Abstract
Shallow lakes are typically found in one of two stable states, a macrophyte-dominated clear water state or a turbid state due to excessive phytoplankton and suspended sediment. Whether shallow backwater lakes in large river floodplains exhibit similar alternate stable states is less understood. This study considers mechanisms, interactions and feedbacks associated with a shift in environmental conditions and biotic community structure in backwater lakes of a hydrologically dynamic floodplain river system. We use long-term data from backwater lakes to show an increase in submersed aquatic vegetation, improved water quality, and resulting shifts in the community structure of aquatic vegetation and fish following a 4 year period of summer low water discharge on the Upper Mississippi River. Backwater lakes in our study span a gradient of environmental conditions. Backwater lakes located in the upper reach of our study area were chronically turbid and support only sparse aquatic macrophytes, whereas those downriver exhibited clearer water and abundant vegetation. An increase in submersed aquatic vegetation in the lower backwater lakes resulted in a fish community shift to more vegetation-associated species. A lesser response in submersed aquatic vegetation abundance and fish community shift was observed in the upper, more turbid backwater lakes. The combination of vegetative cover and turbidity were key environmental variables associated with fish community structure in lower backwater lakes. Turbidity was the key environmental variable associated with submersed aquatic vegetation in both upper and lower backwaters. Providing further insight into the physical, chemical and biological interactions associated with ecological shifts will help guide management and restoration decisions towards more resilient, macrophyte-rich floodplain backwaters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concepts of ecological regime shifts, alternative stable states and ecosystem resilience were introduced decades ago (Lewontin 1969; Holling 1973; May 1977). In freshwater systems, classic examples of regime shifts take place in shallow lakes that alternate between a clear water state dominated by macrophytes and a turbid state characterized by phytoplankton and suspended sediment (Scheffer et al. 1993). Aquatic macrophytes in shallow lakes provide a feedback loop that enhances water clarity and improves conditions for growth. Mechanisms may include phytoplankton suppression from direct competition for nutrients or allelopathy (van Donk and van de Bund 2002), stabilization of bottom sediments (Dieter 1990; James et al. 2004), wind energy reduction (James and Barko 1994) and refuge from fish predation for zooplankton that consume phytoplankton (Timms and Moss 1984; Schriver et al. 1995). Without feedback mechanisms that provide resilience, shallow eutrophic lakes are susceptible to excessive phytoplankton growth and sediment resuspension, which will increase turbidity, reduce light penetration and may lead to the loss of submersed aquatic vegetation (SAV) and inhibit its reemergence. Aquatic macrophytes also provide structural habitat complexity and their presence can structure invertebrate (Chilton 1990; Beckett et al. 1992; Burdis and Hoxmeier 2011) and fish communities (Bettoli et al. 1993; Crowder and Cooper 1982). Thus aquatic macrophyte dynamics are key to the resilience of shallow lake systems existing in one stable state or another.
While many studies of freshwater regime shifts have reported on the relatively closed systems of shallow inland lakes, few studies have identified whether these sorts of ecological shifts occur in large rivers (but see Sparks et al. 1990; Ibáñez et al. 2012; Giblin 2017). Large floodplain-river systems often consist of an array of lateral off-channel areas including shallow backwater lakes (hereafter referred to as backwaters). Shifts in closed lake systems are generally thought to be biotically driven (Dent et al. 2002); however, the dynamics of biotic and abiotic drivers of aquatic macrophytes in backwaters of a large floodplain river are less understood. Backwaters are diverse and may be driven by both physical and biological mechanisms. Water discharge has been described as a master variable that controls many processes in river ecosystems (Doyle et al. 2005). Discharge directly affects current velocity, water residence time, water elevation, depth, and sediment and nutrient loading. While discharge regime and associated physical attributes may be important to macrophytes in river systems, consideration must also be given to the potential of biotic interactions and feedback (Dent et al. 2002; Franklin et al. 2008).
Most large rivers in the world have been altered by human activity (Gore and Shields 1995) including the Upper Mississippi River (UMR) (Sparks et al. 1998). Hydrological characteristics of the UMR have been modified by a low-head lock and dam system built for commercial navigation and levees for flood protection. Inundation of large areas of the floodplain caused by the lock and dam system created new and productive backwater habitat of ecological and recreational importance (Fremling 2005). However, over time some backwaters began to lose aquatic macrophytes and became turbid, unvegetated lakes vulnerable to wind and wave energy. In addition, much of the UMR basin has been converted to intensive agriculture resulting in water quality impairments due to excessive nutrients and sediments associated with row crop agriculture (Tuner and Rabalais 2003) raising concerns of cumulative effects on the state of the river (Bouska et al. 2018). Despite these alterations and stressors to the system, much of the floodplain remains connected in upper reaches of the river providing a complex assemblage of habitats that support diverse biological communities (Bouska et al. 2018).
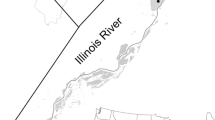
Our study area on the UMR contains a long mainstem natural riverine lake that retains suspended material, substantially improving water clarity downriver (Houser et al. 2010). Long-term data from contrasting environmental conditions upriver and downriver of the mainstem lake provide a unique opportunity to study long term ecological dynamics of two different groups of shallow, floodplain backwaters located in close proximity. The objective of this study was to use our 23 years of monitoring data to illustrate that the macrophyte-turbidity relationship identified by Scheffer et al. (1993) as driving dynamics in shallow inland lakes is also present in backwaters of a large floodplain river system. We examined spatiotemporal responses in water quality, SAV, and patterns in fish community structure to a period of low water discharge. Lastly, we attempt to identify whether there are a subset of particularly important environmental variables associated with changes in SAV and fish community composition.
