Abstract
Atmospheric nitrogen deposition predominantly influences ecosystems by shifting their available nutrient budgets towards excess nitrogen conditions. In temperate lakes nitrogen is often naturally in excess and phosphorus is deficient, when compared with the optimal Redfield ratio of 16:1. To investigate effects of future increasing nitrogen conditions on lake plankton communities, we performed mesocosm experiments in three different nitrogen rich lakes, all characterised by high nitrogen to phosphorus ratios. In order to determine functional responses to increased nitrogen loading, we conducted six nitrogen fertilization treatments. Nitrogen fertilization was based upon existing nitrate and ammonium concentrations in natural wet deposition and multiple loadings of these concentrations. Despite the initial conditions of excess nitrogen, removal of additional nitrogen by the plankton community was observed in all of the lakes. In one lake, an increasing phosphorus limitation became visible in seston stoichiometry. Over all of the lakes and within each lake’s experimental nitrogen gradient, we found evidence for decreased mesozooplankton due to nitrogen enrichment. The negative responses of mesozooplankton to N enrichment were mainly restricted to cladocerans and nauplii. The results indicate that nitrogen enrichment within the magnitudes of projected future atmospheric nitrogen depositions may lead to a long-term reduction of mesozooplankton in phosphorus deficient lakes. The transfer of nitrogen enrichment effects on lower food-web dynamics could have consequences for higher trophic levels, such as fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of continuously rising global economic activity, together with the energy and the food demands of the human population (Galloway et al. 2008), the nitrogen (N) cycle is considered to be the most anthropogenically altered biogeochemical nutrient cycle. Nitrogen excess is one of the critical risks for a sustainable human life on earth (Rockström et al. 2009; Steffen et al. 2015). Over the last century, a three- to fivefold increase in the reactive forms of nitrogen emissions (nitrate and ammonium) has been observed (Denman et al. 2007; Ciais et al. 2013). Due to their widespread atmospheric distribution, the subsequent locally uncontrollable deposition leads to rising concentrations of reactive N in ecosystems, even in remote regions (Bergström et al. 2005; Crowley et al. 2012; Kim et al. 2014). It has been shown that an increased N deposition is able to alter the nutrient status of ecosystems (Vitousek et al. 1997; Aber et al. 2003) due to the increment of nitrification rates (Aber et al. 2003), as well as to an increase of N leaching from forests and soils (Gundersen et al. 1998; Reay et al. 2008; Lovett et al. 2013).
An increasing N supply is of biological importance since it leads to changes in the available nutrient ratios, and eventually, to excess N conditions and a phosphorus (P) deficiency in (terrestrial and aquatic) ecosystems (Bergström and Jansson 2006; Elser et al. 2009a, b; Crowley et al. 2012). An increment of N is the first and foremost concern for ecosystems in which the primary producers are limited by N, potentially increasing biomass and primary production (Tyrrell 1999; Reich et al. 2001; Elser et al. 2009b; Hessen 2013). In addition, autotrophs, such as phytoplankton, have the flexibility to alter their elemental (stoichiometric) composition according to the surrounding resource conditions (Sterner et al. 1997; Klausmeier et al. 2004). It has recently been shown that for predominantly N-limited lakes, in a gradient of atmospheric N deposition, the availability of higher dissolved N:P ratios can result in higher seston biomass N:P ratios (Elser et al. 2009b; Hessen 2013).
Seston stoichiometry is an indicator of food quality for aquatic herbivores (Andersen and Hessen 1991; Hessen 1992), whose performance is related to both the producer food quantity and quality (Hessen et al. 2002). Food quality needs to meet specific nutrient requirements for each herbivore, which include seston stoichiometry in general (Andersen and Hessen 1991; Hessen 1992; Elser et al. 2000), fatty acid composition (Müller-Navarra 1995; Müller-Navarra et al. 2000), as well as the edibility of algae (Sommer et al. 1986). In terms of mineral nutrient limitation, a copepod’s growth is rather limited by N-deficient food through the higher organismic demands of N (Hessen 1992; Sterner and Hessen 1994). In contrast, the growth and the reproduction of the abundant freshwater cladoceran Daphnia are commonly limited by the amount of available P (Sommer 1992; Sterner and Hessen 1994; Urabe et al. 1997). There is evidence from natural lake systems that an algal P limitation, expressed in high seston C:P ratios, is transferred to the trophic level of secondary producers and affects the growth of Daphnia (Elser et al. 2001; Berger et al. 2006). Besides seston stoichiometry, the fatty acid content of phytoplankton is essential for herbivore growth and may be reduced under an increased P limitation (Müller-Navarra 1995; Müller-Navarra et al. 2000). Hence, effects of increased N load in traditionally P deficient temperate lakes (Schindler 1977) would not be expected to be of great magnitude. It is conceivable that the growth and the reproduction of P-demanding herbivores should be derogated to a greater extent, and thus might lead to lower herbivore abundance under increasing N:P conditions.
In order to estimate the degree to which effects of an increased N load in P deficient lakes are transferred to the zooplankton trophic level, we experimentally investigated the consequences of N enrichment on epilimnion plankton communities in mesocosm field studies. To be able to detect overarching effects of an increased N load in P deficient systems, we performed experiments in three lakes with different trophic conditions. Experiments were performed synchronously and covered a broad N:P nutrient supply range. We hypothesised that the zooplankton groups with high P requirements would be the first to be negatively affected by an additional N enrichment. We did not expect that increased N loads would primarily lead to changes in the phytoplankton biomass, but rather to shifts in the seston stoichiometry.
