary and the Quaternary (Sala et al. 1983). Relief is flat (0–200 m above sea level) in most parts of the province. Climate is temperate humid, with a mean annual temperature between 13 and 17 °C and a mean annual rainfall between 700 and 1200 mm evenly distributed throughout the year (Matteucci 2012). Due to the expansion of agriculture and cattle breeding, natural vegetation (mostly grasslands) is confined to small patches in less-productive lands and in riparian zones. Stream sediments consist mainly of fine materials (silt and clay).
To cover a wide range in physicochemical conditions, we selected streams from three ecoregions of the Buenos Aires province: Salado River and tributaries, tributaries of the Paraná and Río de la Plata Rivers, and tributaries of the Atlantic Ocean [regions A, C, and D, respectively, according to Feijoó and Lombardo (2007)]. Ecoregions differ in geomorphologic and hydrological features (Frenguelli 1956). The Salado River system runs along a tectonic depression forming wandering meanders. Tributaries of the Paraná and Río de la Plata Rivers have well defined drainage networks and steeper banks compared to the other systems. Tributaries of the Atlantic Ocean are characterized by scarcely marked channels in the upper and middle reaches that become deeper downstream, forming steep banks close to the mouth. Rivers of this region tend to run separately, without forming common branches.
The catchments of the selected streams differed significantly in cropland (cropland = 5.8–92.6%) and cattle (0.4–72.6%) land use (Supplementary Table 1). Land-use data were derived from Landsat V Thematic Mapper (TM) satellite images (provided by the Instituto Nacional de Pesquisas Espaciais, INPE, Brazil).
Stream reach characterization
We sampled between February and March 2019 (austral summer). Each stream was visited on one occasion and sampled between 12 and 16 h, when metabolic activity is highest (Martí et al. 2020).
We selected a 100-m reach at each stream. At the end of the reach, we measured stream cross-sectional area with a ruler and tape measure as well as current velocity with a Schiltknecht MiniAir20 anemometer (Schiltknecht Messtechnik, Gossau, Switzerland) to estimate stream discharge by the velocity–area method (Gordon et al. 1992). We measured wetted width in ten transects separated 10 m away to determine reach surface area. In each transect, we also measured depth in triplicate (at the center and close to the margins) to calculate mean reach depth.
We mapped macrophyte abundance by visually estimating the percent of the stream reach covered by each plant species in 5-m sub-reaches. These data were then used to calculate the percent of reach surface covered by different life forms (macroalgae and emergent, submerged, and floating macrophytes) (Feijoó et al. 2011).
Water physicochemistry
We measured water temperature, conductivity, and pH in situ at the middle of the reach of each stream with a Hach HQ40D multiprobe (Hach Company, Loveland, CO, USA). We calibrated the probes following the manufacturer’s instructions at the start of the sampling camping and checked them before every visit to the field. In the same spot, we also collected three water samples (250-mL acid-washed polyethylene bottles), which were stored in an ice chest and transported to the laboratory for later analyses.
We used one unfiltered sample to determine alkalinity (carbonates and bicarbonates) by titration (APHA 2005, method code 2320 B) within the same day of collection.
We filtered the second sample through pre-ashed glass fiber filters (Whatman GF/F, nominal pore size = 0.7 μm) preserved it with 0.1% chloroform, and stored it at 4 °C until nutrient analysis (Gardolinski et al. 2001). We estimated soluble reactive phosphorus (SRP) and ammonium concentrations by the molybdate–ascorbic method and the indophenol blue method, respectively (APHA 2005). We determined nitrate and nitrite concentrations with a FUTURA Autoanalyzer (Alliance Instruments, Frepillon, France) through a reaction with sulfanilamide with a previous Cu–Cd reduction for nitrate (APHA 2005).
We filtered the third sample through pre-ashed glass fiber filters (Whatman GF/F, nominal pore size = 0.7 μm), and divided it into two subsamples. We stored the first subsample in amber-colored glass bottles to minimize light exposure, acidified it with HCl 10% to reach pH 2, and analyzed it for dissolved organic carbon (DOC) by high-temperature catalytic oxidation on a Shimadzu TOC-V CSH Analyzer (Shimadzu Corporation, Kyoto, Japan). We filtered the second subsample through 0.2-µm nylon membrane filters (GVS Magna, Bologna, Italy) and kept it in cool and dark conditions for the spectroscopic analysis of dissolved organic matter (DOM) composition (Spencer et al. 2014).
We measured the absorbance spectra (200–800 nm) on a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) using a 1-cm quartz cuvette. We used Milli-Q water as blank and subtracted the average sample absorbance between 700 and 800 nm from the spectrum to correct for offsets due to instrument baseline drift (Green and Blough 1994). We determined the specific UV absorbance at 254 nm (SUVA, L mg−1 m−1) by dividing the UV absorbance measured at 254 nm by the DOC concentration (Weishaar et al. 2003). The SUVA index is associated with bulk aromaticity in the sample, with higher values being indicative of a high content of aromatic carbon. We determined the spectral slopes for the interval 275–295 and 350–400 nm (S275–295 and S350–400, respectively) by fitting the single absorbance spectra to an exponential decay function. We calculated the slope ratio (SR) as the ratio of S275–295 to S350–400, with high values of the index indicative of low molecular weight compounds (Helms et al. 2008).
