Abstract
Abnormal mossy fiber connections in the hippocampus have been implicated in schizophrenia. However, it remains unclear whether this abnormality in the patients is genetically determined and whether it contributes to the onset of schizophrenia. Here, we showed that iPSC-derived hippocampal NPCs from schizophrenia patients with the A/A allele at SNP rs16864067 exhibited abnormal NPC polarity, resulting from the downregulation of SOX11 by this high-risk allele. In the SOX11-deficient mouse brain, abnormal NPC polarity was also observed in the hippocampal dentate gyrus, and this abnormal NPC polarity led to defective hippocampal neurogenesis—specifically, irregular neuroblast distribution and disrupted granule cell morphology. As granule cell synapses, the mossy fiber pathway was disrupted, and this disruption was resistant to activity-induced mossy fiber remodeling in SOX11 mutant mice. Moreover, these mutant mice exhibited diminished PPI and schizophrenia-like behaviors. Activation of hippocampal neurogenesis in the embryonic brain, but not in the adult brain, partially alleviated disrupted mossy fiber connections and improved schizophrenia-related behaviors in mutant mice. We conclude that disrupted mossy fiber connections are genetically determined and strongly correlated with schizophrenia-like behaviors in SOX11-deficient mice. This disruption may reflect the pathological substrate of SOX11-associated schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a severe mental illness resulting from malformation of neural circuits [1] in the brain. For example, mossy fiber connections, a key component of the trisynaptic circuit in the hippocampus, have been reported to be altered in the brain of schizophrenia patients [2, 3]. Notably, however, mossy fibers are under constant remodeling with high dynamics in the adolescent brain [4] and vulnerable to disease conditions [5]. Therefore, it is difficult to determine whether the alterations of mossy fibers are just an outcome, or, instead, an etiological factor of schizophrenia.
Mossy fibers are hippocampal granule cell axons that primarily connect to pyramidal neurons in the CA3 region [6]. The development of mossy fibers involves both embryonic and adult neurogenesis in the hippocampus. Hippocampal neurogenesis starts from embryonic day 14.5 (E14.5) in the mouse brain [7]. At this stage, the dentate neuroepithelium begins to migrate along the dentate migration stream (DMS) to form the primitive dentate gyrus (DG). During DMS migration, SOX2+ dentate neuroepithelium differentiates into TBR2 + intermediate precursors (IPCs). In turn, these precursors differentiate further into DCX+ neuroblasts, which eventually become PROX1+ granule cells at the primitive DG [8, 9]. In the neonatal mouse brain, embryonic neurogenesis shifts to a slower adult neurogenesis [8]. During the transition from embryonic to adult neurogenesis, the DG granule cells project mossy fibers to CA3 pyramidal neurons to form the trisynaptic circuit in the hippocampus [10]. It has been reported that abnormal mossy fiber connections to CA3 neurons were observed in patients with psychiatric disorders including schizophrenia [5, 11].
The formation of appropriate mossy fiber connections is composed of two phases: genetically determined assembly of synapses and activity-dependent remodeling [12]. In the P0 mouse brain, the DG granule cells begin to project mossy fibers [13]. At P7, mossy fibers reach the stratum lucidum (SL), stratum oriens (SO), stratum pyramidale (SP), and other layers [14] in the hippocampal CA3 region, and their maturation in the neonatal brain is completed around P21 [15]. After maturation in the neonatal brain, mossy fiber connections are mostly restricted to the SL region. Non-mature mossy fiber connections to either the SO or SP region must be pruned during circuit remodeling to avoid excessive excitatory connections, which were linked with schizophrenia [5]. Both embryonic and adult neurogenesis contribute to the formation of appropriate mossy fiber connections, failure of which leads to defects in memory [16] and cognition [17] and schizophrenia-like behaviors [18].