Materials and methods
Study sites and experimental design
The mesocosm field experiments were performed during the spring of 2013 in three lakes with different trophic statuses (Lake Brunnsee 27.03.13–31.05.13, Lake Klostersee and Lake Thalersee 27.03.13–28.05.13) in Bavaria, Germany. The lakes were chosen for their different nutrient backgrounds (Table 1), in which the dissolved N:P ratios (nitrate and ammonium compared to total phosphorus concentrations) varied from >50:1 N:P (Lake Klostersee), >400:1 N:P (Lake Thalersee), to >1000:1 N:P (Lake Brunnsee). This was far higher than the classical Redfield ratio of 16:1 N:P. All of the lakes are hard water lakes with bedrocks of rubble and lie in a nature reserve. The total N supply of the lakes is continuously affected by atmospheric deposition and leaching, but also, the groundwater discharges and the surface runoffs contribute to the total N in the lakes. Lake Brunnsee (18.6 m max. depth, 5.9 ha, 502 × 103 m3) is mainly groundwater fed, whereas Lake Klostersee (16 m max. depth, 47 ha, 2762 × 103 m3) and Lake Thalersee (7 m max. depth, 3.8 ha, 166 × 103 m3) have small streams running through them. Lake Brunnsee and Lake Klostersee are typically dimictic, with a stable stratification from spring to autumn, and Lake Thalersee is a shallow polymictic lake.
In the mesocosm experiments at each lake, we simulated a continuous N supply on a stratified pelagic water column. The natural phytoplankton and zooplankton communities were enclosed in 12 mesocosm bags (4 m deep, 0.95 m in diameter, made of white polyethylene foil, 150 µm, Biofol Film GmbH, Germany), which were sealed at the bottom and open to the atmosphere. Those mesocosms were filled by lowering the bags into the water column (6–8 m, if possible) and then lifting them back to the surface. The mesocosm bags were fixed to anchored rafts and were equipped with a transparent covering to control for the N wet deposition, while ensuring that there were natural light conditions, with regard to light quantity and spectrum. The N supply was manipulated in a gradient of six N treatments (two replicates per treatment), starting from a zero N deposition, to 1, 2, 8, 16, and 32-fold the natural regional N wet deposition. Since we could not foresee the range of N deposition amounts, where responses in the plankton community could be expected, we chose a log-linear experimental design to cover a reasonably wide magnitude of N deposition. The natural N wet deposition was based on average amounts, which are effectively supplied to the lake surfaces in about 25 l m−2 of weekly precipitation in the region (German Meteorological Survey) with 3 mg l−1 nitrate (NO3), and 1 mg l−1 ammonium (NH4) (~1:1 mol:mol). Thus, this ambient wet deposition contained an average supply of 75 mg m−2 NO3 and 25 mg m−2 NH4 per week (Bavaria regional state office), with yearly peak values of 490 mg m−2 NO3 and 245 mg m−2 NH4 per week. Only a doubling of the maximum N deposition, combined with a doubling in the weekly precipitation (Denman et al. 2007), would result in conceivable future N depositions in the range of 32 times the average wet deposition.
The N fertilization of the mesocosms was carried out twice a week by the addition of 1 l of an appropriate N solution per mesocosm and by a thorough mixing using a Secchi disc. The respective N solutions were freshly prepared from a stock solution prior to fertilization (41.1 mg ml−1 NaNO3, 29.7 mg ml−1 NH4Cl). These were calculated according to the natural N deposition in relation to the enclosure surface. For each treatment, 1, 2, 8, 16, and 32 ml of stock solution was placed into a 1-l PE bottle, which was then filled up with distilled water. The control treatment (0) received 1 l of distilled water only. An initial fertilization, equivalent to 4 weeks of a fertilization amount, was given on 28 March 2013, the first day of the experiment. This was in order to mimic the high availability of dissolved nutrient levels after the winter/spring circulation, and to ensure the immediate treatment differences at the start of the algal bloom phase in early spring. The sampling was performed once a week for water chemistry and zooplankton analyses, and twice a week for the phytoplankton analyses. Temperature was monitored in 20 randomly chosen mesocosms during the experiment by a sensor below the water surface and connected to a data logger (SE-309; Conrad Electronic, Germany).
Laboratory and data analyses
The samples for water chemistry and phytoplankton analyses were taken with an integrated tubular water sampler (2 m; KC Denmark A/S research equipment) from a water depth of between 1 and 3 m and then were pre-filtered over a 250 µm mesh to exclude the mesozooplankton. The weekly water chemistry analyses started on 30 March 2013 and included measurements for NO3 by ion chromatography (Dionex ICS-1100, Thermo Scientific, USA), and measurements of NH4 by fluorometry (Trilogy Laboratory Fluorometre Module CDOM/NH4; Turner Designs, USA), using the orthophthalate method (Holmes et al. 1999). This was done in addition to the measurements of total phosphorus levels (TP) by spectrophotometry (Shimadzu UV-1700, Shimadzu Cooperation, Germany), using the molybdenum blue method (Wetzel and Likens 1991). For particulate C, N, and P analyses, between 100 and 350 ml of enclosure water was filtered in duplicates onto a pre-combusted acid-washed GF/F filter (Whatman) and then frozen (−20 °C) until further analyses. The measurements for particulate organic carbon (POC) and particulate N were accomplished with an elemental analyzer (vario Micro cube, Elementar, Germany)—and for the particulate P (PP) with a spectrophotometer applying the molybdenum blue method. In weeks 4, 6, 8, and 10, biogenic silicate (Si) was analyzed by filtering between 50 and 200 ml of enclosure water onto cellulose-acetate filters (0.6 µm pore size, Satorius) and then frozen (−20 °C) until further analyses. The filters were subsequently extracted in a water bath (95 °C, for 4 h, in 0.2 mol NaOH) (Ragueneau and Tréguer 1994) and measured spectrophotometrically using the molybdenum blue method. From the particulate measurements, atomic seston C:N, C:P, N:P, and N:Si ratios, were calculated.
The phytoplankton groups were optically analyzed twice a week in vivo with an AlgaeLabAnalyser (bbe Moldaenke, Germany), which measures the total of chlorophyll a, and additionally separates the excitation spectra of four pigment groups into spectrally characterised algae groups. These spectrally characterised algae groups are named “green algae” (chlorophyta), “chromophytes” (with heterokontophyta, haptophyta, and also dinophyta), “blue-green algae” (cyanobacteria) and “cryptophytes” (Beutler et al. 2002).
