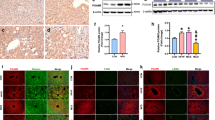
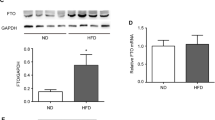
Abstract
Non-alcoholic steatohepatitis (NASH) is associated with obesity and increased expression of hepatic peroxisome proliferator-activated receptor γ (PPARγ). However, the relevance of hepatocyte PPARγ in NASH associated with obesity is still poorly understood. In this study, hepatocyte PPARγ was knocked out (PpargΔHep) in male and female mice after the development of high-fat diet-induced obesity. The diet-induced obese mice were then maintained on their original diet or switched to a high fat, cholesterol, and fructose (HFCF) diet to induce NASH. Hepatic PPARγ expression was mostly derived from hepatocytes and increased by high fat diets. PpargΔHep reduced HFCF-induced NASH progression without altering steatosis, reduced the expression of key genes involved in hepatic fibrosis in HFCF-fed male and female mice, and decreased the area of collagen-stained fibrosis in the liver of HFCF-fed male mice. Moreover, transcriptomic and metabolomic data suggested that HFCF-diet regulated hepatic amino acid metabolism in a hepatocyte PPARγ-dependent manner. PpargΔHep increased betaine-homocysteine s-methyltransferase expression and reduced homocysteine levels in HFCF-fed male mice. In addition, in a cohort of 102 obese patients undergoing bariatric surgery with liver biopsies, 16 cases were scored with NASH and were associated with increased insulin resistance and hepatic PPARγ expression. Our study shows that hepatocyte PPARγ expression is associated with NASH in mice and humans. In male mice, hepatocyte PPARγ negatively regulates methionine metabolism and contributes to the progression of fibrosis.









Similar content being viewed by others
Data availability
Transcript profiling: Gene Expression Omnibus (GEO) # GSE200352.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- LF/LFD:
-
Low fat diet
- HF/HFD:
-
High fat diet
- HFCF:
-
High fat, cholesterol and fructose
- BMI:
-
Body mass index
- HbA1c:
-
Glycated hemoglobin A1c
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- ALT:
-
Alanine aminotransferase
- TG:
-
Triglycerides
- NEFA:
-
Non-esterified fatty acids
- GTT:
-
Glucose tolerance test
- ITT:
-
Insulin tolerance test
- H&E:
-
Hematoxylin and eosin
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- DNL:
-
De novo lipogenesis
- Pparg ΔHep :
-
Adult-onset hepatocyte-specific PPARγ knockout
- TZD:
-
Thiazolidinediones
- NAS:
-
NAFLD Activity Score
- DEG:
-
Differentially expressed gene
- GO:
-
Gene ontology
- SAM:
-
S-adenosylmethionine
- Hcy:
-
Homocysteine
References
Younossi ZM, Tampi RP, Racila A, Qiu Y, Burns L, Younossi I, Nader F (2020) Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the US. Diabetes Care 43(2):283–289
Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, Singal AG (2018) Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the united states: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 16(2):198-210 e2
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ (2018) Mechanisms of NAFLD development and therapeutic strategies. Nat Med 24(7):908–922
Loomba R, Friedman SL, Shulman GI (2021) Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184(10):2537–2564
Tsuchida T, Friedman SL (2017) Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14(7):397–411
Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77:289–312
Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM (2001) PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7(1):48–52
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM (2013) PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med 19(5):557–566
Vidal-Puig AJ, Considine RV, Jimenez-Linan M, Werman A, Pories WJ, Caro JF, Flier JS (1997) Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 99(10):2416–2422
Pettinelli P, Videla LA (2011) Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab 96(5):1424–1430
Lima-Cabello E, Garcia-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillon J, Lozano-Rodriguez T, Fernandez-Bermejo M, Olcoz JL, Gonzalez-Gallego J, Garcia-Monzon C, Sanchez-Campos S (2011) Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond) 120(6):239–250
Nakamuta M, Kohjima M, Morizono S, Kotoh K, Yoshimoto T, Miyagi I, Enjoji M (2005) Evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 16(4):631–635