Methods
Study area
Pool 4 of the UMR (Fig. 1) is one of 29 pools created primarily by a low-head lock and dam system built for navigation. The floodplain remains mostly connected to the mainstem river and has an extensive off-channel system of side channels and shallow backwaters. Pool 4 is unique among the navigation pools in that a 35 km long mainstem natural riverine lake, Lake Pepin, is located in the middle and separates the pool into an upper and lower reach of which each are about 19 km long. There are large physical and biological differences between the two reaches due in part to Lake Pepin, which creates a semi-lentic environment that retains suspended material, improving water clarity downriver by twofold (Houser et al. 2010). Backwaters in the upper reach are chronically turbid during most of the year and aquatic vegetation is sparse, whereas the lower reach backwaters have abundant aquatic vegetation and clearer water. The lock and dam system has stabilized water elevations relative to the historical, unconstrained river (Theiling and Nestler 2010), especially at the lower ends of the pool. However, a gradient of water elevation fluctuation now extends through the pool so that when water discharge increases, the upper backwaters experience much greater water level fluctuations than lower backwaters.
Sampling design
Data were collected as part of a multi-agency cooperative partnership known as Upper Mississippi River Restoration program’s Long Term Resource Monitoring element https://www.umesc.usgs.gov/ltrm-home.html. Water quality, aquatic vegetation and fish have been sampled annually in Pool 4 since the early 1990’s. All three monitoring components utilized a stratified random sampling (SRS) design whereby strata represent the major habitat types on the UMR. Water quality SRS is conducted during four seasonal episodes that generally occur within a 2 week window (winter—late January, spring—late April, summer—late July, fall—early October). Fish were sampled over three SRS episodes during the midsummer (June 15th–July 31st); late summer (August 1st–September 15th) and fall (September 16th–October 31st). SAV was sampled during one midsummer episode (June 15th–August 15th). In addition, water quality was sampled at 14 fixed site locations on a bi-weekly to monthly frequency, depending on time of the year. Standardized sampling was conducted from 1993 through 2015 for the fish and water quality components and from 1998 through 2015 for the vegetation component. For this study, only data collected from the backwater stratum was used in the analysis where backwaters upriver of Lake Pepin were identified as upper backwaters and those below Pepin as lower backwaters.
Fifty random backwater sites were allocated for water quality sampling during each seasonal SRS episode (Soballe and Fischer 2004). Water temperature and current velocity were taken in situ. Water grab samples were collected for laboratory analysis of turbidity, chlorophyll-a (CHL), total suspended solids (TSS), volatile suspended solids (VSS), total phosphorus (TP) and total nitrogen (TN).
A minimum of 200 backwater sites per year over the 23 years of the study were allocated for standardized annual aquatic vegetation sampling using a rake method (Yin et al. 2000). At each site, six subsites, each approximating a quadrat of 150 cm long by 35 cm wide (i.e. width of rake), were sampled around the perimeter of the boat. Sampling included an initial visual scan of each quadrat followed by the dragging of a two-sided rake along the sediment. All species observed during the visual scan were recorded as present. The relative abundance of SAV species captured in each rake was assigned a six-level ordinal plant density score where increasing values represented increasing thickness of plant material on the rake teeth. If plants were not captured, the sample received a plant density score of 0. If submersed plants were captured the entire sample was assigned a value of 1, 2, 3, 4 or 5 which corresponded to the amount of plant material on the rake.
For each of the 23 years of the study a minimum of 42 sites were allocated in backwaters for fish sampling during the late summer (August 1st–September 15th) and fall (September 16th–October 31st) episodes. The fish community was sampled using fyke nets, mini-fyke nets, and day electrofishing. In addition, a visual assessment of percent vegetation coverage at each site was assigned one of four categorical values (0 = 0, 1 = 1–50%, 2 = 51–75%, 3 = 76–100%) (Ratcliff et al. 2014).
Data analysis
Environmental conditions
Multiple independent environmental variables were analyzed for spatial and temporal trends, and were used as explanatory variables in multivariate analyses of biological community structure (Table 1). Environmental variables were obtained from three sources: routine water quality monitoring, lock and dams (i.e., discharge, elevation), or collected as part of fish sampling (i.e., vegetation cover). Median values of environmental variables were used in most multivariate analyses, except for water elevation, which used calculated coefficient of variation, and vegetation cover, where categorical data was averaged. P < 0.05 was set as the level of significance for all statistical analyses.
To justify separation of upper and lower backwaters for analysis, environmental data were analyzed using multivariate techniques with PRIMER 6 and PERMANOVA + software (PRIMER_E Ltd. Plymouth, UK). Principle component analysis (PCA) was used to examine spatiotemporal patterns using a subset of environmental data (Table 1). To test for significant environmental differences between upper and lower backwaters a one-way permutational multivariate analysis of variance (PERMANOVA) was performed. Data were transformed when necessary to approximate normality.