The zooplankton sampling started on 17 April 2014 in order to allow for an initially undisturbed population growth. From then on, a 105 µm net (12 cm diameter) was hauled through the 4 m water column once a week. The zooplankton were immediately fixed to a 70% ethanol final concentration. The zooplankton communities were subsequently analyzed under a stereo microscope by counting splits of the fixed samples that contained at least 400 specimens and by determining those individuals to a species level if possible. For biomass calculation, the length of up to 30 individuals of the most abundant zooplankton taxa of cladocerans and copepods were measured if available and applied to established length-weight regression equations from the literature (Watkins et al. 2011; Bottrell et al. 1976). Rotifer biomass was estimated with given dry weights from the literature (Pauli 1989). As a direct and counting independent second biomass proxy, carbon measurements were performed weekly on the mesozooplankton (>250 µm) from the water samples that were filtered through a 250 µm gauze. The samples were taken with an integrated tubular water sampler from a water depth of between 1 and 3 m. The mesozooplankton were first counted alive (copepods and cladocerans) and then it was brought onto a pre-combusted GF/F filter for POC measurements using an elemental analyzer (vario Micro cube, Elementar, Germany).
Following the logarithmic fertilization design, the statistical analyses were performed with regression models (linear or unimodal) using SigmaPlot 11.0 (Systat Software 2008) against the log-transformed N fertilization loadings or, against the observed dissolved reactive N or dissolved N:P ratios. In the case of the phytoplankton development, the maximum chlorophyll a values were defined as being the highest values after the growth phase. The “peak phase” was defined according to the maximum of total chlorophyll a values in Lake Brunnsee and Lake Thalersee (chromophytes in the case of Lake Klostersee), including two measurements before and after the maximum chlorophyll a values. Before and after the peak phase, the exponential growth phase and the subsiding phase were determined. We statistically analyzed the total chlorophyll a levels and all of the spectral algal classes for their maximum and average chlorophyll a levels. For zooplankton from microscopic counting, the abundances and biomass of copepods, cladocerans, and rotifers, together with the total zooplankton species, were analyzed. Calculations were performed for the average, and the maximum (“peak”) during the experimental period. Zooplankton biomass parameters of all lakes (mesozooplankton carbon measurements, copepod and cladoceran carbon estimated by their relative biomass abundances and calculated total zooplankton biomass from microscopic countings) were analysed as averages for the second half of the experiment (after day 43), when responses of mesozooplankton communities to bottom up driven effects of N fertilization would be expected to be visible. Multivariate analyses were performed as canonical correspondence analyses (CCA, Legendre and Legendre 1998) using PAST 2.17 Software (Hammer et al. 2001). Explanatory variables included the TP levels, the dissolved reactive N levels, and the dissolved N:P ratios. Log-transformed abundances of calanoid copepods, nauplii, cladocerans, and rotifers, were used as species variables.
Results
Nitrogen enrichment
In all of the three lakes, the required nitrogen gradient was established in the mesocosms (linear regression over all of the lakes versus N fertilization by the end of the experiment: NO3: R 2 = 0.69, F = 316, P < 0.001; NH4: R 2 = 0.25, F = 23.7, P < 0.001). It varied between 3.8 and 5.7 mg l−1 NO3–N (0.01–1.7 mg l−1 NH4–N) in Lake Brunnsee, 0.3–2 mg l−1 NO3–N (0.14–.8 mg l−1 NH4–N) in Lake Klosterse, and 2.8–4.9 mg l−1 NO3–N (0.004–1.7 mg l−1 NH4–N) in Lake Thalersee. In contrast, the TP concentrations in the mesocosms were not correlated with N fertilization (R 2 = 0.01, F = 1.99, P = 0.16) and ranged between 3 and 8 µg l−1 in Lake Brunnsee, 6–15 µg l−1 in Lake Klostersee, and 6–17 µg l−1 in Lake Thalersee. Over the experimental duration, the temperatures in the mesocosms were on average about 15.2 ± 0.1 °C in Lake Brunnsee, 15.3 ± 0.1 °C in Lake Klostersee and 15.5 ± 0.1 °C in Lake Thalersee.
When comparing the fertilization treatments at the end of the experiments and comparing the observed versus the predicted concentrations of NH4, the final NH4 concentrations were consistently below the predicted concentrations in all of the N treatments (negative intercepts of linear regressions, Supplementary Table S1). The lower than predicted NH4 concentrations indicated a significant NH4 conversion into biomass or other oxidized molecular forms in all of the lakes. For Lake Thalersee, the observed NO3 concentrations for the higher N treatments were higher than what would be expected from the fertilization alone (slope >1, Supplementary Table S1), whereas in Lake Klostersee, an increasing NO3 removal could be observed in the applied 8–32-fold N treatments (slope <1, Supplementary Table S1). In Lake Brunnsee, the NO3 removal occurred only in the lower N treatments (0–2-fold) but this was reduced in the 8–32-fold N treatments (slope >1, Supplementary Table S1).
Phytoplankton
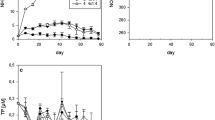
In terms of the phytoplankton growth, the mesocosms in oligotrophic Lake Brunnsee reached the highest chlorophyll a values between the days 28 and 35 (Fig. 1a). The mesocosms in Lake Thalersee had already reached the highest chlorophyll a values between days 7 and 10 (Fig. 1b) and it lacked an exponential growth phase. Over the entire experimental duration, the phytoplankton communities in Lake Thalersee consisted equally of green algae and chromophytes and in Lake Brunnsee, it was mainly chromophytes (Supplementary Fig. S2). The experiment in Lake Klostersee was dominated by high green algae concentrations during the first 3 weeks, after which the adjoining “peak phase” of the spring bloom between the days 31 and 35 (Fig. 1c) was primarily due to chromophytes. In Lake Klostersee, the highest chlorophyll a concentration levels after the growth phase of the chromophytes were lower than at the start of the experiment in some of the mesocosms.