We further analyzed DOM composition via Excitation–Emission Matrices (EEMs) using a Perkin Elmer LS 55 fluorescence spectrophotometer (Perkin Elmer, Waltham, MA, USA) with a 1-cm quartz cuvette. We measured fluorescence intensity across the excitation range set from 240 to 449 nm (3 nm increments) and the emission range set from 250 to 598 nm (3 nm increments). Before measuring the samples, we calibrated the fluorometer with a Rhodamine B solution to correct instrument-specific biases. We allowed water samples to warm to room temperature prior to measurements. EEMs were blank subtracted using the EEM of Milli-Q water, determined every ten samples. We corrected spectra for inner filter effects using UV–Vis absorbance spectra (Kothawala et al. 2013). We calculated three DOM descriptors: (i) humification index (HIX), with higher values indicating higher humification degree (Fellman et al. 2010); (ii) biological index (BIX), related to the proportion of recently produced DOM, for which higher values are indicative of a higher contribution of recently produced DOM (Hughet et al. 2009); and (iii) fluorescence index (FI), related to DOM sources, with values ranging between ~ 1.2 for higher plant DOM sources to ~ 1.8 for microbial DOM sources (Cory and McKnight 2005).
Whole-stream metabolism
We estimated whole-stream rates of gross primary production (GPP) and ecosystem respiration (ER) with the open-channel one-station technique (Odum 1956; Uehlinger and Naegeli 1998). The day before the sampling, we installed a field-calibrated oximeter (HQ40D, Hach, Loveland, Colorado; miniDOT, PME, Vista, California) at the end of the stream reach. We measured changes in dissolved oxygen (DO) concentration (mg L−1) and water temperature (˚C) in 5-min intervals for 24 h. For all sites situated in the north of the province (all A and C sites, except C8; Fig. 1), we obtained photosynthetically active radiation (PAR, 400–700 nm, μmol m−2 s−1) from the meteorological station in Lujan. For the rest of the sites (C8 and all D sites; Fig. 1), we modeled light based on latitude and longitude (Appling et al. 2017). We also obtained barometric pressure (mb) from nearby meteorological stations (Lujan for the A and C sites in the north, Balcarce and Miramar for the D sites in the south of the province). The distance from the meteorological station to the furthest stream was 194 km. We used barometric pressure together with water temperature to estimate the DO saturation concentration (i.e., the theoretical concentration of DO if the air and water were at equilibrium; mg L−1) (García and Gordon 1992; Colt 2012). Mean stream depth (m) was obtained from transects (see above).
We estimated GPP (g O2 m−2 d−1), ER (g O2 m−2 d−1) and K600 (i.e., the gas exchange rate coefficient after normalization for molecular properties and temperature to a Schmidt number of 600, corresponding to DO at 17.5˚C; d−1) fitting models of DO dynamics to DO data, using inverse fitting and Bayesian inference (Holtgrieve et al. 2010). The gas transfer velocity (k600, m d−1) was calculated multiplying K600 (d−1) by mean stream depth (m). The model we used corresponded to the model variant b_np_oipi_tr_plrckm.stan in the streamMetabolizer R package (Appling et al. 2017), which accounts for both observation and process errors in diel DO dynamics using a state space formulation. The most likely sources of process error in our streams are inputs of low-O2 groundwater, the high patchiness of primary producers and light-associated misspecifications (in particular, cloudiness at sites C8 and D15-D20, where we modeled light based on latitude and longitude). The process error accounts for these misspecifications and besides, including both observation and process errors, which provides lower bias and more accurate estimates of parameter error than assuming either process error or observation error alone (Appling et al. 2018).
Prior probability values for parameters were based on literature range from Hall et al. (2016), following Appling et al. (2018). Priors were GPP ~ Ɲ (3.1,6.0), ER ~ Ɲ(7.1,7.1), and K600 ~ Lognormal(2.48,1.0). Tests on subsets of the data showed that the posterior parameter distribution was robust to varying these priors.
We solved for model parameters using program Stan, a Markov chain Monte Carlo (MCMC) simulation program (Stan Development Team 2016). This method was embedded in streamMetabolizer version 0.11.4 and R version 3.6.3. We ran four MCMC chains in parallel on four cores, with 1000 warmup steps and 500 saved steps on each chain.
To assess model fit, we ensured parameter convergence as the Gelman-Rubin Rhat < 1.1. We also checked how well modeled DO fitted observed DO. Although all models converged, we excluded estimates at A3, A4, and D16 because of poor model fit.
pCO2 and CO2 exchange
We measured the partial pressures of CO2 in water and air (pCO2,w and pCO2,a, respectively) in three points along the stream reach. To capture stream reach heterogeneity, each selected point presented different biological community composition (algae, macrophytes, or open water) and hydraulic conditions (current velocity, measured with a Schiltknecht MiniAir20 anemometer).
We made measurements by triplicate at each location using an infrared gas analyzer (EGM-4, PP-Systems, USA). For pCO2,w measurements, we circulated water samples at a rate of ~ 300 mL min−1 through a membrane contactor (MiniModule, Liquid-Cel, USA) coupled to the gas analyzer (Gómez-Gener et al. 2016). For pCO2,a, we circulated atmospheric air approximately 1 m above the water surface through the gas analyzer.
We determined CO2 exchange across the water–air interface (mmol m−2 d−1) using an indirect and a direct approach. The CO2 exchange estimated with the indirect approach (CO2 exchange(indirect)) provided an average estimation of the CO2 exchange at reach scale, whereas the CO2 exchange estimated with the direct approach (CO2 exchange(direct)) allowed exploring the spatial heterogeneity in CO2 exchange along the reach. Positive values of CO2 exchange indicate that the stream behaved as a net CO2 emitter (i.e., there is an efflux of the gas to the atmosphere), whereas negative values indicate that the stream was a CO2 sink.