Water quality trends
Water quality variables collected as part of routine monitoring (Table 1) were analyzed for monotonic trends over the period of study (1993–2015) with SAS software version 9.4 (SAS Institute Inc. Cary, NC, USA). Kendall’s tau test (Kendall 1975) was used to test for significant trends and the associated Sen’s slope estimates (Sen 1968) were used to capture the magnitude of the trend. Data were not summarized or transformed for these nonparametric tests. Sites were grouped into upper or lower backwaters for analysis.
Submersed aquatic vegetation
The annual percent frequency of occurrence of all SAV and individual species were calculated for both upper and lower backwaters as the number of sites where observed divided by the number of sample sites. In addition, SAV biomass at each site was estimated based on a quadratic relationship between rake density scores and fresh weight (Drake and Lund 2020). SAV community structure was analyzed using multivariate techniques with PRIMER software version 6. Annual percent frequency of occurrence of thirteen commonly occurring SAV species were square root transformed prior to the creation of a Bray–Curtis similarity matrix and analysis with non-metric multidimensional scaling (NMDS) (Clarke 1993). Cluster analysis along with similarity profile permutation tests (SIMPROF) were used to identify groups of years that were significantly similar based on SAV community structure. Similarity percentages method (SIMPER) (Clarke and Gorley 2006) was performed to determine individual SAV species most responsible for differences between year groupings identified in cluster analysis. Lastly, the BIOENV method (Clarke and Gorley 2006) was performed to identify environmental variables that may explain SAV community structure and patterns using relevant available data (Table 1).
Fish
Fish community structure was also analyzed using multivariate techniques with PRIMER. In order to analyze multiple gear types together, a multi-gear mean standardization method (Gibson-Reinemer et al. 2016) was used to combine the catch per unit effort of fyke nets, mini-fyke nets and electrofishing into a single index of abundance (hereafter referred to as CPUE). A total of 46 fish species were included in the community analysis. Rare species captured at a frequency of less than 10% over the study period were excluded from this analysis based on the findings of Poos and Jackson (2012). Fish CPUE data were square root transformed prior to the creation of a Bray–Curtis similarity matrix to minimize the impact of more abundant species. NMDS was performed using annual mean abundances calculated from late summer and fall sampling episodes.
Cluster analysis and SIMPROF were used to identify groups of years that were significantly similar based on fish community structure within upper and lower backwaters. To determine if there were significant differences in the fish community structure between upper and lower backwaters a one-way analysis of similarity (ANOSIM) was performed. The ANOSIM procedure provides an R statistic, which is a comparative measure of the degree of separation between communities (Clarke and Warwick 2001). Values of R near zero indicate assemblages are similar and support the null hypothesis of no differences between communities, whereas values close to 1 indicate greater differences. Next, SIMPER was performed to determine the individual species responsible for the differences between the groups identified in the cluster analysis and in differences in the fish community between the upper and lower backwaters. Finally, BIOENV was performed to identify environmental variables that may explain fish community structure and patterns using relevant available data (Table 1).
Results
Environmental and hydrologic conditions
PCA biplot of environmental variables revealed a distinct spatial pattern separating upper and lower backwaters (Fig. 2) that was significant based on PERMANOVA analysis (P = 0.001). Vegetation cover, turbidity and water elevation variability contributed the most to the first principle component in PCA analysis, which accounted for 47% of the total variance (Table 2). Vegetation cover was most associated with lower backwaters, while turbidity and water elevation variability were most associated with upper backwaters. The environmental spatial pattern was consistent over the study period, aside from one outlier year that included flood-level conditions, justifying separation of upper and lower backwaters for community analysis of SAV and fish.
Principal component biplot for environmental variables illustrating the relationship between upper and lower backwaters. Variables are abbreviated as follows: chlorophyll-a (Chl), total phosphorus (TP), total nitrogen (TN), turbidity (Turb), water elevation (Elv), current velocity (Vel), vegetation cover (Veg)
Pool 4 of the UMR experienced unusually low water discharge (below 50% of the 23 year median) during the summer months of 2006, 2007 and 2009 (Fig. 3a). Tailwater elevation at Lock and Dam 3, which indicate water elevation conditions in upper backwaters, were lower and less variable during those same three years (Fig. 3b). Water elevation near Lock and Dam 4, which indicate conditions in lower backwaters, were more stable over the entire study period. With the exception of the low water discharge period, upper backwaters experienced more variability in water elevation than lower backwaters. Current velocity in backwaters is positively correlated to water discharge at the lock and dams. This relationship was most noticeable in the lower backwaters when summer median current velocity was zero from 2005 through 2010 (Fig. 4a).
Water quality differed between the upper and lower backwaters over the 23-year period of record (Table 3; Fig. 4). Turbidity, TSS, and CHL concentrations during summer SRS episodes were approximately three times higher in the upper backwaters compared to the lower. Nutrient concentrations were also higher in the upper backwaters; total nitrogen and phosphorus were over 15% greater on average.
Water quality trends
Water quality trends (Table 3) included a decrease in turbidity and TSS in both upper and lower backwaters over the study period. In lower backwaters, a decrease in VSS, CHL (Fig. 4b) and turbidity (Fig. 4c) occurred in 2007. There was also an obvious decrease in turbidity at a fixed site that coincided with the increase in SAV (Fig. 5). Total phosphorus concentrations had no significant change in upper backwaters and a slight decrease in lower backwaters. There was a similar decrease in total nitrogen in both upper and lower backwaters.