Development of the chlorophyll a concentration (µg l−1) (a–c) and the zooplankton densities (Ind l− 1) (d–f) over the experimental duration. Displayed are the average concentrations (N = 2) of each of the six N fertilization treatments for lakes Brunnsee (left), Klostersee (middle) and Thalersee (right). The legend in c applies to all panels
In terms of the N fertilization, different responses of the chlorophyll a concentration levels could be observed between the lakes. In two of the lakes (Brunnsee and Thalersee), the chlorophyll a levels increased with the increment of N fertilization during the peak phase (Table 2). In Lake Brunnsee, we observed a positive relationship between the maximum chlorophyll a concentrations and the N fertilization (Table 2), as well as with the average chlorophyll a concentrations of the peak phase (Table 2; Fig. 2a). This trend was mainly driven by the chromophytes, which made up >95% of the total chlorophyll a concentration levels, and therefore, this group also showed a positive relationship of the chlorophyll a peaks with N fertilization (R 2 = 0.41, F = 6.82, P < 0.05). In Lake Thalersee, the average chlorophyll a concentrations increased with the increment of N fertilization during the peak phase (Table 2; Fig. 2c). In contrast, Lake Klostersee showed a decrease of average chlorophyll a concentrations during the peak phase with increasing N fertilization. The regression analyses revealed a significant negative relationship of maximum and average chlorophyll a levels with the increment of the N fertilization (Table 2; Fig. 2b).
Observed relationships due to the experimental nitrogen fertilization in lakes Brunnsee (left), Klostersee (middle) and Thalersee (right): the average chlorophyll a concentrations (µg l−1) of the peak phase (a–c) and the total zooplankton biomass (µg l−1) (d average biomass, e, f peak biomass). Displayed are the significant regression curves (solid P < 0.05; equations in Table 2 for chlorophyll a and Table 6 for zooplankton) and the 95% confidence bands (dotted); in f without the outlier (cross)
In terms of the seston stoichiometry, we observed significant changes in the meocosms of Lake Brunnsee and Lake Thalersee due to the N fertilization, but none in Lake Klostersee (Table 3). For Lake Brunnsee, we observed an increase in the particulate C (R 2 = 0.04, F = 4.79, P < 0.05) and in the particulate N (R 2 = 0.04, F = 3.97, P < 0.05) with the N fertilization, as well as an increase in the seston N:Si ratio (Table 3). A higher seston stoichiometric P limitation with an increasing N fertilization was observed at the time of the chlorophyll a peak, where the seston C:P ratios (day 28: R 2 = 0.42, F = 7.38, P < 0.05; day 35: R 2 = 0.49, F = 7.58, P < 0.05), as well as the seston N:P ratios (day 35: R 2 = 0.42, F = 5.81, P < 0.05), significantly increased with the N fertilization. In Lake Brunnsee, the seston C:P ratios over the entire experimental duration correlated significantly with the dissolved N:P ratios (R 2 = 0.05, F = 5.37, P < 0.05). In Lake Thalersee, we observed decreasing seston C:N ratios with the N fertilization (Table 3), which might indicate a higher N uptake in the higher fertilized treatments—but there were no changes in the seston C:P, in the N:P, or in the N:Si ratios, when increasing the N load. Over all of the lakes including all of the experimental treatments, a general response of the seston stoichiometry was observed for average seston C:P and N:P ratios, which increased with the dissolved reactive N and the dissolved N:P ratios (Table 4; Fig. 3a), as well as for seston C:N ratios, which decreased respectively (Table 4).
Regression analyses of a the seston C:P ratios, b the total zooplankton biomass (C µg l−1), c the cladoceran biomass (C µg l−1), and the copepod biomass (C µg l−1) versus the dissolved reactive N:P ratios in the mesocosms (n = 36). Linear regressions: a R 2 = 0.49, P < 0.001 (Table 4); b R 2= 0.13, P < 0.05 (Table 7); c R 2= 0.53, P < 0.001 (Table 7). Black squares Lake Klostersee, circles Lake Thalersee, dots Lake Brunnsee
Zooplankton
The community composition in all of the lakes included copepods, cladocerans, and rotifers. In the mesocosms of Lake Brunnsee, the copepods were on average the most abundant taxonomical group and were followed by the cladocerans (Table 5). On the contrary, in Lake Klostersee and Lake Thalersee, the cladocerans were the most abundant group, followed by the copepods. In all of the lakes, the rotifers were the least abundant taxonomical group. In the lakes Brunnsee and Klostersee, the zooplankton populations increased in all of the mesocosm treatments over the course of the experiment and they reached peak densities after the chlorophyll a maxima between the days 35 and 45 (Fig. 1d, e). In Lake Brunnsee, the absolute zooplankton densities remained low compared to the other two lakes, but the cladoceran densities increased until the end of the experiment. In Lake Thalersee, no clear growth phase was observed and the zooplankton numbers showed a high variability and fluctuated between the treatments over the experimental duration (Fig. 1f). The CCA analysis of the data revealed that 80.2% of the variance in the entire data set could be explained by the first axis (P < 0.01), which was first correlated with the TP (R = 0.54) and then with the dissolved N:P ratios (R = −0.16) (Fig. 4). The remaining 19.2% of the variance in the data set was explained by the second axis, which was correlated with the highest of the dissolved reactive N (r = −0.25), followed by the dissolved N:P ratios (R = −0.19) and the TP (R = 0.14). The analysis supports that samples from TP-rich and low N:P environments, as in Lake Thalersee, were generally characterised by the presence of cladocerans, whereas the copepods were associated with the higher dissolved N:P ratios, as found in Lake Brunnsee (Fig. 4). The nauplii held an intermediate position and since the Thalersee nauplii started to grow at the end of the experiment under the highest treatment N concentrations, they plot together with the high dissolved reactive N (Fig. 4).