Recently, an independent case–control study reported that single-nucleotide polymorphism (SNP) rs16864067 in 3ʹ UTR of SOX11 had a significant genome-wide association with schizophrenia [19]. SOX11 is a member of the SRY-related high-mobility-group C (Sox-C) subfamily of transcription factors [20]. It has been documented that, sometimes acting in redundancy with other Sox-C members, SOX11 is required for neuronal differentiation during hippocampal neurogenesis [21]. It is known that SOX11 mutations are associated with Coffin–Siris syndrome [22, 23], and some of these patients show mental retardation and have a high risk of psychiatric disorders [24]. However, the mechanism underlying the pathological alteration of both disorders is unclear.
In the present study, we collected blood samples from patients with a high-risk A/A allele or healthy controls with a low-risk G/G allele at SNP rs16864067 (Fig. S1A, B). Using blood cells, we generated induced pluripotent stem cells (iPSCs). Because human SOX11 expression is significantly higher in hippocampal neural progenitor cells (NPCs) than in human cortex NPCs [25], but most iPSC studies of schizophrenia have been performed on cortical neurons [26], we differentiated iPSCs further into hippocampal NPCs [27] to study whether SOX11-associated schizophrenia could result from abnormal hippocampal neurogenesis. To this end, we found that high-risk SNP rs16864067 directly downregulated the expression of SOX11 (Fig. S1A–S1C). Moreover, we observed that Sox11-deficient NPCs exhibited abnormal cell polarity and defective neuronal differentiation. Given that neuronal polarity is associated with abnormal mossy fiber sprouting in the brain [28], we investigated the hippocampal neurogenesis, particularly mossy fiber connections, in the SOX11-deficient mouse brain to probe possible pathological alterations related to SNP rs16864067-associated schizophrenia.
Methods
Generation and characterization of human iPSC lines
Human iPSC lines were established from peripheral blood cells (PMNCs) [29]. PMNCs were isolated by density gradient centrifuging with Ficoll-Paque Plus (GE Healthcare, Waukesha). PMNCs (5 × 106) were introduced with 3 mg of expression plasmid (a combination of plasmids encoding OCT3/4, SOX2, KLF4, L-MYC, LIN28, EBNA1 and shRNA for TP53) mixture by electroporation using Nucleofector 2b Device (Lonza, Basel, Switzerland) and Amaxa Human T-cell Nucleofector kit (Lonza). After transfection, the cells were cultured in X-Vivo10 (Lonza) supplemented with 30 U/ml IL-2 (PeproTech) and 5 μl/well of Dynabeads Human T-activator CD3/CD28 (Thermo Fisher). 2 days after later, an equal volume of ESC medium mTESR (STEM CELL) with 10 μm Y27632 (STEMCELL) was added into the previous medium. 4 days after the transfection, the culture medium was replaced with ESC medium with 10 μm Y27632. The colonies were counted 4 weeks after plating, and were selected for further cultivation and characterization.
Generation of genetically modified isogeneic hESCs mediated by CRISPR–CAS9
Genome editing hESCs (H1) was carried out according to the protocol “CRISPR/CAS9 Gene Editing of Human Induced Pluripotent Stem Cells (iPSCs)” from Sigma. CRISPR single guide RNAs (sgRNAs) were cloned into sgRNA/CAS9 expressing plasmid pX330 tagged with GFP (Addgene, Cambridge, MA, USA). GFP+ cells were FACS-sorted and plated as one cell/per well. Single colony was picked and grew for approximately 5 days before PCR analysis for on-target and off-target mutagenesis.
Differentiation of human PSCs into hippocampal NPCs
Differentiation of hippocampal NPCs was performed as previously published [27]. Briefly, EBs were treated with DKK1 (0.5 µg/ml), SB431542 (10 µM), noggin (0.5 µg/ml), and cyclopamine (1 µM) in NeuroCul NS-A Basal Medium (Human) plus N2 and B27 supplements. The treatment was continued for about 3 weeks followed by plating onto polyornithine/laminin-coated dishes in Neurobasal Medium (N2 and B27).