Annual summer (Jun–Sep) turbidity (box and whiskers) and percent frequency of occurrence of submersed aquatic vegetation (SAV; solid circles and line) in a lower backwater. Box and whiskers represent the median, 5th, 25th, 75th and 95th percentiles. Turbidity data is from a fixed site and SAV is from random sampling sites within this backwater
Submersed aquatic vegetation
Seventeen species of SAV were identified in Pool 4 over the study period, the two most common being coontail (Ceratophyllum demersum) and Canadian waterweed (Elodea canadensis; Table 4). Four species were very rare, found at less than 5% of sites and only in lower backwaters: northern watermilfoil (Myriophylum sibiricum), alpine pondweed (Potamogeton alpinus), common bladderwort (Utricularia macrorhiza), and clasping-leaf pondweed (Potamogeton richardsonii). Annual SAV species richness increased in upper and lower backwaters during and following the low discharge years (Fig. 6a). However, total and annual species richness was greater in lower backwaters (17 total species; 11–13 per year) than in upper backwaters (11 total species; 2–10 per year).
Frequency of occurrence of SAV in lower backwaters increased sharply in 2005 (from 65 to 80%), continued to increase through the low discharge years (2006–2009) and exceeded 80% for the remainder of the study period (Fig. 6b). Frequency of occurrence in the upper backwaters increased sharply in 2007 (from 8 to 23%) and continued to increase steadily through the low discharge years to over 50% (Fig. 6b). The proportion of sites where SAV was observed was greater in lower backwaters during all years though both reaches exhibited similar patterns over the entire study period. Annual SAV biomass estimates generally followed the same increasing and decreasing patterns as percent frequency of occurrence. However, biomass estimates revealed a more pronounced contrast between upper and lower backwaters where annual mean biomass ranged from 4 to 16 times greater in lower backwaters and followed a similar increasing pattern through the low discharge years (Fig. 6c). The very large increase in SAV biomass and percent frequency of occurrence in 2005 coincided with a decrease in CHL (Fig. 4b) and turbidity (Fig. 4c) in lower backwaters.
Community structure of SAV shifted in both upper and lower backwaters (Fig. 7). SIMPROF analysis identified 3 distinct SAV clusters in lower backwaters: 1998–2004, 2005–2007, and 2008–2015 with the exception of 2014 which clustered with the early years. Sampling efficiency for SAV may have been hindered in 2014 due to very high discharge and elevation during the sampling episode. SAV community structure in upper backwaters also had 3 distinct clusters: 2004 on its own, 1998–2008 clustered together with 2012–2014, and 2009–2011 with 2015.
SIMPER analysis revealed that an increase in overall abundance of flat-stem pondweed (Potamogeton zosteriformis), narrow-leaf pondweeds (Potamogeton spp.), Canadian waterweed and coontail were primarily responsible for the shift in SAV composition in lower backwaters. In upper backwaters an increases in coontail was most responsible for the SAV community shift. BIOENV analysis showed turbidity as the environmental variable that best explained SAV community patterns in upper and lower backwaters (Table 5).
Fish community
Of the 46 species of fish used in analysis, bluegill (Lepomis macrochirus) was by far the most common fish in backwaters of Pool 4 (Table 6). Spatial differences in the fish community between the upper and lower backwaters were significant (ANOSIM, R = 0.68, P = 0.001; Fig. 8). Bluegill, yellow perch (Perca flavescens), weed shiner (Notropis texanus) and largemouth bass (Micropterus salmoides) were more abundant in lower backwaters. Emerald shiner (Notropis atherinoides), gizzard shad (Dorosoma cepedianum), and black crappie (Pomoxis nigromaculatus) were more abundant in upper backwaters. These seven species contributed to over 40% of the dissimilarity between upper and lower fish communities (Table 6; Fig. 8). Several species of fish that are routinely collected in lower backwaters were rarely collected in upper backwaters [e.g. pumpkinseed (Lepomis gibbosus), spotted sucker (Minytrema melanops), golden redhorse (Moxostoma erythrurum) and golden shiner (Notemigonus crysoleucas)]. In fact three fish species: pirate perch (Aphredoderus sayanus), yellow bullhead (Ameiurus natalis) and black bullhead (Ameiurus melas) have never been collected in upper backwaters.
NMDS ordination of annual fish assemblages in both upper and lower backwater lakes and vectors of the fish species most responsible for community difference. Fish species are abbreviated as follows: bluegill (BLGL), emerald shiner (ERSN), gizzard shad (GZSD), yellow perch (YWPH), weed shiner (WDSN), black crappie (BKCP), largemouth bass (LMBS)
Fish community structure in both upper and lower backwater lakes separated into two significant clusters (Fig. 9). However, in upper backwaters only two nonconsecutive years (i.e. 2012, 2015) were identified as having distinct community structure (Fig. 9a). The separation of these 2 years was driven by increased abundance of bluegill, spottail shiner (Notropis hudsonius), black crappie and decreased abundance in emerald shiner and gizzard shad. These five species accounted for over 50% of the dissimilarity between clusters (Table 7). In contrast, the fish community in lower backwaters experienced a temporal shift between 2005 and 2008 and revealed two distinct and significantly different clusters (Fig. 9b). This shift was driven in large part by an increase in weed shiner, which had rarely been captured prior to 2006 (Table 8; Fig. 10). Nine species accounted for over 50% of the dissimilarity, including increased abundance in bluegill, pumpkinseed and yellow perch and decreased abundance in gizzard shad, pugnose minnow (Opsopoeodus emiliae), emerald shiner, bullhead minnow (Pimephales vigilax) and black crappie (Table 8; Fig. 10).