We determined CO2 exchange(indirect) using Fick’s law of gas diffusion
where \(k_{{{\text{CO}}_{2} }}\) (m d−1) is the specific gas transfer velocity for CO2, Kh (mmol µatm−1 m−3) is Henry’s constant for CO2 adjusted for salinity and temperature, and pCO2,w (µatm) and pCO2, a (µatm) are the partial pressures of CO2 in water and air, respectively. We made measurements of pCO2 by triplicate at each location. We estimated \(k_{{{\text{CO}}_{2} }}\) using the k600 estimated with the metabolism model and the Schmidt number of CO2 at the stream water temperature during pCO2,w measurements (Raymond et al. 2012).
We determined CO2 exchange(direct) using the enclosed chamber method (Goldenfum 2010) at the same locations where we measured pCO2,w and pCO2,a. We made measurements by triplicate at each location. We monitored the CO2 gas concentration inside an opaque floating chamber every 4.8 s with the infrared gas analyzer. Exchange measurements lasted until we observed a change in CO2 of at least 10 µatm, with a minimum duration of 120 s and a maximum of 240 s. We estimated CO2 exchange(direct) (mmol m−2 d−1) from the rate of change of CO2 inside the chamber as
where \(\left( {\frac{{{\text{dCO}}_{2} }}{{{\text{d}}t}}} \right)\) is the slope of the relationship between CO2 concentration and time (µatm s−1), V and S are the volume and the surface area of the chamber (3.37 dm3 and 4.14 dm2, respectively), T is the air temperature (K), and R is the ideal gas constant (0.08206 L atm K−1 mol−1) (Bodmer et al. 2016; Gómez-Gener et al. 2016).
Statistical analyses
We performed all statistical analyses using the R software version 3.6.3 (R Core Team 2021).
We used a principal component analysis (PCA) to identify stressor gradients in the data, detect the main environmental differences among the study streams, and analyze correlations between variables (Johnson and Hering 2009). Before conducting the PCA, we reduced the number of variables. For land use, we used percent agriculture, because together with cattle, it is the main land use in all the catchments and both were inversely related (i.e., the higher the cover of agriculture, the lower the cover of cattle, and vice versa). For nutrient status, we used dissolved inorganic nitrogen (DIN; the sum of NH4, NO3, and NO2), SRP, and DOC. For hydro-morphology, we used discharge, which strongly correlated with both water depth and current velocity. For primary producers, we used the percent of the stream reach covered by primary producers (i.e., the sum of the percent of algae and emergent, submerged and floating macrophytes). For physicochemical properties, we used conductivity, pH, and water temperature, and excluded concentration of DO and CO2, because they strongly relate to the main response variables of interest (i.e., stream metabolism and CO2 exchange). Finally, for DOM quality, we used SUVA, FI, HIX, and BIX. We did not include SR, because it was strongly correlated with BIX (r = 0.89), and we had to reduce the number of variables from this and further analyses. All variables were scaled to unit variance and the PCA was performed using the rda() function in the vegan package (Oksanen et al. 2020).
We analyzed differences in stream metabolism using linear mixed-effect models with GPP, ER, and NEP as dependent variables, environmental variables as fixed factors, and ecoregion as the random factor to account for potential spatial autocorrelation. We first reduced the number of variables by selecting variables known to affect the response variable based on the literature (Tank et al. 2010; Hoellein et al. 2013; Bernhardt et al. 2018; Marzolf and Ardón 2021). For GPP, the selection included DIN, SRP, water temperature, discharge, and the percent of the stream reach covered by primary producers. For ER, the selection included DOC, water temperature, discharge, SUVA, HIX, BIX, GPP and the percent of the stream reach covered by primary producer. For NEP, the selection included DIN, SRP, DOC, water temperature, discharge, SUVA, HIX, BIX, and the percent of the stream reach covered by primary producers. Then, we selected the best model using backward elimination of fixed-effect terms in the linear mixed-effect models. More specifically, we used the lmer() and step() functions in the lmerTest R package, using Satterthwaite's method for degrees of freedom and t-statistics (Kuznetsova et al. 2017). We also analyzed the Variance Inflation Factor (VIF) for the fixed factors to avoid high multicollinearity between predictors.
We analyzed differences in pCO2,w and CO2 exchange(indirect) following the same procedure described for stream metabolism. For pCO2, w the fixed factors were the ones related to metabolic activity (GPP, ER), gas solubility (water temperature), alkalinity, and gas transfer (discharge and percent of the stream reach covered by macrophytes); and the random factor was again ecoregion. For CO2 exchange (indirect), the fixed factors were k600 and pCO2 and the random effect was ecoregion. In this latter case, we also analyzed the variance explained by each predictor, using the partR2() function in the partR2 R package (Stoffel et al. 2021).
Finally, we examined the spatial heterogeneity in CO2 exchange within each stream using linear mixed-effect models with CO2 exchange (direct) as the dependent variable, habitat type as fixed factor [five levels: open water with high (i), medium (ii), or low (iii) current velocity, and emergent (iv) and submerged (v) macrophytes], and stream as random factor.
In all cases, we analyzed the distribution of the residuals visually inspecting the histograms and performing Shapiro–Wilk test, accepting normality only when p > 0.05. We also analyzed homogeneity of variances by plotting the model's residuals against fitted response values. These model assumptions (i.e., normal distribution of the residuals and homoscedasticity) were violated by the pCO2, w and CO2 exchange (direct) models, so these variables were log-transformed. Additionally, when models included predictor variables on very different scales (i.e., models for ER and NEP), variables were standardized using a z-score normalization.