Statistical analysis
All statistical tests and sample sizes were included in the figure legends. All data were shown as mean ± SEM. Statistical analysis was performed using GraphPad Prism 8.0.1 with either Student’s t test or ANOVA followed by Dunnett’s multiple comparisons test or Tukey’s multiple comparisons test (more than three groups). p < 0.05 was considered statistically significant.
Results
SOX11 deficiency caused abnormal polarity in hippocampal NPCs derived from schizophrenia patients or isogenic human ESCs
We first characterized the pluripotency in the established iPSCs. We did not see any significant difference in morphology and pluripotent markers between the four schizophrenia patients (P1–P4) and the paired cohort controls (C1–C4) (Fig. S1D). In addition, iPSCs from both the patients and the controls efficiently differentiated into neural ectoderm, as indicated by PAX6 and NESTIN immunofluorescence (Fig. S1E). However, a difference emerged when the cells differentiated further into hippocampal NPCs. First, the SOX11 expression decreased significantly in NPCs derived from the patients (Fig. 1A and B). Second, based on immunofluorescent staining of ZO-1, a tight junction protein [30], NPCs from healthy controls formed well-structured neural rosettes (rosette formation), indicated by asymmetric apical localization of ZO-1. In contrast, NPCs from the patients failed to form rosettes (Fig. 1C and D). Because neural rosette structures recapitulate the apical–basal polarity of NPCs in the brain [31], the failure of rosette formation indicated abnormal cell polarity in the hippocampal NPCs derived from schizophrenia patients’ iPSCs. These results indicated that SOX11 deficiency may cause abnormal polarity in hippocampal NPCs.
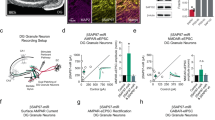
SOX11 deficiency caused abnormal polarity in NPCs derived from schizophrenia patients. SOX11 mRNA (A) and the protein (B) in NPC derived from four schizophrenia patients or the paired controls. Error bars, mean ± SEM, n = 4 cultures, *p < 0.05 (Student’s t test). C Formation of neural rosettes by immunostaining of ZO-1 (red) and PAX6 (green). Scale bar, 20 μm. D Percentage of neural rosettes in NPC colonies from C. Error bars, mean ± SEM, n = 4 cultures, **p < 0.01 (Student’s t test). E Neural rosettes and the intensity of SOX11 in the circled NPCs derived from ESCs with either the A/A- or G/G allele. Error bars, mean ± SEM, n = 32 cells, **p < 0.01 (Student’s t test). F Ectopic expression of SOX11 in NPCs differentiated from ESCs with the A/A allele (upper panel) and knockdown of SOX11 in NPCs differentiated from ESCs with the G/G allele. Scale bar, 20 μm. Quantifications of neural rosettes from F. Error bars, mean ± SEM, n = 3 cultures, *p < 0.05, (Student’s t test). G Immunostaining TUJ1 in young neurons differentiated from isogenic ESCs, patients, or the controls. Scale Bar, 20 μm, and the percentage of TUJ1+ cells in total DAPI + cells. Error bars, mean ± SEM, n = 3 cultures, *p < 0.05 (Student’s t test)
To avoid bias due to genetic variation in patients, we edited human H1 embryonic stem cells (ESCs) using CRISPR–Cas9-based technology to generate isogenic ESCs with either an A/A allele or a G/G allele at SNP rs16864067 (Fig. S2A). We then differentiated these cells into hippocampal NPCs and found that rosette formation was indeed defective (Fig. 1E), and SOX11 mRNA and protein levels (Fig. S2B and S2C) were both compromised in these NPCs derived from A/A allele ESCs. We examined the immunofluorescence of SOX11 in the area of neural rosettes formed by G/G allele NPCs or the same-size area of A/A allele NPCs. SOX11 fluorescence correlated well with rosette formation in these cells (Fig. 1E). Moreover, after introducing SOX11 cDNA without inclusion of SNP rs16864067 into the A/A allele NPCs from ESCs, we observed that the defective rosette formation was significantly corrected (Fig. 1F, upper panels). Downregulation of SOX11 in G/G allele NPCs by SOX11-shRNA resulted in abnormal polarity in NPCs (Fig. 1F, lower panels). Therefore, we concluded that the high-risk allele at SNP rs16864067 led to abnormal polarity in NPCs due to SOX11 deficiency.