Environmental variables were not associated with fish community patterns in upper backwaters as indicated by the BIOENV procedure (Table 5). However, in lower backwaters the multivariate combination of aquatic vegetative cover and turbidity best explained fish community patterns (ρ = 0.47, P = 0.001).
Discussion
We documented an abrupt increase in water clarity and SAV abundance and an associated shift in the structure of a diverse fish community to fewer open water species and more vegetation-associated species following several consecutive years of low discharge. These changes were observed throughout our study area but were more pronounced in lower backwaters downriver of Lake Pepin. We hypothesize that low discharge enhanced a subset of environmental conditions beyond a critical threshold in backwaters and triggered an increase in SAV growth. SAV community composition also shifted towards more lentic species intolerant of high current velocity. Feedback associated with increased abundance of SAV was detected in lower backwaters as water clarity increased two years following a large increase in SAV abundance. Turbidity was the environmental variable most associated with changes in SAV in both upper and lower backwaters, yet the cause or effect relationship between SAV and turbidity could differ between the two sets of backwaters. Vegetation cover and turbidity were the environmental variables most temporally associated with fish community patterns in lower backwaters. There were no significant associations between environmental variables and fish community patterns in upper backwaters.
In their review, Franklin et al. (2008) identified discharge, light availability, substrate, and nutrient availability, in addition to current velocity, as the most important variables governing macrophyte growth in river systems. Current velocity negatively affects macrophytes by uprooting or causing physical damage to the plant, but it can also influence indirect physiological mechanisms affecting nutrient uptake, gas exchange and photosynthesis (Madsen et al. 2001). While current velocity is important, it is understood that other environmental variables also determine the distribution and abundance of SAV. Prevalence and composition of SAV in lower backwaters during the early years of our study suggests that light was adequate. Water-column nutrient concentrations and water elevation, both of which are known to control SAV communities (Franklin et al. 2008), remained relatively stable in lower backwaters throughout the study period suggesting these variables were not primary drivers of the increase in SAV. Our analysis identified turbidity as the variable most strongly associated with changes in SAV community patterns. While water clarity improved as SAV increased, we believe this may have been more of a feedback provided by SAV in lower backwaters. The other notable environmental change in lower backwaters was the distinct period of low current velocity. We suspect that the low current velocity may have acted as a catalyst to start the increase in SAV abundance and shift towards more lentic species similar to what has been observed in nearby areas on the UMR (Carhart and De Jager 2019).
Gurnell et al. (2006) found altered patterns and reductions in current velocities within and near SAV stands. We suspect that current velocities may have been further reduced in lower backwaters following the increase in SAV. Sand-Jensen and Mebus (1996) noted SAV species with large leaf areas on bushy shoots (e.g. Canadian waterweed) reduced current more than species with narrower streamlined leaves. Noticeable increases in coontail and Canadian waterweed along with flat-stem and narrow-leaf pondweeds and only modest increases in lotic species such as wild celery (Vallisneria americana) and water stargrass (Heteranthera dubia) are another indication that discharge and current velocity are potential key drivers of an overall increase in SAV abundance and community shift. Carhart and De Jager (2019) found similar results in Pool 8 of the UMR where SAV shifted towards a community dominated by coontail and narrow-leaf pondweeds in areas where current velocity was reduced due to island construction. In our study, the SAV shift in lower backwaters appeared to include a transition community (2005–2007) before stabilizing in the later years. Lentic species of narrow-leaf pondweeds and Canadian waterweed accounted for most of the dissimilarly between early and late SAV communities. While our analysis did not uncover a significant link between SAV and current velocity, prevalence of lentic species warrants further investigation into this relationship.
In contrast to lower backwaters where water clarity is good, light penetration in upper backwaters remained chronically impaired by turbid conditions. Lund (2019) estimated the mean summer depth of 1% surface light to be 1.2 m in upper backwaters over a similar period of study. Turbidity, combined with water elevation fluctuations that are inherent in upper backwaters, may limit light availability for SAV growth during typical discharge regimes. However, SAV abundance did increase and composition was more diverse in upper backwaters during and following low discharge years. Water elevation and depth are positively linked to discharge, and during years of low discharge adequate light penetration for SAV growth reaching bottom sediments likely increased. Hilt et al. (2011) described a similar occurrence on the Spree River in Germany where an increase in light availability due to lower water elevation and or lower seston resulted in rapid macrophyte development. A concurrent study in Pool 4 identified increases in SAV occurrence correlated to low summer discharge in upper backwaters and negatively correlated to high summer discharge (Lund 2019). This supports our notion that discharge regime may have played a role in SAV community structure even though our analysis identified turbidity as most associated with changes in SAV. While upper backwaters did have a modest increase in clarity, the two most common SAV species (coontail and sago pondweed (Stuckenia pectinatus)), were canopy-formers capable of surviving in poor light conditions (Lougheed et al. 2001). Moreover, five species of SAV found in lower backwaters have never been collected in upper backwaters. Without substantial sediment load reduction and further improvements in water clarity in upper backwaters, any gains achieved from short-term low discharge events may not be sustainable and an ecological shift similar to what transpired in lower backwaters is unlikely. For example, in an agricultural dominated landscape, backwaters on the Illinois River shifted from clear macrophyte-driven lakes to turbid non-vegetated waters in the late-1950s due to chronic sediment loads and have never recovered (Sparks et al. 1990). These patterns in SAV illustrate the importance of habitat conditions, particularly the effects of water clarity and hydrological conditions.