Canonical correspondence analysis of the log transformed absolute zooplankton abundances (Ind l−1) (calanoid copepods, cladocerans, rotifers, nauplii) and the explanatory variables (TP, dissolved N:TP ratios, dissolved reactive N) over the entire experimental duration (n = 252, P < 0.01). Black squares Lake Klostersee, circles Lake Thalersee, dots Lake Brunnsee
We found decreasing zooplankton densities and their biomasses with a higher N fertilization in all the lakes (mainly negative slopes in Table 6, Supplementary Table S3). However, significant zooplankton parameters differed between the lakes. In Lake Klostersee and Lake Thalersee, the peak zooplankton was linearly decreasing over the N fertilization gradient (Table 6, Supplementary Table S3; Fig. 2e, f). In Lake Brunnsee, declining average zooplankton was observed in the N treatments higher than 8 times the natural wet deposition due to a unimodal relationship (Fig. 2d; Table 6). For Lake Brunnsee, this unimodal trend was also found in the nauplii abundances (Supplementary Table S3). In Lake Klostersee, the nauplii (Table 6, Supplementary Table S3) and the cladocerans (Table 6, Supplementary Table S3 and the carbon biomass average: R 2 = 0.38, F = 6.14, P < 0.05, and peak: R 2= 0.59, F = 14.46, P < 0.01) were negatively affected and showed a significant decrease with the N fertilization in the average, and in case of cladocerans the peak parameter. In Lake Thalersee, only the cladocerans were negatively affected by the N fertilization and declined (Table 6, Supplementary Table S3). Interestingly, in Lake Brunnsee and in Lake Klostersee, the rotifer biomass showed some positive relationship with the increased N fertilization (Table 6, Supplementary Table S3). The average zooplankton biomass after day 43 (carbon biomass data from elemental analyses as well as the calculated zooplankton biomass from microscopic countings) showed a declining relationship over all of the lakes across a wide range of dissolved N:P ratios (wider than experimentally achieved within each lake) (Table 7; Fig. 3b, dry weight µg l−1 versus dissolved N:P: R 2= 0.72, P < 0.001). This overarching pattern was observed in the total carbon biomass of cladocerans (Fig. 3c) but not in the total carbon biomass of copepods (Fig. 3d).
Discussion
Our study indicates that an increased N load influences the food-web dynamics in pelagic ecosystems that are P deficient. All mesocosm experiments showed measurable effects of N enrichment, although they were conducted in three lakes with differences in nutrient concentrations, dissolved N:P ratios ranging from >50:1–1000:1, and differences in other lake parameters such as depth, water exchange and plankton community assemblages. An in situ bioassay study (Ilic 2014) characterized the lakes Brunnsee and Klostersee as primary P limited (classification of Andersen et al. 2007) with synergistic N + P co-limitation effects. This characterization is in accordance with findings in most freshwater systems (Elser et al. 1990, 2007). The experiments showed effects on zooplankton and chlorophyll a, although they received much lower N fertilization amounts in contrast to previous eutrophication studies (0.33 mg l−1 N per week in the highest 32 N treatment compared to 6 mg l−1 N per week in Donald et al. 2013). The resulting N concentrations lie within the naturally observed total N concentrations in Scandinavian lake systems under an increased N deposition (Elser et al. 2009a).
It was not necessarily expected that under the already high environmental N:P conditions effects of N enrichment on the zooplankton would be measurable. During spring succession each individual lake experiences a diversity of interacting mechanisms, which are affecting the lake’s biology over all trophic levels (Sommer et al. 2012). Nevertheless, we observed declines of zooplankton at high fertilization levels in all lake experiments. The qualitative relationships of N load and zooplankton were markedly lake specific (two were linear, one was unimodal) and restricted to distinct zooplankton parameters (2-times peak, once average biomass). Thereby, Lake Brunnsee, with highest dissolved N:P ratios, showed weakest responses of zooplankton to N fertilization, and Lake Klostersee, with lowest dissolved N:P ratios, showed strongest responses compared to the other lakes (Table 6). Additionally, a negative correlation of the total zooplankton biomass with dissolved N:P ratios was seen over the entire data set including all treatments from the three lakes (Table 7; Fig. 3b). Despite this experimental variability, the observed relationship of N load and zooplankton also seems to occur across all three investigated lakes. This general relationship supports the idea that trophic transfer of increasing N load to the zooplankton community follows an overarching trend but does so via lake-specific mechanisms.
A negative effect of the N enrichment was primarily expected for the cladocerans, which have typically higher P requirements, but to a lesser degree for the copepods with typically higher N requirements (Sterner and Hessen 1994). Indeed, a negative relationship between N load (and dissolved N:P ratios) and cladocerans existed over all of the lakes (Table 7; Fig. 3c) but was expressed in different magnitudes in two lakes (Table 6). Strongest effects of N enrichment on cladoceran dry weights were observed in the peak biomass of the mesotrophic Lake Klostersee (Table 6). The generally supposed constraints of the N and P requirements of cladocerans compared to copepods are supported by the multivariate analysis. In the data set over all three lakes, the cladocerans clearly correlate to a low N:P ratio and the copepods to higher N:P conditions (Fig. 4). Subsequently, with increasing N:P ratios, the cladocerans are expected to be the first zooplankton group to be negatively affected. This is indeed true for Lake Thalersee and Lake Klostersee (Table 6, adjoining lakes in Fig. 4), which were richer in cladocerans (Table 5). In case of Lake Brunnsee and Lake Klostersee (adjoining lakes in Fig. 4) nauplii were sensitive to increased N load (Table 6, Supplementary Table S3 ). There is evidence for commonalities between the cladocerans and the nauplii, which both have low N:P body stoichiometry (<12:1), indicating high P demands (Andersen and Hessen 1991; Carrillo et al. 2001; Sterner and Elser 2002; Meunier et al. 2015), and similar optimal food size spectra (Hansen et al. 1994). The seston N:P ratios in this study were >44:1 (Table 3) indicating non-favourable food conditions for filter feeders with low biomass N:P ratios. Therefore, by increasing the P deficiency due to N enrichment, it is conceivable that this directly affects these two zooplankton groups, although it remains to be verified if the copepod egg production and/or the hatching success indirectly affect the nauplii abundances.