Results
Physicochemical characteristics
The study streams differed widely in water physicochemical characteristics, with ecoregion driving some of these differences (Table 1, Fig. 2). The first component of the PCA explained 28.8% of the variance. This PCA component was largely driven by site D18, which appeared far from the rest of the sites mainly because of its extremely high DIN concentration and maximum values of FI, BIX, and macrophyte cover. The second component of the PCA accounted for 27.8% of the variance. According to this PCA component, the tributaries of the Atlantic Ocean (D sites) were characterized by higher discharge, higher percent of agricultural land use in their basin, and higher SRP concentration with respect to the streams from the Salado basin (A sites) and tributaries of the Paraná and Río de la Plata (C sites).
Principal component analysis (PCA) of selected variables. The percent values on each axis represent the amount of variance explained by each PCA component. See Table 1 and Supplementary Table 1 for a more detailed description of the variables included in the PCA
Metabolism
Metabolic rates varied considerably among the study sites (Fig. 3 and Supplementary Table 2). GPP ranged from 0.4 to 6.7 g O2 m−2 d−1 and ER from 2.1 to 11.9 g O2 m−2 d−1. NEP ranged from – 10.2 to 1.6 g O2 m−2 d−1, with 8 sites being net heterotrophic, two sites (A4 and C5) in balance, and only one site (D15) being net autotrophic.
Environmental drivers of metabolism differed among processes (Table 2). None of the explanatory fixed variables had a significant effect on GPP. However, there were differences among ecoregions, GPP being higher in the Paraná & Río de la Plata region (C sites). These differences explained 65.5% of the variance in GPP (Table 2). For ER, the best model (75.5% variance explained) included SUVA and the percent of the stream reach covered by primary producers (Table 2). Both were positively related to ER: the higher the content of aromatic carbon and the cover by primary producers, the higher the ER. Overall, ER was highest in the tributaries of the Atlantic Ocean (D sites) and lowest in the Salado River and tributaries (A sites), ecoregion accounting for 16.4% of the variance. Results for NEP were consistent, the final model (74.8% variance explained) including SUVA and the percent of the stream reach covered by primary producers (Table 2). In this case, the higher the content of aromatic carbon and the cover by primary producers, the lower the NEP (i.e., the higher the degree of heterotrophy). There were no significant differences among ecoregions (variance explained < 3%).
pCO2 and CO2 exchange
All streams were CO2 supersaturated, with pCO2,w values ranging from 1255 to 9999 µatm (Fig. 4a and Supplementary Table 2). Both alkalinity and GPP had a significant effect on pCO2,w: the higher the alkalinity the higher the pCO2,w, and the higher the GPP the lower the pCO2,w (Table 2). This model explained 52.3% of the variance, ecoregion accounting for none of it.
Mean and standard deviation of a the partial pressure of CO2 in water (pCO2,w) and b the CO2 exchange estimated with the indirect approach (CO2 exchange indirect)) in the sampled streams. Note that some streams (A3, A4, and D16) have no CO2 efflux due to the lack of k600 estimates. Also, note that some standard deviations are so small that they are hidden in the bar charts
Supersaturation was reflected in positive CO2 exchange (i.e., all streams were net emitters of CO2 to the atmosphere), with CO2 exchange (indirect) values ranging from 29.1 to 367.6 mmol m−2 d−1 (Fig. 4b and Supplementary Table 2). As expected, both predictor variables introduced in the model (k600 and pCO2,w) had a significant positive effect on CO2 exchange (Table 2, Fig. 5). However, k600 explained a higher proportion of the variance than pCO2,w (63.7% and 21.9%, respectively).
The spatial variability in CO2 exchange within stream reaches was substantial (Fig. 6). CO2 exchange(direct) tended to be higher in sub-reaches with high current velocity and lower in sub-reaches with presence of emerged macrophytes, variability being higher within other sub-habitat types (i.e., medium and low current velocity, and submerged macrophytes; Table 3, Fig. 6).
Discussion
Our results confirmed that all study streams were CO2 supersaturated and net emitters of CO2. Internal metabolism contributed to CO2 dynamics, although external DIC inputs via groundwater were likely important drivers of pCO2,w. Despite generally high pCO2,w, the slow current velocities and low water turbulence characteristic of these streams restricted CO2 exchange with the atmosphere. Moreover, variability in both current velocity and coverage of primary producers enhanced the spatial heterogeneity of CO2 exchange within streams.
The fact that the study streams were CO2 supersaturated relative to the atmosphere and thus net emitters of CO2 was expected, as most streams and rivers behave in this manner, resulting in a substantial contribution to the global carbon cycle (Raymond et al. 2013; Lauerwald et al. 2015). Our model showed that pCO2,w increased with alkalinity and decreased with GPP, providing evidence that both external and internal sources of DIC drive pCO2,w variation in the study streams. On the one hand, the positive relationship between pCO2,w and alkalinity suggests that mineral weathering in the catchment is an important source of DIC, which enters streams via groundwater (Feijoó et al. 2018), and probably contributes to CO2 supersaturation in stream water (Marcé et al. 2015; Stets et al. 2017). In fact, alkalinity in the study streams varied between 3.79 and 11.08 meq L−1. On the other hand, the negative relationship between pCO2,w and GPP suggests that CO2 uptake via photosynthesis by primary producers (e.g., macrophytes and algae) can reduce to some extent the concentration of CO2 in stream water (Wallin et al. 2020). We might have expected CO2 undersaturation in streams that were net autotrophic (GPP > ER). However, all study streams except three were net heterotrophic, and even the only net autotrophic stream (D15) was CO2 supersaturated (pCO2,w = 2561 µatm) compared to the atmosphere. These observations suggest that relative to external sources (i.e., groundwater DIC inputs originated from soil respiration and mineral weathering in the catchment), internal metabolism might be a minor driver of CO2 concentration and exchange in these streams. This supposition is in line with previous studies, showing that internal metabolism typically accounts for a minor proportion of CO2 outgassing from streams to the atmosphere (Butman and Raymond 2011; Hotchkiss et al. 2015; Gómez-Gener et al. 2016; Hill et al. 2017; Demars 2019).