Aquatic macrophytes are a key component of fish habitat and can be important in early life history (Holland and Huston 1984; Werner and Hall 1988) and adult stages of fishes (Diehl 1988). Aquatic macrophytes typically increase abundance and diversity of the fish community by providing structurally complex habitat (Killgore et al. 1989; Petry et al. 2003). Presence or absence of aquatic vegetation can influence fish community structure, especially open water species. For example, gizzard shad are generally less numerous in lakes with abundant vegetation (Allen et al. 2000; Michaletz and Bonneau 2005), possibly due to lower food abundance of appropriate size, reduced foraging efficiency in structurally complex vegetation and greater risk of predation. Like gizzard shad, emerald shiners also tend to occupy and feed in open water (Becker 1983) and have been reported to avoid dense aquatic vegetation. Johnson and Jennings (1998) did find a positive correlation between emerald shiners and vegetation in UMR backwaters; however, percent frequency of occurrence and biomass of vegetation in their study was far less than what we measured in lower backwaters during the later years of our study. Emerald shiners and gizzard shad, both key species driving the fish community shift in upper and lower backwaters, declined in abundance following the increase in SAV.
In contrast to the decline in open water fish species, several sight feeding and vegetation-associated species increased in abundance. Most noticeable was the increase in weed shiners in lower backwaters, where the species was exceedingly rare prior to the increase in SAV. Weed shiners were shown by our analysis to be the species most responsible for the fish community shift in lower backwaters. DeLain and Popp (2014) found significant positive correlations between SAV and weed shiner abundance in multiple habitats on Pool 4 of the UMR. Pumpkinseed also increased in abundance along with SAV in lower backwaters. Hinch and Collins (1993) reported macrophyte cover as one of the major environmental factors influencing pumpkinseed and bluegill abundance in Ontario lakes. They surmised enhanced food abundance in the macrophytes and reduction in predator efficiency may have increased the growth and survival of both juveniles and adults. Sight feeding piscivores such as largemouth bass and bowfin (Amia calva) also increased in abundance during our study. Rodríguez and Lewis (1997) found transparency was an excellent predictor of fish community structure in floodplain lakes of the Orinoco River. Sight feeding fishes dominated in clear lakes, whereas fish adapted to low light dominated in turbid lakes. Similarly, Diehl (1988) found that European perch (Perca fluviatilis) were more efficient foragers in vegetation compared to bream (Abramis brama) and roach (Rutilus rutilus) which were superior in turbid non-vegetated water. In our study, yellow perch abundance increased greatly in lower backwaters with an increase in SAV and this species has become a major component of the sport fishery on the UMR. We speculate that both improved clarity and the physical structure provided by aquatic vegetation were key to the fish community shift. This is consistent with our finding that aquatic vegetation cover and turbidity were the environmental variables most strongly associated with changes in fish community structure. Similarly, Giblin (2017) found TSS and aquatic vegetation key to fish assemblage changes in Pool 8 of the UMR. Although fish community structure did change in upper backwaters, it was not to the extent observed in lower backwaters. Vegetation-associated species such as bluegill and black crappie did increase in abundance; however, other species found in lower backwaters such as pumpkinseed, spotted sucker, golden redhorse and golden shiner remained extremely rare in upper backwaters. These patterns in fish community structure illustrate the importance of aquatic macrophytes in backwaters.
Conclusions
Several consecutive years of low summer discharge on the UMR appears to have altered environmental conditions and triggered biological changes that represent a shift in the ecology of floodplain backwaters. A substantial increase in SAV abundance added structural complexity to backwaters and feedback mechanisms improved water clarity beyond what was already supporting SAV. Fish community structure shifted away from open water species to those associated with aquatic macrophytes and sight feeders dependent on clear water. SAV community structure also shifted towards a greater abundance of lentic species less tolerant of current. Habitat conditions between upper and lower backwaters differed in quality and consequently fish and SAV community structure as well. Continued stability of SAV abundance, water clarity and the new fish assemblage in lower backwaters indicates a sufficient level of resilience in the system to withstand the recurrence of less favorable hydrological conditions. Physical and biological response in upper backwaters was less noticeable and arguably did not represent an ecological regime shift. Upper backwaters endure a chronic influx of turbid water from the main channel and bottom sediment resuspension that impairs clarity. Without a large reduction in suspended solids load, it may be unrealistic to expect a clear water macrophyte driven system in these backwaters. While hydrological conditions that reduced physical stress on SAV and enhanced light penetration may have initiated the shift, it appears biological processes are providing resilience to the ecosystem via SAV feedback. Finally, we confirmed the macrophyte-turbidity relationship common in shallow inland lakes occurs in this system as well. This is also one of the few studies to document ecological shifts in a hydrologically dynamic large floodplain river system.