In stoichiometric theory, a trophic transfer effect of an N enrichment to the zooplankton can be related to a decrement of food quality in terms of seston stoichiometry (Hessen et al. 2013). This negatively affected the zooplankton in Lake Brunnsee, since in this lake increasing seston C:P ratios were observed. However, previous investigations of other lakes also revealed that higher dissolved N:P resource supply ratios, together with an increased P deficiency, were not necessarily reflected in a significantly higher seston N:P stoichiometry (Elser et al. 2009a). Similarly, it has been shown that zooplankton nutrient recycling can diminish the seston stoichiometric signatures despite having very different initial nutrient supply ratios (Trommer et al. 2012). Therefore, the absence of clear responses of seston stoichiometry to an N enrichment within the lakes Klostersee and Thalersee, does not necessarily mean that trophic food quality effects were not present. The role of seston stoichiometry as an underlying ecological mechanism for the observed negative zooplankton response in our study was supported by the increasing C:P (and N:P) ratios with dissolved N:P ratios over all of the lakes (Table 4; Fig. 3a). The negative effects of seston C:P ratios >300:1 (Urabe et al. 1997) and across a range of similar seston C: P ratios of natural lakes (Brett et al. 2000) have already been observed for the growth of the daphnid species.
Other than the seston stoichiometry, the food quality transfer to the higher trophic levels can also be related to other energetic pathways. It is for example known that the fatty acid composition of algae plays an essential role for food quality and can significantly influence the growth of Daphnia sp. (Müller-Navarra et al. 2000; Wacker and Elert 2001). There are indications that the abundance of fatty acids correlates with the relative P availability for algae to some extent, since some species grown in P-limited media produce less essential fatty acids than in P saturated media (Müller-Navarra 1995). Additionally, Daphnia growth is seasonally stronger correlated to specific fatty acid concentrations than seston C:P ratios (Wacker and Elert 2001), which might also be related to physiological adaptations to high energy and low nutrient environments (Mulder and Bowden 2007). Higher metabolic costs from higher feeding activity (Plath and Boersma 2001), higher respiration rates (Darchambeau et al. 2003) or higher alkaline phosphatase activity (Elser et al. 2010; McCarthy et al. 2010) could also have contributed to an energetic mismatch for cladocerans in the lakes Klostersee and Thalersee through an increasing P deficiency.
The immediate causes for unfavourable growth conditions for the zooplankton under a high N fertilization might also be related to the toxic effects of high N concentrations. However, the highest NO3 concentrations (5.7 mg l−1 NO3–N in the Lake Brunnsee) did not reach critical values for the cladocerans (>14 mg l−1 NO3–N, Camargo et al. 2005). In terms of an NH4 toxicity, concentrations >0.6 mg l−1 ammonia nitrogen (NH3–N) can result in chronic toxicity for Daphnia magna (Gersich and Hopkins 1986). These are higher than the NH3–N concentrations in our experiments (<0.2 mg l−1 NH3–N at 1.7 mg l− 1 NH4–N, pH 8, <20 °C).
In terms of effects of food quantity for zooplankton, the phytoplankton biomass was hypothesised not to change with higher N fertilization, since the dissolved N:P ratios were clearly above Redfield ratios in all of the lakes. Nevertheless, we found positive relationships of chlorophyll a with N fertilization in Lake Brunnsee and Lake Thalersee. However, the chlorophyll a content per cell can be variable (Paasche 1971; Levasseur et al. 1993; Poxleitner et al. 2016), and chlorophyll a per phytoplankton biomass does vary with the community composition (Felip and Catalan 2000; Schindler et al. 2008). Besides the indirect responses of phytoplankton to herbivore grazing pressure (Sommer et al. 1986), the addition of NH4 in our fertilization solution might have promoted the growth of certain algal taxa (Donald et al. 2013; Glibert et al. 2016) or altered the chlorophyll a content per cell (Collos and Harrison 2014). It is known that the utilisation of NH4 and NO3 is highly species specific. It can range from a preference of NH4 to the inhibition of the NO3 uptake (Dortch 1990), and thus, may change the community depending on the original phytoplankton composition. Therefore, detailed investigations on the phytoplankton community composition shifts that are caused by different NO3:NH4 supply ratios are required.
This study suggests that N amounts in the range of an increased atmospheric deposition can lead to changes of food-web dynamics in P deficient ecosystems. Effects of increased N load are well known with focus on phytoplankton; mainly in studies with regard to eutrophication (e.g. Paerl 2009; Rabalais et al. 2009; Donald et al. 2013) with fewer studies in unproductive systems (Bergström et al. 2005; Poxleitner et al. 2016). Here, we observed a decline in the mesozooplankton with N enrichment across all of the three lakes and within the experimental manipulations. The data indicate that increasing dissolved N:P ratios due to N enrichment are able to negatively affect zooplankton, most notably, the cladocerans. The observed lake-specific responses of the different taxa would imply that N enrichment could result in community composition changes, towards a more copepod and rotifer dominated community, containing fewer cladocerans. Since the cladocerans typically represent the most convenient food source for the planktivorous fish (Brooks 1968; Vanni et al. 1987), the predicted progressing P deficiency in ecosystems (Vitousek et al. 2010; Goll et al. 2012) may cause deteriorating food conditions for fish populations.