To further evaluate the functional consequences of impaired NPC polarity, we calculated TUJ1 + neuron numbers after NPCs’ neuronal differentiation. There were much fewer TUJ1 + young neurons (YN) (Fig. 1G) differentiated from patient iPSCs or A/A allele ESCs than from the G/G control samples, which instead had many more cPARP + apoptotic cells (Fig. S2D). We therefore concluded that high-risk SNP rs16864067 downregulated SOX11 expression, leading to abnormal NPC polarity and defective neuronal differentiation.
Abnormal NPC polarity, irregular neuroblast distribution, and disrupted morphology of granule cells in SOX11-mutant hippocampus
The polarity of NPCs is crucial for brain development [32]. To investigate whether SOX11 deficiency causes abnormal NPC polarity in the mouse brain, we deleted Sox11 selectively from the telencephalon, since Sox11 is mainly expressed in this region [33]. Thinking about the dose effect of SNP rs16864067 on the expression of SOX11, we studied the brain development in both Sox11cHET and Sox11cKO mice. Consistent with the observation by Shim and colleagues [34], we did not find any obvious changes in brain size. There were no apparent gross defects in either the cerebrum or the olfactory bulb (Fig. S3A–S3C) in the adult mutant brain. Immunostaining for SOX2 in the subventricular zone (SVZ) and DCX in the SVZ-RMS-OB region [35], did not show an obvious difference between the mutant and the control mice (Fig. S3D and S3E), suggesting that the cerebral cortex neurogenesis had changed little. In contrast, there was abnormal neuron distribution in the DG region and a significant reduction in PROX1-positive granule cells in the hippocampus of heterozygous (Sox11cHET) or homogeneous (Sox11cKO) mice (Fig. S3F and S3G).
Furthermore, we performed behavioral measurements to evaluate functional alterations in the hippocampus of mutant mice. Non-hippocampus-dependent novel object recognition (NOR) [36] did not differ between the mutant mice and the controls (Fig. S3H), whereas hippocampus-dependent object–place recognition (OPR) [36] was significantly altered in the mutant mice (Fig. S3I). These data suggested that SOX11 deficiency may cause defects in the development and related functions of the hippocampus.
To investigate the cellular mechanisms in the SOX11-deficient hippocampus, we performed immunofluorescence and found that most HOPX + NPCs in the wild-type mouse brain lined up along the subgranular zone (SGZ). In contrast, in Sox11cKO mice, 22% of NPCs stayed at the hilus and 27% of the cells migrated from SGZ to GCL (Fig. 2A and B). Sox11cHET mice also exhibited similar alterations (Fig. 2A and B). NPCs were not restricted to SGZ and were distributed randomly in the granule cell layer (GCL) and hilus. Notably, unlike NPCs of wild-type mice, which showed a radial shape, about half of NPCs in the mutant brains showed multipolar morphology (Fig. 2C and D), indicating that SOX11 is required for appropriate NPC polarity in the hippocampus.