Results from metabolism models showed that there was a positive relationship between ER (and NEP) and the cover by primary producers. Thus, and contrary to our expectations, streams with a higher abundance of macrophytes showed a higher degree of heterotrophy. This could be explained by the presence of dense bacterial communities in benthic and epiphytic biofilms. Punctual estimations made in one of the study streams indicated that bacterial density in the epipelon and in the epiphyton–macrophyte complex can be high (2.17E + 08 and 1.37E + 07 cell L−1, respectively) (Ana Torremorell, pers. comm.). In addition, macrophytes tend to accumulate fine organic matter, which together with the decomposition of macrophyte tissues may enhance benthic respiration (O’Brien et al. 2014). Moreover, shading by macrophytes may limit algal productivity on sediments (Sand-Jlnsen et al. 1989). Together, these different macrophyte-linked processes could explain the heterotrophic character of the study streams despite the high irradiance and the abundance of primary producers.
ER was also positively related to the SUVA index, an indicator of the aromatic carbon content (Helms et al. 2008). Several studies have shown that aromatic compounds of high molecular weight can contribute to the pool of reactive DOC, representing a relevant component of the carbon cycle in streams (Kaplan and Cory 2016). Complex molecules with aromatic groups can be photodegraded to simple compounds, increasing the bioavailability of residual DOC that is not converted into CO2 (Fasching et al. 2014; Fovet et al. 2020). In addition, Jones et al. (2016) observed that DOM composed of humic materials is more sensitive to photodegradation. In Pampean streams, the relative contribution of humic materials to DOM is high (Messetta et al. 2018), and high irradiance conditions may favor the photodegradation of humic compounds that may be bioavailable to microbial communities. This could explain the strong relationship between ER and SUVA found in our study, and suggests that the presence of aromatic dissolved organic compounds in water fueled microbial respiration in these streams. Macrophytes may have contributed to the release of at least part of this colored DOM (Lürig et al. 2021).
Regarding CO2 exchange across the air–water interface of streams, as expected, we observed a significant effect of both k600 and pCO2. However, in our case, k600 explained significantly more variance than pCO2. This result was likely due to the extremely low turbulence of these low-gradient and slow-flowing streams (Feijoó and Lombardo 2007). The k600 values modeled in our study streams (0.61 to 4.47 m d−1) are in the lower range of k600 values reported in a recent review of gas exchange in streams (range = 0.100 to 4117.8 m d−1, mean = 45.0 m d−1, n = 718; Ulseth et al. 2019). Additionally, macrophytes can form dense mats, further hampering gas exchange with the atmosphere (Attermeyer et al. 2016). Under these conditions of extremely slow gas exchange, water pCO2 can be far from equilibrium with the atmosphere. The CO2 in the stream, which might be fueled by groundwater DIC inputs and, to a lesser extent, by internal ER, is not compensated by the output via turbulence-limited CO2 exchange with the atmosphere. Accordingly, as stated earlier, stream water becomes supersaturated with CO2, even in the net autotrophic streams. Thus, overall variation in CO2 is mostly governed by differences in turbulence, which is the limiting factor. In fact, changes in turbulence did not only explain variation among study sites, but also variation within sites. In each stream, current velocity and the presence of macrophytes controlled the spatial heterogeneity in CO2 exchange. Consequently, we observed highest CO2 exchange values in open water areas with the highest flow velocity, and lowest CO2 exchange values in areas with emergent macrophytes.
Several studies have reported that macrophyte mats, and especially floating species, act as important sinks of CO2 (O’Brien et al. 2014; Attermeyer et al. 2016; Peixoto et al. 2016). However, although generally lower than in other parts of the streams, we always observed net emission of CO2 above macrophyte mats. In our study, macrophytes were predominantly submerged (Myriophyllum sp., Elodea nuttalli, and Stuckenia striata) and emergent (Ludwigia peploides, and Hydrocotyle ranunculoides) species, and only in one spot of one stream (D18), we measured CO2 flux on a floating species (Lemna minima). As stated earlier, macrophytes may have strongly contributed to in-stream pCO2 in the studied streams through their attached heterotrophic epiphytic biofilms, their shading effect on benthic algae, and the enhancement of benthic respiration via decomposing organic matter (Sand-Jlnsen et al. 1989; O’Brien et al. 2014; Levi et al. 2017). It is worth mentioning here that the contribution of macrophytes to CO2 emissions may be even larger after their senescence, when large quantities of their organic tissues are decomposed within these streams.
The relative abundance of the different primary producers could also determine CO2 fluxes. Although it was not included in the analysis, because it was a single case, we observed that in one spot of the A2 stream, where algae and cyanobacteria were dominating, CO2 was negative, suggesting that uptake was higher than release because of photosynthesis. Consequently, the dynamics of CO2 exchange in the air–water interface would also be influenced by the type (algae vs. macrophytes) and specific composition (in the case of macrophytes) of the dominant autotrophic community in the stream reach.