References
Allen MS, Hoyer MV, Canfield DE Jr (2000) Factors related to gizzard shad and the threadfin shad occurrence and abundance in Florida lakes. J Fish Biol 57:291–302
Becker GC (1983) Fishes of Wisconsin. The University of Wisconsin Press, Madison
Beckett DC, Aartila TP, Miller AC (1992) Contrasts in density of benthic invertebrates between macrophyte beds and open littoral patches in Eau Galle Lake, Wisconsin. Am Midl Nat 127:77–90
Bettoli PW, Maceina MJ, Noble RL, Betsill RK (1993) Response of a reservoir fish community to aquatic vegetation removal. N Am J Fish Manage 13:110–124
Bouska K, Houser J, De Jager N, Hendrickson J (2018) Developing a shared understanding of the Upper Mississippi River: the foundation of an ecological resilience assessment. Ecol Soc 23:6. https://doi.org/10.5751/ES-10014-230206
Burdis RM, Hoxmeier RJH (2011) Seasonal zooplankton dynamics in main channel and backwater habitats of the Upper Mississippi River. Hydrobiologia 667:69–87
Carhart AM, De Jager NR (2019) Spatial and temporal changes in species composition of submersed aquatic vegetation reveal effects of river restoration. Restor Ecol. https://doi.org/10.1111/rec.12911
Chilton EW (1990) Macroinvertebrate communities associated with three aquatic macrophytes (Ceratophyllum demersum, Myriophyllum spicatum, and Vallisneria americana) in Lake Onalaska, Wisconsin. J Freshw Ecol 5:455–466
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd., Plymouth
Clarke KR, Warwick RM (2001) Change in marine communities. An approach to statistical analysis and interpretation. PRIMER-E Ltd., Plymouth
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63:1802–1813
DeLain SA, Popp WA (2014) Relationship of weed shiner and young-of-the year bluegill and largemouth bass abundance to submersed aquatic vegetation in Navigation Pool 4, 8, and 13 of the Upper Mississippi River, 1998-2012: a technical report submitted to the U.S. Army Corps of Engineers’ Upper Mississippi River Restoration-Environmental Management Program from the U.S Geological Survey, Technical Report 2014-T001. p 29
Dent CL, Cumming GS, Carpenter SR (2002) Multiple states in river and lake ecosystems. Philos Trans R Soc Lond B Biol Sci 357:635–645
Diehl S (1988) Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53:207–214
Dieter CD (1990) The importance of emergent vegetation in reducing sediment resuspension in wetlands. J Freshw Ecol 5:467–473
Doyle MW, Stanley EH, Strayer DL, Jacobson RB, Schmidt JC (2005) Effective discharge analysis of ecological processes in streams. Water Resour Res 41:W11411. https://doi.org/10.1029/2005WR004222
Drake D, EM Lund (2020) Developing methods of estimating submersed aquatic vegetation biomass in the Upper Mississippi River: Expanding capabilities within the Upper Mississippi River Restoration program and improve the utility of the long-term vegetation data. U.S. Army Corps of Engineers’ Upper Mississippi River Restoration Long Term Resource Monitoring Element Completion Report LTRMP-2018BI03, p 45
Franklin P, Dunbar M, Whitehead P (2008) Flow controls on lowland river macrophytes: a review. Sci Tot Environ 400:369–378
Fremling CR (2005) Immortal river: the Upper Mississippi in ancient and modern times. Univ of Wisconsin Press, Wisconsin
Giblin SM (2017) Identifying and quantifying environmental thresholds for ecological shifts in a large semi-regulated river. J Freshw Ecol 32:433–453
Gibson-Reinemer DK, Ickes BS, Chick JH (2016) Development and assessment of a new method for combining catch per unit effort data from different fish sampling gears: multigear mean standardization (MGMS). Can J Fish Aquat Sci 74:8–14
Gore JA, Shields FD (1995) Can large rivers be restored? Bioscience 45:142–152
Gurnell AM, Van Oosterhout MP, De Vlieger B, Goodson JM (2006) Reach-scale interactions between aquatic plants and physical habitat: River Frome, Dorset. River Res Appl 22:667–680
Hilt S, Köhler J, Kozerski HP, van Nes EH, Scheffer M (2011) Abrupt regime shifts in space and time along rivers and connected lake systems. Oikos 120:766–775
Hinch SG, Collins NC (1993) Relationships of littoral fish abundance to water chemistry and macrophyte variables in central Ontario lakes. Can J Fish Aquat Sci 50:1870–1878
Holland LE, Huston ML (1984) Relationship of young-of-the-year northern pike to aquatic vegetation types in backwaters of the Upper Mississippi River. N Am J Fish Manage 4:514–522
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23
Houser JN, Bierman DW, Burdis RM, Soeken-Gittinger LA (2010) Longitudinal trends and discontinuities in nutrients, chlorophyll, and suspended solids in the Upper Mississippi River: implications for transport, processing, and export by large rivers. Hydrobiologia 651:127–144
Ibáñez C, Alcaraz C, Caiola N, Rovira A, Trobajo R, Alonso M, Duran C, Jimennez PJ, Munne A, Prat N (2012) Regime shift from phytoplankton to macrophyte dominance in a large river: top-down versus bottom-up effects. Sci Total Environ 416:314–322
James WF, Barko JW (1994) Macrophyte influences on sediment resuspension and export in a shallow impoundment. Lake Reserv Manag 10:95–102