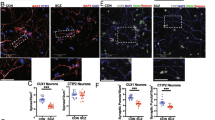
SOX11 deficiency results in random distribution and irregular morphology of hippocampal granule cells and causes diminished PPI. A Abnormal distribution of HOPX + NPCs in the DG in the SOX11-mutant adult brain. Scale bar, 100 μm. B Quantifications of HOPX + NPCs in the granule cell layer, SGZ, and hilus within the DG in adult mouse brain, n = 3 mice. Morphology (C) and quantification (D) of radial or multipolar NPCs in the DG in the SOX11-deficient or control mouse brain. Scale bar, 20 μm. Error bars, mean ± SEM, n = 5 mice. (Student’s t test). Distribution (E), quantification (F) and average dendrite length (G) of DCX + neuroblasts in the SOX11-deficient or control mouse hippocampus. Scale bar, 50 μm. Error bars, mean ± SEM (n = 7, *p < 0.05, one-way ANOVA). H Golgi–Cox staining of the hippocampus (coronal section) from the SOX11-deficient or control mouse brain. Scale bar, 50 μm. I–J Sholl analyses of dendritic complexity of dentate granule cells in the SOX11-deficient or control mouse brain (I). Cumulative distribution of branch numbers of neurons in the DG granule cells in SOX11-deficient or control mouse brain. Error bars, mean ± SEM, n = 27, *p < 0.05, **P < 0.01 versus the indicated group (Student’s t test). K Mossy fiber connections decreased to the SL region but increased to the SP and SO regions in the SOX11-deficient hippocampal CA3 region. Error bars, mean ± SEM, n = 3 mice, *p < 0.05, (one-way ANOVA). L PPI was diminished in the SOX11-deficient mice. Error bars, mean ± SEM, n = 13, *p < 0.05 (one-way ANOVA)
To assess the impact of NPC polarity on hippocampal neurogenesis, we examined the number of DCX + neuroblasts in the DG region. Neuroblasts were significantly reduced, and their distribution was obviously disrupted (Fig. 2E and F), in the SOX11 mutant hippocampus. In addition, the average dendrite length in these neuroblasts significantly decreased (Fig. 2G). To investigate the cause of the reduction in hippocampal cells, we measured cell proliferation and apoptosis by immunostaining Ki67 and cPARP, respectively. All the Ki67 + cells decreased significantly (Fig. S4A), with both HOPX + NPCs (Fig. S4B) and TBR2+ IPCs (Fig. S4C) decreased significantly in the mutant hippocampus. Given that there was no increase in cPARP + cells (Fig. S4D) in the hippocampus of the mutant brain, we concluded that abnormal NPC polarity caused defects in the differentiation of neuroblasts and granule cells (Fig. S3G) in the hippocampal DG region.
Disrupted mossy fiber connection, diminished PPI, and behavior disorders in SOX11-deficient mice
To further investigate the pathological changes in the mutant brain, we examined neuronal dendrite structures through Golgi–Cox staining. The hippocampal DG neurons showed an abnormal distribution (Fig. 2H) in the mutant mice. The branch number and the complexity of hippocampal neurons in the mutant mice significantly decreased (Fig. 2I and J). In contrast, there were no obvious differences in morphology, distribution, and the dendrite structure of pyramidal neurons in cortical areas between the control and SOX11-mutant mice (Fig. S5A–S5D). In the mutant mice, while pyramidal neurons exhibited disrupted dendrite structure in the hippocampal CA3 region (Fig. S5F), the CA1 region did not show obvious defects (Fig. S5E).
Given the irregular distribution and the morphology of DG granule cells in the SOX11-deficient hippocampus (Fig. 2H–J and S3G), as granule cell synapses, the mossy fibers were evaluated in the DG–CA3 pathway. In the normal hippocampus, mossy fibers selectively projected to the SL region (> 80%) (Fig. 2K, blue). In the mutant hippocampus, however, mossy fibers were not restricted to the SL region; instead, more than half of the mossy fibers projected to the wrong targets in the SO and SP regions, indicated by staining of KA-1, a glutamate receptor that is mainly expressed on pyramidal cells in the SP layer [37] (Fig. 2K). To examine if these misconnected mossy fibers were functional abnormal, the mossy fibers at the DG–hilus border were stimulated, and excitatory postsynaptic potentials (EPSPs) were recorded in the CA3 pyramidal cell layer. Compared with the wild-type controls, we found that, with increasing frequency of stimulations, neural adaption was altered in the hippocampal DG–CA3 pathway of SOX11-mutant brain (Fig. S6B–S6D), indicating abnormal mossy fiber functions in the mutant mice [38].