References
Aber JD, Goodale CL, Ollinger SV, Smith M-L, Magill AH, Martin ME, Hallett RA, Stoddard JL (2003) Is nitrogen deposition altering the nitrogen status of northeastern forests? Bioscience 53:375–389
Andersen T, Hessen DO (1991) Carbon, nitrogen, and phosphorus content of freshwater zooplankton. Limnol Oceanogr 36:807–814
Andersen T, Saloranta TM, Tamminen T (2007) A statistical procedure for unsupervised classification of nutrient limitation bioassay experiments with natural phytoplankton communities. Limnol Oceanogr Methods 5:111–118
Berger SA, Diehl S, Kunz TJ, Albrecht D, Oucible AM, Ritzer S (2006) Light supply, plankton biomass, and seston stoichiometry in a gradient of lake mixing depths. Limnol Oceanogr 51:1898–1905
Bergström A-K, Jansson M (2006) Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Glob Change Biol 12:635–643
Bergström A-K, Blomqvist P, Jansson M (2005) Effects of atmospheric nitrogen deposition on nutrient limitation and phytoplankton biomass in unproductive Swedish lakes. Limnol Oceanogr 50:987–994
Beutler M, Wiltshire KH, Meyer B, Moldaenke C, Lüring C, Meyerhöfer M, Hansen U-P, Dau H (2002) A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth Res 72:39–53
Bottrell HH, Duncan A, Gliwicz ZM, Grygierek E, Herzig A, Hillbricht-Ilkowska A, Kurasawa H, Larsson P, Weglenska T (1976) A review of some problems in zooplankton production studies. Norw J Zool 24:419–456
Brett MT, Müller-Navarra DC, Park S-K (2000) Empirical analysis of the effect of phosphorus limitation on algal food quality for freshwater zooplankton. Limnol Oceanogr 45:1564–1575
Brooks LJ (1968) The effects of prey size selection by lake planktivores. Syst Biol 17:273–291
Camargo JA, Alonso A, Salamanca A (2005) Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58:1255–1267
Carrillo P, Villar-Argaiz M, Medina-Sanchez JM (2001) Relationship between N:P ratio and growth rate during the life cycle of calanoid copepods: an in situ measurement. J Plankton Res 23:537–547
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P (2013) Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Collos Y, Harrison PJ (2014) Acclimation and toxicity of high ammonium concentrations to unicellular algae. Marine Poll Bull 80:8–23
Crowley KF, McNeil BE, Lovett GM, Canham D, Driscoll CT, Rustad LE, Denny E, Hallett RA, Arthur MA, Boggs JL, Goodale CL, Kahl JS, Nulty SG, Ollinger SV, Pardo LH, Schaberg PG, Stoddard JL, Weand MP, Weathers KC (2012) Do nutrient limitation patterns shift from nitrogen toward phosphorus with increasing nitrogen deposition across the northeastern United States? Ecosystems 15:940–957
Darchambeau F, Faerovig PJ, Hessen DO (2003) How Daphnia copes with excess carbon in its food. Oecologia 136:336–346
Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, Lohmann U, Ramachandran S, da Silva Dias PL, Wofsy SC, Zhang X (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Donald D, Bogard M, Finlay K, Bunting L, Leavitt PR (2013) Phytoplankton-specific response to enrichment of phosphorus-rich surface waters with ammonium, nitrate, and urea. PLoS One 8:e53277
Dortch Q (1990) The interaction between ammonium and nitrate uptake in phytoplankton. Mar Ecol Prog Ser 61:183–201
Elser JJ, Marzolf ER, Goldman CR (1990) Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Can J Fish Aquat Sci 46:1468–1477
Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham SS, McCauley E, Schulz KL, Siemann EH, Sterner RW (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580
Elser JJ, Hayakawa K, Urabe J (2001) Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology 82:898–903
Elser JJ, Bracken MES, Cheland EE, Gruner DS, Harpole WS, Hildebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1–8
Elser JJ, Kyle M, Steger L, Nydick KR, Baron JS (2009a) Nutrient availability and phytoplankton nutrient limitation across a gradient of atmospheric nitrogen deposition. Ecology 90:3062–3073
Elser JJ, Andersen T, Baron JS, Bergström AK, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO (2009b) Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326:835–837
Elser JJ, Peace AL, Kyle M, Wojewodzic M, McCrackin ML, Andersen T, Hessen DO (2010) Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol Lett 13:1256–1261
Felip M, Catalan J (2000) The relationship between phytoplankton biovolume and chlorophyll in a deep oligotrophic lake: decoupling spatial and temporal maxima. J Plankton Res 22:91–105
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gersich FM, Hopkins DL (1986) Site-specific acute and chronic toxicity of ammonia to Daphnia magna Straus. Environ Toxicol Chem 5:443–447
Glibert PM, Wilkerson FP, Dugdale RC, Raven JA, Dupont CL, Leavitt PR, Parker AE, Burkholder JM, Kana TM (2016) Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol Oceanogr 61:165–197
Goll DS, Brovkin V, Parida BR, Reick CH, Kattge J, Reich PB, van Bodegom PM, Niinemets Ü (2012) Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 9:3547–3569
Gundersen P, Callesen I, De Vries W (1998) Nitrate leaching in forest ecosystems is related to forest floor C/N ratios. Environ Pollut 102:403–407
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological Statistics software package for education and data analysis, version 2.17. http://folk.uio.no/ohammer/past/. Accessed 5 Jan 2014
Hansen B, Bjornsen PK, Hansen PJ (1994) The size ratio between planktonic predators and their prey. Limnol Oceanogr 39:395–403
Hessen DO (1992) Nutrient element limitation of zooplankton production. Am Nat 140:799–814
Hessen DO (2013) Inorganic nitrogen deposition and its impacts on N:P-ratios and lake productivity. Water 5:327–341
Hessen DO, Faerovig PJ, Andersen T (2002) Light, nutrients, and P:C ratios in algae: grazer performance related to food quality and quantity. Ecology 83:1886–1898
Hessen DO, Elser JJ, Sterner RW, Urabe J (2013) Ecological stoichiometry: an elementary approach using basic principles. Limnol Oceanogr 58:2219–2236
Holmes RM, Aminot A, Kerouel R, Hooker BA, Peterson BJ (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56:1801–1808