James WF, Barko JW, Butler MG (2004) Shear stress and sediment resuspension in relation to submersed macrophyte biomass. Hydrobiologia 515:181–191
Johnson BL, Jennings CA (1998) Habitat associations of small fishes around islands in the upper Mississippi River. N Am J Fish Manage 18:327–336
Kendall M (1975) Multivariate analysis. Charles Griffin & Company Ltd., Glasgow
Killgore KJ, Morgan RP, Rybicki NB (1989) Distribution and abundance of fishes associated with submersed aquatic plants in the Potomac River. N Am J Fish Manage 9:101–111
Lewontin RC (1969) The meaning of stability. Brookhaven Symp Biol 22:13–24
Lougheed VL, Crosbie B, Chow-Fraser P (2001) Primary determinants of macrophyte community structure in 62 marshes across the Great Lakes basin: latitude, land use, and water quality effects. Can J Fish Aquat Sci 58:1603–1612
Lund EM (2019) Time lag investigation of physical conditions and submersed macrophyte prevalence in upper navigation pool 4, Upper Mississippi River. U.S. Army Corps of Engineers’ Upper Mississippi River Restoration Program Long Term Resource Monitoring Element Completion Report LTRM-2015A8, p 23
Madsen JD, Chambers PA, James WF, Koch EW, Westlake DF (2001) The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444:71–84
May RM (1977) Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature 269:471–477
Michaletz PH, Bonneau JL (2005) Age-0 gizzard shad abundance is reduced in the presence of macrophytes: implications for interactions with bluegills. Trans Am Fish Soc 134:149–159
Petry P, Bayley PB, Markle DF (2003) Relationships between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain. J Fish Biol 63:547–579
Poos MS, Jackson DA (2012) Addressing the removal of rare species in multivariate bioassessments: the impact of methodological choices. Ecol Indic 18:82–90
Ratcliff EN, Gittinger EJ, O’Hara TM, Ickes BS, (2014) Long term resource monitoring program procedures: fish monitoring, 2nd edition. Program Report LTRMP 2014-P001. https://pubs.usgs.gov/mis/ltrmp2014-p001/pdf/ltrmp2014-p001.pdf. Accessed 1 June 2019
Rodríguez MA, Lewis WM (1997) Structure of fish assemblages along environmental gradients in floodplain lakes of the Orinoco River. Ecol Monogr 67:109–128
Sand-Jensen K, Mebus JR (1996) Fine-scale patterns of water velocity within macrophyte patches in streams. Oikos 76:169–180
Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E (1993) Alternative equilibria in shallow lakes. Trends Ecol Evol 8:275–279
Schriver P, Bogestrand J, Jeppesen E, Sondergaard M (1995) Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshw Biol 33:255–270
Sen PK (1968) Estimates of the regression coefficient based on Kendall’s tau. J Am Stat Assoc 63:1379–1389
Soballe DM, Fischer JR (2004) Long term resource monitoring program procedures: water quality monitoring. U.S. Geological Survey, Upper Midwest Environmental Sciences Center, La Crosse, Wisconsin Technical Report LTRMP 2004-T002-1. https://www.umesc.usgs.gov/documents/reports/2004/04t00201.pdf. Accessed 1 June 2019
Sparks RE, Bayley PB, Kohler SL, Osborne LL (1990) Disturbance and recovery of large floodplain rivers. Environ Manage 14:699–709
Sparks RE, Nelson JC, Yin Y (1998) Naturalization of the flood regime in regulated rivers. Bioscience 48:706–720
Theiling CH, Nestler JM (2010) River stage response to alteration of Upper Mississippi River channels, floodplains, and watersheds. Hydrobiologia 640:17–47
Timms RM, Moss B (1984) Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing, in the presence of zooplanktivorous fish, in a shallow wetland ecosystem. Limnol Oceanogr 29:472–486
Tuner RE, Rabalais NN (2003) Linking landscape and water quality in the Mississippi River Basin for 200 years. Bioscience 53:563–572
van Donk E, van de Bund WJ (2002) Impact of submerged macrophytes including charophytes on phyto-and zooplankton communities: allelopathy versus other mechanisms. Aquat Bot 72:261–274
Werner EE, Hall DJ (1988) Ontogenetic habitat shifts in bluegill: the foraging rate-predation risk trade-off. Ecology 69:1352–1366
Yin Y, Winkelman JS, Langrehr HA (2000) Long term resource monitoring program procedures: aquatic vegetation monitoring. U.S. Geological Survey, Upper Midwest Environmental Sciences Center, La Crosse, Wisconsin. LTRMP 95-P002-7. https://www.umesc.usgs.gov/documents/reports/1995/95p00207.pdf. Accessed 1 June 2019
Acknowledgements
This study was funded as part of the Upper Mississippi River Restoration Program, Long Term Resource Monitoring element; a cooperative effort between the U.S. Army Corps of Engineers, U.S. Geological Survey, U.S. Fish and Wildlife Survey, and the states of Illinois, Iowa, Minnesota, Missouri, and Wisconsin. Thank you to Doug Dieterman and Jeff Houser for their helpful review of the manuscript and a special thanks to an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burdis, R.M., DeLain, S.A., Lund, E.M. et al. Decadal trends and ecological shifts in backwater lakes of a large floodplain river: Upper Mississippi River. Aquat Sci 82, 27 (2020). https://doi.org/10.1007/s00027-020-0703-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-0703-7