In conclusion, our findings provide interesting insights on the role of Pampean streams in CO2 fluxes, which could be representative of other lowland streams elsewhere in the world. Overall, our results indicate that CO2 emission in these extremely low turbulence streams is controlled by a combination of external and internal biogeochemical processes and limited atmospheric exchange (Aho et al. 2021). External groundwater inputs of DIC might be more important than internal metabolic processes in determining CO2 concentration and fluxes at the air–water interface in these lowland streams. However, more detailed studies considering mass–balance approaches and groundwater contributions are needed to elucidate the relative importance of these and other processes (e.g., anaerobic metabolism and calcite precipitation) for controlling CO2 dynamics in these ecosystems. Second, open-canopy streams with dense macrophyte cover may not necessarily act as net sinks of CO2. Primary production by macrophytes may not be able to counteract the effect of external DIC inputs. In addition, the degree of heterotrophy and the presence of abundant microbial communities may also determine whether they are net sinks or emitters of CO2 to the atmosphere. Finally, CO2 exchange does not only vary significantly among different streams but also within the same stream reach. In this study, CO2 exchange was greatly influenced by reach heterogeneity, which is generated by flow variability and by the different algal, bacterial, and macrophyte communities. This highlights the need of measuring CO2 fluxes in multiple sites within the same reach to represent the hydrological and biological heterogeneity. Similarly, it is fundamental that future studies cover broader time scales. There is a strong seasonal and diel variability in the metabolic activity of biological communities in Pampean streams (García et al. 2017; Martí et al. 2020), which, together with temporal changes in external sources, is expected to also generate high temporal variability in CO2 concentrations. In this study, we measured CO2 fluxes between 12 and 16 h on 1 day of the most productive season in each stream, when CO2 concentration could be expected to be lowest in these streams. Therefore, it is crucial that future studies more accurately account for both spatial and temporal variability to better understand the role of these ecosystems in the carbon cycle.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. Any additional data are available from the corresponding author on request.
Code availability
The R code generated during the current study is available from the corresponding author on request.
References
Aho KS, Hosen JD, Logozzo LA et al (2021) Highest rates of gross primary productivity maintained despite CO2 depletion in a temperate river network. Limnol Oceanogr Lett 6:200–206. https://doi.org/10.1002/lol2.10195
Alin SR, Rasera MDFFL, Salimon CI et al (2011) Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J Geophys Res Biogeosci. https://doi.org/10.1029/2010JG001398
Allen GH, Pavelsky TM (2018) Global extent of rivers and streams. Science 361:585–588
Alnoee AB, Levi PS, Baattrup-Pedersen A, Riis T (2021) Macrophytes enhance reach-scale metabolism on a daily, seasonal and annual basis in agricultural lowland streams. Aquat Sci 83:1–12
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association
Appling AP, Hall RO, Arroita M, Yackulic CB (2017) streamMetabolizer: Models for estimating aquatic photosynthesis and respiration. https://github.com/USGS-R/streamMetabolizer/tree/v0.10.1. Accessed 15 Sept 2020
Appling AP, Hall RO, Yackulic CB, Arroita M (2018) Overcoming equifinality: Leveraging long time series for stream metabolism estimation. J Geophys Res G Biogeosci 123:624–645
Attermeyer K, Flury S, Jayakumar R et al (2016) Invasive floating macrophytes reduce greenhouse gas emissions from a small tropical lake. Sci Rep 6:1–10. https://doi.org/10.1038/srep20424
Aufdenkampe AK, Mayorga E, Raymond PA et al (2011) Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front Ecol Environ 9:53–60. https://doi.org/10.1890/100014
Bernhardt ES, Heffernan JB, Grimm NB et al (2018) The metabolic regimes of flowing waters. Limnol Oceanogr 63:S99–S118. https://doi.org/10.1002/lno.10726
Bodmer P, Heinz M, Pusch M et al (2016) Carbon dynamics and their link to dissolved organic matter quality across contrasting stream ecosystems. Sci Total Environ 553:574–586. https://doi.org/10.1016/j.scitotenv.2016.02.095
Butman D, Raymond PA (2011) Significant efflux of carbon dioxide from streams and rivers in the United States. Nat Geosci 4:839–842. https://doi.org/10.1038/ngeo1294
Cole JJ, Prairie YT, Caraco NF et al (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10:172–185
Colt J (2012) Computation of dissolved gas concentration in water as functions of temperature, salinity and pressure, 2nd edn. Elsevier
Cory RM, McKnight DM (2005) Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ Sci Technol 39:8142–8149
Demars BOL (2019) Hydrological pulses and burning of dissolved organic carbon by stream respiration. Limnol Oceanogr 64:406–421. https://doi.org/10.1002/lno.11048
Duarte CM, Prairie YT (2005) Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 8:862–870
Fasching C, Behounek B, Singer GA, Battin TJ (2014) Microbial degradation of terrigenous dissolved organic matter and potential consequences for carbon cycling in brown-water streams. Sci Rep 4:4981