Lee SM, Pusec CM, Norris GH, de Jesus A, Diaz-Ruiz A, Muratalla JT, Sarmento-Cabral A, Guzman G, Layden BT, Cordoba-Chacon J (2021) Hepatocyte-specific loss of PPARγ protects mice from NASH, and increases the therapeutic effects of rosiglitazone in the liver. Cell Mol Gastroenterol Hepatol 11(5):1291–1311
Lee SM, Muratalla J, Diaz-Ruiz A, Remon-Ruiz P, McCann M, Liew CW, Kineman RD, Cordoba-Chacon J (2021) Rosiglitazone requires hepatocyte PPARgamma expression to promote steatosis in male mice with diet-induced obesity. Endocrinology 162(11):bqab175
Cordoba-Chacon J (2020) Loss of hepatocyte-specific PPARgamma expression ameliorates early events of steatohepatitis in mice fed the methionine and choline-deficient diet. PPAR Res 2020:9735083
Wolf Greenstein A, Majumdar N, Yang P, Subbaiah PV, Kineman RD, Cordoba-Chacon J (2017) Hepatocyte-specific, PPARgamma-regulated mechanisms to promote steatosis in adult mice. J Endocrinol 232(1):107–121
Gao M, Ma Y, Alsaggar M, Liu D (2016) Dual outcomes of rosiglitazone treatment on fatty liver. AAPS J 18(4):1023–1031
Moran-Salvador E, Titos E, Rius B, Gonzalez-Periz A, Garcia-Alonso V, Lopez-Vicario C, Miquel R, Barak Y, Arroyo V, Claria J (2013) Cell-specific PPARgamma deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J Hepatol 59(5):1045–1053
Moran-Salvador E, Lopez-Parra M, Garcia-Alonso V, Titos E, Martinez-Clemente M, Gonzalez-Periz A, Lopez-Vicario C, Barak Y, Arroyo V, Claria J (2011) Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J 25(8):2538–2550
Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, Suzuki Y, Saito H, Kohgo Y, Okumura T (2005) Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 336(1):215–222
Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML (2003) Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278(36):34268–34276
Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B Jr, Reitman ML, Gonzalez FJ (2003) Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 111(5):737–747
de Conti A, Tryndyak V, Willett RA, Borowa-Mazgaj B, Watson A, Patton R, Khare S, Muskhelishvili L, Olson GR, Avigan MI, Cerniglia CE, Ross SA, Sanyal AJ, Beland FA, Rusyn I, Pogribny IP (2020) Characterization of the variability in the extent of nonalcoholic fatty liver induced by a high-fat diet in the genetically diverse Collaborative Cross mouse model. FASEB J 34(6):7773–7785
Drescher HK, Weiskirchen R, Fulop A, Hopf C, de San Roman EG, Huesgen PF, de Bruin A, Bongiovanni L, Christ A, Tolba R, Trautwein C, Kroy DC (2019) The influence of different fat sources on steatohepatitis and fibrosis development in the western diet mouse model of non-alcoholic steatohepatitis (NASH). Front Physiol 10:770
Gaskell H, Ge X, Desert R, Das S, Han H, Lantvit D, Guzman G, Nieto N (2020) Ablation of Hmgb1 in intestinal epithelial cells causes intestinal lipid accumulation and reduces NASH in mice. Hepatol Commun 4(1):92–108
Clapper JR, Hendricks MD, Gu G, Wittmer C, Dolman CS, Herich J, Athanacio J, Villescaz C, Ghosh SS, Heilig JS, Lowe C, Roth JD (2013) Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol 305(7):G483–G495
Cordoba-Chacon J, Gahete MD, McGuinness OP, Kineman RD (2014) Differential impact of selective GH deficiency and endogenous GH excess on insulin-mediated actions in muscle and liver of male mice. Am J Physiol Endocrinol Metab 307(10):E928–E934
Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K (2012) Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56(5):1751–1759
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, N. Nonalcoholic Steatohepatitis Clinical Research (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6):1313–1321
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Cordoba-Chacon J, Gahete MD, Castano JP, Kineman RD, Luque RM (2011) Somatostatin and its receptors contribute in a tissue-specific manner to the sex-dependent metabolic (fed/fasting) control of growth hormone axis in mice. Am J Physiol Endocrinol Metab 300(1):E46-54
Cordoba-Chacon J, Sarmento-Cabral A, Del Rio-Moreno M, Diaz-Ruiz A, Subbaiah PV, Kineman RD (2018) Adult-onset hepatocyte GH resistance promotes NASH in male mice, without severe systemic metabolic dysfunction. Endocrinology 159(11):3761–3774
Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques PE, Li S, Xia J (2021) MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 49(W1):W388–W396
Kineman R, Majumdar N, Subbaiah PV, Cordoba-Chacon J (2016) Hepatic PPARγ is not essential for the rapid development of steatosis following loss of hepatic GH signaling, in adult male mice. Endocrinology 157(50):1728–1735
Lee YJ, Ko EH, Kim JE, Kim E, Lee H, Choi H, Yu JH, Kim HJ, Seong JK, Kim KS, Kim JW (2012) Nuclear receptor PPARgamma-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci USA 109(34):13656–13661
Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK (2003) Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem 278(1):498–505
Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W, Yang XF, Wang H (2010) Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J 24(8):2804–2817
Dai H, Wang W, Tang X, Chen R, Chen Z, Lu Y, Yuan H (2016) Association between homocysteine and non-alcoholic fatty liver disease in Chinese adults: a cross-sectional study. Nutr J 15(1):102
Dai Y, Zhu J, Meng D, Yu C, Li Y (2016) Association of homocysteine level with biopsy-proven non-alcoholic fatty liver disease: a meta-analysis. J Clin Biochem Nutr 58(1):76–83
Torres L, Garcia-Trevijano ER, Rodriguez JA, Carretero MV, Bustos M, Fernandez E, Eguinoa E, Mato JM, Avila MA (1999) Induction of TIMP-1 expression in rat hepatic stellate cells and hepatocytes: a new role for homocysteine in liver fibrosis. Biochim Biophys Acta 1455(1):12–22
Pacana T, Cazanave S, Verdianelli A, Patel V, Min HK, Mirshahi F, Quinlivan E, Sanyal AJ (2015) Dysregulated hepatic methionine metabolism drives homocysteine elevation in diet-induced nonalcoholic fatty liver disease. PLoS ONE 10(8):e0136822
Dubois V, Eeckhoute J, Lefebvre P, Staels B (2017) Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest 127(4):1202–1214
Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, Lefebvre P, Taskinen MR, Van Hul W, Mertens I, Hubens G, Van Marck E, Michielsen P, Van Gaal L, Staels B (2015) PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol 63(1):164–173
Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T, Group LS (2010) Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51(2):445–453
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362(18):1675–1685
Jia X, Zhai T (2019) Integrated analysis of multiple microarray studies to identify novel gene signatures in non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 10:599
Namjou B, Lingren T, Huang Y, Parameswaran S, Cobb BL, Stanaway IB, Connolly JJ, Mentch FD, Benoit B, Niu X, Wei WQ, Carroll RJ, Pacheco JA, Harley ITW, Divanovic S, Carrell DS, Larson EB, Carey DJ, Verma S, Ritchie MD, Gharavi AG, Murphy S, Williams MS, Crosslin DR, Jarvik GP, Kullo IJ, Hakonarson H, Li R, Xanthakos SA, Harley JB (2019) GWAS and enrichment analyses of non-alcoholic fatty liver disease identify new trait-associated genes and pathways across eMERGE Network. BMC Med 17(1):135
Frohlich J, Kovacovicova K, Mazza T, Emma MR, Cabibi D, Foti M, Sobolewski C, Oben JA, Peyrou M, Villarroya F, Soresi M, Rezzani R, Cervello M, Bonomini F, Alisi A, Vinciguerra M (2020) GDF11 induces mild hepatic fibrosis independent of metabolic health. Aging (Albany NY) 12(20):20024–20046
Wang W, Xu MJ, Cai Y, Zhou Z, Cao H, Mukhopadhyay P, Pacher P, Zheng S, Gonzalez FJ, Gao B (2017) Inflammation is independent of steatosis in a murine model of steatohepatitis. Hepatology 66(1):108–123
Kawashita E, Ishihara K, Nomoto M, Taniguchi M, Akiba S (2019) A comparative analysis of hepatic pathological phenotypes in C57BL/6J and C57BL/6N mouse strains in non-alcoholic steatohepatitis models. Sci Rep 9(1):204
Kiourtis C, Wilczynska A, Nixon C, Clark W, May S, Bird TG (2021) Specificity and off-target effects of AAV8-TBG viral vectors for the manipulation of hepatocellular gene expression in mice. Biol Open 10(9):bio058678
Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE (1996) Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97(11):2553–2561
Zhang YL, Hernandez-Ono A, Siri P, Weisberg S, Conlon D, Graham MJ, Crooke RM, Huang LS, Ginsberg HN (2006) Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem 281(49):37603–37615