Since abnormal mossy fiber function may induce hippocampal hyperactivity [5] that causes diminished prepulse inhibition (PPI) [39], we measured PPI and found that PPI was significantly diminished in all of the stimulating conditions in SOX11-mutant mice (Fig. 2L). It has been documented that disrupted neural circuitry and diminished PPI are the pathological and behavioral hallmarks of schizophrenia [40, 41]. Therefore, we proposed that SOX11 deficiency may contribute to the susceptibility to schizophrenia due to the disrupted mossy fiber connections.
Anxiety and social dysfunction are prominent features of schizophrenia [42, 43]. We further measured animal anxiety using elevated plus maze [44]. In this test, a preference for enclosed space and avoidance of open height indicate anxiety. As expected, SOX11-deficient mice spent more time than the controls in the closed arms of the maze (Fig. S7A), indicating that SOX11 deficiency exacerbated animal anxiety. We also measured social novelty recognition as well as social challenge between the controls and SOX11-mutant mice using a three-chamber test [45]. SOX11-mutant mice had poor social recognition (Fig. S7B); they tended to avoid unfamiliar mice (Fig. S7C). We concluded that pathological alterations from SOX11 deficiency caused hippocampal dysfunction in the brains of mutant mice.
Disturbed neurogenesis at E13.5 and disrupted mossy fiber connections at P7 were discovered in the SOX11-deficient hippocampus
To determine when these pathological defects were initiated in the mutant hippocampus, we first examined postnatal DG at P7, the beginning of adult neurogenesis [8]. In the normal hippocampus, more than half of NPCs were concentrated at the SGZ region. In contrast, NPCs in the mutant hippocampus were randomly distributed, and about 68% of these cells were scattered from the SGZ (Fig. 3A and B). Unlike the appropriate distribution of granule cells in the hippocampal DG region of the control mice, the hippocampal DG area in the Sox11 cKO mice shrank and showed an irregular shape (Fig. 3C and D). More importantly, in P7 mutant mice, 80% of mossy fibers projected to the wrong target areas from the very beginning before the start of adult neurogenesis (Fig. 3E) [13]. At E18.5, the time for establishment of the primitive DG, abnormal distribution of NPCs (Fig. 3F) and defective neurogenesis (Fig. 3G) were also present at the primitive DG in the mutant brains. The dentate neuroepithelium at E12.5 appeared to be normal (Fig. S7D). However, the development of the primitive DG at E15.5 was obviously altered, given that the ventricle was enlarged and the primitive DG shrank (Fig. 3H and I). Although the total number of SOX2 + cells was not obviously different (Fig. 3J), the percentage of double-positive IPCs (SOX2 + and TBR2 +) among the total SOX2 + cells significantly decreased at the primitive DG (Fig. 3K) but not the SVZ region (Fig. S7E). In addition, the number of PROX1 + neurons significantly decreased (Fig. 3L). These results indicated that the defects in hippocampal development were obvious at E15.5 in SOX11-mutant brains.