Feijoó CS, Lombardo RJ (2007) Baseline water quality and macrophyte assemblages in Pampean streams: a regional approach. Water Res 41:1399–1410. https://doi.org/10.1016/j.watres.2006.08.026
Feijoó C, Giorgi A, Ferreiro N (2011) Phosphate uptake in a macrophyte-rich Pampean stream. Limnologica 41:285–289. https://doi.org/10.1016/j.limno.2010.11.002
Feijoó C, Messetta ML, Hegoburu C, Gómez-Vázquez A, Guerra-López J, Mas-Pla J, Rigacci L, García V, Butturini A (2018) Retention and release of nutrients and dissolved organic carbon in a nutrient-rich stream: a mass balance approach. J Hydrol 566:795–806. https://doi.org/10.1016/j.jhydrol.2018.09.051
Fellman JB, Hood E, Spencer RGM (2010) Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: a review. Limnol Oceanogr 55:2452–2462. https://doi.org/10.4319/lo.2010.55.6.2452
Fovet O, Cooper DM, Jones DL, Jones TG, Evans CD (2020) Dynamics of dissolved organic matter in headwaters: comparison of headwater streams with contrasting DOM and nutrient composition. Aquat Sci 82:29. https://doi.org/10.1007/s00027-020-0704-6
Frenguelli J (1956) Rasgos generales de la hidrografía de la Provincia de Buenos Aires. LEMIT II:1–19
García HR, Gordon LI (1992) Oxygen solubility in seawater: better fitting equations. Limnol Oceanogr 37:1307–1312
García VJ, Gantes P, Giménez L et al (2017) High nutrient retention in chronically nutrient-rich lowland streams. Freshw Sci 36:26–40. https://doi.org/10.1086/690598
Gardolinski PCFC, Hanrahan G, Achterberg EP et al (2001) Comparison of sample storage protocols for the determination of nutrients in natural waters. Water Res 35:3670–3678. https://doi.org/10.1016/S0043-1354(01)00088-4
Goldenfum JA (2010) GHG measurement guidlines for freshwater reservoirs. IHA, Sutton
Gómez-Gener L, Schiller D, Marcé R et al (2016) Low contribution of internal metabolism to carbon dioxide emissions along lotic and lentic environments of a Mediterranean fluvial network. J Geophys Res Biogeosci 121:3030–3044. https://doi.org/10.1002/2016JG003549
Gordon ND, McMahon TA, Finlayson BL (1992) Stream hydrology. Wiley, Chichester
Green SA, Blough NV (1994) Optical absorption and fluorescence properties of chromophoric dissolved organic matter in natural waters. Limnol Oceanogr 39:1903–1916
Hall RO, Ulseth AJ (2020) Gas exchange in streams and rivers. Wiley Interdiscip Rev Water 7:e1391
Hall RO, Tank JL, Baker MA et al (2016) Metabolism, gas exchange, and carbon spiraling in rivers. Ecosystems 19:73–86. https://doi.org/10.1007/s10021-015-9918-1
Helms JR, Stubbins A, Ritchie JD et al (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53:955–969
Hill BH, Elonen CM, Herlihy AT et al (2017) A synoptic survey of microbial respiration, organic matter decomposition, and carbon efflux in US streams and rivers. Limnol Oceanogr 62:S147–S159
Hoellein TJ, Bruesewitz DA, Richardson DC (2013) Revisiting Odum (1956): a synthesis of aquatic ecosystem metabolism. Limnol Oceanogr 58:2089–2100
Holtgrieve GW, Schindler DE, Branch TA, A’mar ZT, (2010) Simultaneous quantification of aquatic ecosystem metabolism and reaeration using a Bayesian statistical model of oxygen dynamics. Limnol Oceanogr 55:1047–1063
Hotchkiss ER, Hall RO Jr, Sponseller RA et al (2015) Sources of and processes controlling CO2 emissions change with the size of streams and rivers. Nat Geosci 8:696
Hughet A, Vacher L, Relexans S et al (2009) Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org Geochem 40:706–719
Johnson RK, Hering D (2009) Response of taxonomic groups in streams to gradients in resource and habitat characteristics. J Appl Ecol 46:175–186
Jones JB, Mulholland PJ (1998) Carbon dioxide variation in a hardwood forest stream: an integrative measure of whole catchment soil respiration. Ecosystems 1:183–196. https://doi.org/10.1007/s100219900014
Jones TG, Evans CD, Jones DL, Hill PW, Freeman C (2016) Transformations in DOC along a source to sea continuum; impacts of photo-degradation, biological processes and mixing. Aquat Sci 78:433–446. https://doi.org/10.1007/s00027-015-0461-0
Kaplan LA, Cory RM (2016) Dissolved organic matter in stream ecosystems: forms, functions, and fluxes of watershed Tea. In: Stream ecosystems in a changing environment. Elsevier, London, pp 241–320
Kothawala DN, Murphy K, Stedmon CA et al (2013) Inner filter correction of dissolved organic matter fluorescence. Limnol Oceanogr Methods 11:616–630
Kuznetsova A, Brockhoff PB, Christensen RHB et al (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26
Lauerwald R, Laruelle GG, Hartmann J et al (2015) Spatial patterns in CO2 evasion from the global river network. Glob Biogeochem Cycles 29:534–554
Levi PS, Starnawski P, Poulsen B et al (2017) Microbial community diversity and composition varies with habitat characteristics and biofilm function in macrophyte-rich streams. Oikos 126:398–409
Lu Y, Bauer JE, Canuel EA et al (2013) Photochemical and microbial alteration of dissolved organic matter in temperate headwater streams associated with different land use. J Geophys Res Biogeosci 118:566–580
Lürig MD, Best RJ, Dakos V, Matthews B (2021) Submerged macrophytes affect the temporal variability of aquatic ecosystems. Freshw Biol 66:421–435
Marcé R, Obrador B, Morguí J-A et al (2015) Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat Geosci 8:107–111