Ilic M (2014) Nutrient limitation under increased phosphorus limitation in two Bavarian lakes. Master Thesis, Ludwig-Maximilians University Munich pp. 61
Kim I-N, Lee K, Gruber N, Karl DM, Bullister JL, Yang S, Kim T-W (2014) Increasing anthropogenic nitrogen in the North Pacific Ocean. Science 346:1102–1106
Klausmeier C, Litchman E, Daufresne T, Levin SA (2004) Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429:171–174
Legendre P, Legendre L (1998) Numerical ecology. 2nd English. Elsevier, Amsterdam
Levasseur M, Thompson PA, Harrison PJ (1993) Physiological acclimation of marine phytoplankton to different nitrogen sources. J Phycol 29:587–595
Lovett GM, Arthur MA, Weathers KC, Fitzhugh RD, Templer PH (2013) Nitrogen addition increases carbon storage in soils, but not in trees, in an Eastern U.S. deciduous forest. Ecosystems 16:980–1001
McCarthy SDS, Rafferty SP, Frost PC (2010) Responses of alkaline phosphatase activity to phosphorus stress in Daphnia magna. J Exp Biol 213:256–261
Meunier CL, Boersma M, Wiltshire KH, Malzahn AM (2015) Zooplankton eat what they need: copepod selective feeding and potential consequences for marine systems. Oikos 0:001–009
Mulder K, Bowden WB (2007) Organismal stoichiometry and the adaptive advantage of variable nutrient use and production efficiency in Daphnia. Ecol Modell 202:427–440
Müller-Navarra DC (1995) Biochemical versus mineral limitation in Daphnia. Limnol Oceanogr 40:1209–1214
Müller-Navarra DC, Brett MT, Liston AM, Goldman CR (2000) A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403:64–77
Paasche E (1971) Effect of ammonia and nitrate on growth, photosynthesis, and Ribulose diphosphate carboxylase content of Dunaliella tertiolecta. Physiol Plant 25:294–299
Paerl H (2009) Controlling eutrophication along the freshwater-marine continuum: dual nutrient (N and P) reductions are essential. Estuaries Coasts 32:593–601
Pauli H-R (1989) A new method to estimate individual dry weights of rotifer. Hydrobiologia 186/187:355–361
Plath K, Boersma M (2001) Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology 82:1260–1269
Poxleitner M, Trommer G, Lorenz P, Stibor H (2016) The effect of increased nitrogen load on phytoplankton in a phosphorus-limited lake. Freshw Biol 61:1966–1980
Rabalais NN, Turner RE, Diaz RJ, Justic D (2009) Global change and eutrophication of coastal waters. ICES J Mar Sci 66:1528–1537
Ragueneau O, Tréguer P (1994) Determination of biogenic silica in coastal waters: applicability and limits of the alkaline digestion method. Mar Chem 45:43–51
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1:430–437
Reich PB, Knops J, Tilman D, Craine J, Ellsworth D, Tjoelker M, Lee T, Wedin D, Naeem S, Bahauddin D, Hendrey G, Jose S, Wrage K, Goth J, Bengston W (2001) Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410:809–810
Rockström J, Steffen W, Noone K, Persson A, Chapin FS, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley JA (2009) A safe operating space for humanity. Nature 461:472–475
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 195:260–262
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. PNAS 105:11254–11258
Sommer U (1992) Phosphorus-limited Daphnia: Intraspecific facilitation instead of competition. Limnol Oceanogr 37:966–973
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 4:433–471
Sommer U, Adrian A, De Senerpont Domis L, Elser JJ, Gaedke U, Ibelings B, Jeppesen E, Lürling M, Molinero JC, Mooij WM, van Donk E, Winder M (2012) Beyond the plankton ecology group (PEG) model: mechanisms driving plankton succession. Annu Rev Ecol Evol Syst 43:429–448
Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM, Biggs R, Carpenter SR, de Vries W, de Wit CA, Folke C, Gerten D, Heinke J, Mace GM, Persson LM, Ramanathan V, Reyers B, Sorlin S (2015) Planetary boundaries: guiding human development on a changing planet. Science. doi:10.1126/science.1259855
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton, NJ
Sterner RW, Hessen DO (1994) Algal nutrient limitation and the nutrition of aquatic herbivores. Annu Rev Ecol Syst 25:1–29
Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH (1997) The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am Nat 150:663–684
Trommer G, Pondaven P, Siccha M, Stibor H (2012) Zooplankton-mediated nutrient limitation patterns in marine phytoplankton: an experimental approach with natural communities. Mar Ecol Prog Ser 449:83–94
Tyrrell T (1999) The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400:525–531
Urabe J, Clasen J, Sterner RW (1997) Phosphorus limitation of Daphnia growth: Is it real? Limnol Oceanogr 42:1436–1443
Vanni MJ (1987) Effects of food availability and fish predation on a zooplankton community. Ecol Monogr 57:61–87
Vitousek PM, Aber JD, Howarth RW, Likens GE, Pamela A, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15
Wacker A, von Elert E (2001) Polyunsaturated fatty acids: evidence for non-substitutable biochemical resources in Daphnia galeata. Ecology 82:2507–2520
Watkins J, Rudstam L, Kristen H (2011) Length-weight regression for zooplankton biomass calculations—a review and a suggestion for standard equations. eCommons Cornell. http://hdl.handle.net/1813/24566. Accessed 1 July 2013
Wetzel RG, Likens GE (1991) Limnological analyses. 2nd edn. Springer, New York
Acknowledgements
We wish to thank A. Wild and A. Weigert for technical support and laboratory analyses. We are grateful for the help of colleagues, students and the commune of Seeon for setting up the experiments. We thank the editors U. Sommer and S. Findlay, and two anonymous reviewers for their helpful remarks to improve this manuscript. This study was funded by the German Research Foundation to G.T. (DFG Tr 1126/1–1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trommer, G., Poxleitner, M., Lorenz, P. et al. Altered food-web dynamics under increased nitrogen load in phosphorus deficient lakes. Aquat Sci 79, 1009–1021 (2017). https://doi.org/10.1007/s00027-017-0551-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-017-0551-2