Schadinger SE, Bucher NL, Schreiber BM, Farmer SR (2005) PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab 288(6):E1195–E1205
Zhang H, Leveille M, Courty E, Gunes A, Bich NN, Estall JL (2020) Differences in metabolic and liver pathobiology induced by two dietary mouse models of nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 319(5):E863–E876
Harris SE, Poolman TM, Arvaniti A, Cox RD, Gathercole LL, Tomlinson JW (2020) The American lifestyle-induced obesity syndrome diet in male and female rodents recapitulates the clinical and transcriptomic features of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 319(3):G345–G360
Pafili K, Paschou SA, Armeni E, Polyzos SA, Goulis DG, Lambrinoudaki I (2022) Non-alcoholic fatty liver disease through the female lifespan: the role of sex hormones. J Endocrinol Invest 2022:1–15
Hansen HH, Ægidius HM, Oro D, Evers SS, Heeboll S, Eriksen PL, Thomsen KL, Bengtsson A, Veidal SS, Feigh M, Suppli MP, Knop FK, Gronbaek H, Miranda D, Trevaskis JL, Vrang N, Jelsing J, Rigbolt KTG (2020) Human translatability of the GAN diet-induced obese mouse model of non-alcoholic steatohepatitis. BMC Gastroenterol 20(1):210
Boland ML, Oro D, Tolbol KS, Thrane ST, Nielsen JC, Cohen TS, Tabor DE, Fernandes F, Tovchigrechko A, Veidal SS, Warrener P, Sellman BR, Jelsing J, Feigh M, Vrang N, Trevaskis JL, Hansen HH (2019) Towards a standard diet-induced and biopsy-confirmed mouse model of non-alcoholic steatohepatitis: Impact of dietary fat source. World J Gastroenterol 25(33):4904–4920
Radhakrishnan S, Yeung SF, Ke JY, Antunes MM, Pellizzon MA (2021) Considerations when choosing high-fat, high-fructose, and high-cholesterol diets to induce experimental nonalcoholic fatty liver disease in laboratory animal models. Curr Dev Nutr 5(12):nzab138
Bjorkhem I (2002) Do oxysterols control cholesterol homeostasis? J Clin Invest 110(6):725–730
Zhang Y, Breevoort SR, Angdisen J, Fu M, Schmidt DR, Holmstrom SR, Kliewer SA, Mangelsdorf DJ, Schulman IG (2012) Liver LXRalpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest 122(5):1688–1699
Bhushan B, Banerjee S, Paranjpe S, Koral K, Mars WM, Stoops JW, Orr A, Bowen WC, Locker J, Michalopoulos GK (2019) Pharmacologic inhibition of epidermal growth factor receptor suppresses nonalcoholic fatty liver disease in a murine fast-food diet model. Hepatology 70(5):1546–1563
Li Z, Wang F, Liang B, Su Y, Sun S, Xia S, Shao J, Zhang Z, Hong M, Zhang F, Zheng S (2020) Methionine metabolism in chronic liver diseases: an update on molecular mechanism and therapeutic implication. Signal Transduct Targ Ther 5(1):280
Radziejewska A, Muzsik A, Milagro FI, Martinez JA, Chmurzynska A (2020) One-carbon metabolism and nonalcoholic fatty liver disease: the crosstalk between nutrients, microbiota, and genetics. Lifestyle Genom 13(2):53–63
Acknowledgements
We thank Angelie Bacon, Danielle Pins, Dr. Gregory Norris for their technical assistance. Fixed samples and H&E-stained slides were processed by the Research Histology Core at the University of Illinois at Chicago. Metabolomics services were performed by the Metabolomics Core Facility at Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Funding
This work was supported by the National Institutes of Health K01DK125525, R01DK131038, R03DK129419 [JCC], Talento Grant 2018-T1/BMD-11966 [ADR], Institute of Health Carlos III (ISCIII) PI20/00505, CP19/00098 [BRM].
Author information
Authors and Affiliations
Contributions
JCC conceived and designed experiments. JCC and SML wrote the manuscript. JCC, SML, JM, and ADR performed the experiment and analyzed the data. SML, SK, and GG performed a pathological analysis of the liver sections. MDF and BRM performed the experiments in patient samples and analyzed the data. SML, JM, ADR, and BRM revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preprint server: An early version of this study was uploaded to bioRxiv, https://doi.org/10.1101/2022.06.06.494901.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S.M., Muratalla, J., Karimi, S. et al. Hepatocyte PPARγ contributes to the progression of non-alcoholic steatohepatitis in male and female obese mice. Cell. Mol. Life Sci. 80, 39 (2023). https://doi.org/10.1007/s00018-022-04629-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04629-z