Martí E, Feijoó C, Vilches C et al (2020) Diel variations of nutrient retention and metabolism are coupled for ammonium but not for phosphorus in a lowland stream. Freshw Sci 39:268–280
Marx A, Dusek J, Jankovec J et al (2017) A review of CO2 and associated carbon dynamics in headwater streams: a global perspective. Rev Geophys 55:560–585. https://doi.org/10.1002/2016RG000547
Marzolf NS, Ardón M (2021) Ecosystem metabolism in tropical streams and rivers: a review and synthesis. Limnol Oceanogr 66:1627–1638
Matteucci SD (2012) Ecorregión Pampa. In: Morello J et al (eds) Ecorregiones y complejos ecosistémicos argentinos. Editorial Orientación Gráfica Argentina, pp 391–445
Messetta ML, Hegoburu C, Casas-Ruiz JP, Butturini A, Feijoó C (2018) Characterization and qualitative changes in DOM chemical characteristics related to hydrologic conditions in a Pampean stream. Hydrobiologia 808:201–217
O’Brien JM, Lessard JL, Plew D et al (2014) Aquatic macrophytes alter metabolism and nutrient cycling in lowland streams. Ecosystems 17:405–417. https://doi.org/10.1007/s10021-013-9730-8
Odum HT (1956) Primary production in flowing waters. Limnol Ocean 1:102–117
Oksanen J, Guillaume Blanchet F, Friendly M, et al (2020) vegan: Community Ecology Package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan. Accessed 15 Sept 2020
Peixoto RB, Marotta H, Bastviken D, Enrich-Prast A (2016) Floating aquatic macrophytes can substantially offset open water CO2 emissions from tropical floodplain lake ecosystems. Ecosystems 19:724–736. https://doi.org/10.1007/s10021-016-9964-3
Pu J, Li J, Khadka MB et al (2017) In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream. Sci Total Environ 579:1343–1355. https://doi.org/10.1016/j.scitotenv.2016.11.132
R Core Team (2021) R: a language and environment for statistical computing. The R Foundation
Raymond PA, Zappa CJ, Butman D et al (2012) Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnol Oceanogr Fluids Environ 2:41–53
Raymond P, Hartmann J, Lauerwald R et al (2013) Global carbon dioxide emissions from inland waters. Nature 503:355–359. https://doi.org/10.1038/nature12760
Regnier P, Friedlingstein P, Ciais P et al (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 6:597–607. https://doi.org/10.1038/ngeo1830
Sala JM, González N, Kruse E (1983) Generalización hidrológica de la Pcia. de Buenos Aires. En: Coloquio Internacional de Hidrología de Grandes Llanuras. Azul, pp 974–1009
Sand-Jlnsen KAJ, Jeppesen E, Nielsen K et al (1989) Growth of macrophytes and ecosystem consequences in a lowland Danish stream. Freshwat Biol 22:15–32
Spencer RGM, Coble PG (2014) Design for organic matter fluorescence analysis in aquatic environments. In: Coble P, Lead J, Baker A et al (eds) Aquatic organic matter fluorescence. Cambridge University Press, pp 125–147
Stets EG, Butman D, McDonald CP et al (2017) Carbonate buffering and metabolic controls on carbon dioxide in rivers. Global Biogeochem Cycles 31:663–677
Stoffel MA, Nakagawa S, Schielzeth H (2021) partR2: Partitioning R2 in generalized linear mixed models. PeerJ 9:e11414
Tank JL, Rosi-Marshall EJ, Griffiths NA et al (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J North Am Benthol Soc 29:118–146
Team SD (2016) Stan modeling language users guide and reference manual. Team SD
Uehlinger U, Naegeli MW (1998) Ecosystem metabolism, disturbance, and stability in a prealpine gravel bed river. J North Am Benthol Soc 17:165–178. https://doi.org/10.2307/1467960
Ulseth AJ, Hall RO, Boix Canadell M et al (2019) Distinct air–water gas exchange regimes in low- and high-energy streams. Nat Geosci 12:259–263. https://doi.org/10.1038/s41561-019-0324-8
Wallin MB, Audet J, Peacock M et al (2020) Carbon dioxide dynamics in an agricultural headwater stream driven by hydrology and primary production. Biogeosciences 17:2487–2498
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Acknowledgements
We thank GERSOLAR and María José De Negri for providing radiation and PAR data, and INTA Balcarce and INTA Miramar for meteorological data. We also thank the Universidad Nacional de Luján for providing vehicle and driver (special thanks to Mario Pighin and Nacho Casas). Special thanks to Adonis Giorgi for helping with the sampling campaign and the methodology. Comments from the Associate Editor and the reviewers greatly helped to improve the manuscript.
Funding
DvS. is a Serra Húnter Fellow and was additionally supported by the Catalan Government through the FORESTREAM (Forest and Stream Ecological Links: Watershed Management and Restoration) grant (2017 SGR 976). The study was funded by CONICET through two grants (Subsidio de financiamiento para Reuniones Científicas y Tecnológicas 2018 and P-UE0017).
Author information
Authors and Affiliations
Contributions
Conceptualization: DvS and CF. Methodology: DvSc and MA. Formal analysis and investigation: CF, MA, DvS, LM, JA, and LR. Writing—original draft preparation: CF, MA, and DvS; writing—review and editing: MLM, JA, and LR. Funding acquisition: CF; resources: DvS and CF. Supervision: DvS.
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feijoó, C., Arroita, M., Messetta, M.L. et al. Patterns and controls of carbon dioxide concentration and fluxes at the air–water interface in South American lowland streams. Aquat Sci 84, 23 (2022). https://doi.org/10.1007/s00027-022-00852-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00852-9