Pathological defects in the SOX11-deficient hippocampus are initiated from abnormal progenitor polarity and defective differentiation in the E13.5–E15.5 embryonic brain. A Staining of SOX2 + NPCs in the postnatal dentate gyrus at P7. Scale bar, 100 μm. B Distribution of SOX2 + NPCs in the postnatal dentate gyrus in the P7 brain from the SOX11-mutant or control mice. GCL, SGZ and Hilus. n = 3 mice, *p < 0.01 (Student’s t test). Staining (C) and quantification (D) of PROX1 + granule cells at the DG in P7 brain from SOX11-mutant or control mice. Scale bar, 100 μm. E Mossy fiber connections decreased to the SL region but increased to the SP and SO regions in the SOX11-deficient hippocampal CA3 region at P7. Scale bar, 100 μm, Error bars, mean ± SEM, n = 3, *p < 0.05, (Student’s t test). Immunostaining of SOX2 + NPCs (F) and PROX1 + granule cells (G) at the primitive dentate gyrus in the E18.5 brain from the SOX11-mutant or control mice. Scale bar, 50 μm. H and I SOX11 deficiency leads to decreased primitive DG region and enlarged ventricles in the developing primitive dentate gyrus in the E15.5 mouse brain. Scale bar, 200 μm. Error bars, mean ± SEM, n = 3 mice, *p < 0.05, (Student’s t test). The number of SOX2 + cells did not show reduction (J), but the percentage of double-positive cells (SOX2 and TBR2) in total SOX2 + cells (K) was significantly decreased in the developing primitive dentate gurus in the E15.5 mutant hippocampus. Error bars, mean ± SEM, n = 3 mice, *p < 0.05, (Student’s t test). L The area of PROX1 + granule cells decreased significantly in the developing primitive dentate gyrus in the E15.5 mutant hippocampus. Scale bar, 200 μm. Error bars, mean ± SEM, n = 3 mice. *p < 0.05, (Student’s t test)
To address how SOX11 deficiency disrupts dentate epithelium, we performed transcriptional sequencing of the dentate neuroepithelium at E13.5, the time when the dentate neuroepithelium is ready to migrate along the dentate migratory stream for differentiation. To this end, we found that 409 genes (Fig. S7F) were increased in the mutant dentate epithelium, which was related to “the immune system process” and “positive regulation of cell death” (Fig. S7G). In contrast, 50 genes (Fig. S7F) decreased in SOX11-deficient dentate epithelium. We were particularly interested in the genes that belong to both “cell adhesion” and “neuroepithelial cell differentiation” categories (Fig. S7H). These genes included Cdh2, Dcx, and Wnt3a, which are all involved in hippocampal neurogenesis [46, 47]. Notably, the expression of SOX4, another highly related member of SOX gene family, was not obviously changed (Fig. S7I). Given the difficulty delivering these SOX11-downstream genes simultaneously to the fetal brains during the mothers’ pregnancy, we maternally injected P7C3-A20, an activator of neurogenesis [48], to examine whether it could improve pathological conditions in the SOX11-deficient hippocampus because we found that P7C3-A20 upregulated the expression of Sox11 and, most likely through the SOX11 transcriptional activity, enhanced the production of Cdh2, Dcx and Wnt3a in the hippocampus of Sox11cHET mice (Fig. S8A), but not in the Sox11cKO mice.
Intervention neurogenesis in the embryonic brain, but not the adult brain, improved pathological conditions in the hippocampus, alleviated PPI, and attenuated behavioral disorders only in Sox11 cHET mice
Hippocampal neurogenesis starts at E13.5 in the brain and persists into adulthood [49]. Although schizophrenia-related pathological alterations, such as disrupted mossy fibers, may occur in the neonatal brain (Fig. 3E), the onset of disease often takes place during adolescence [50]. Therefore, we induced neurogenesis through injection of P7C3-A20 to activate neurogenesisat in three different time windows: embryonic, adolescent, and adult.
As expected, in comparison with the nontreated Sox11cHET mice, the volume of the PROX1+ DG area (Fig. 4A and B) and total number of DCX+ cells (Fig. S8B and S8C) were significantly higher in animals treated with P7C3-A20 in the embryonic or adolescent period. Interestingly, although PPI was not improved (Fig. S8D), OPR behavior was enhanced in Sox11cHET mice treated during adolescence (Fig. 4D). Impressively, if treated in the embryonic period, PPI (Fig. 4C), OPR behaviors (Fig. 4D), and anxiety-related behaviors (Fig. 4E) significantly improved in Sox11cHET mice. Notably, if treated in adulthood, Sox11cHET mice did not show any positive change in any of these regards (Fig. 4A and D, and S8E). This was also true for Sox11cKO mice (Fig. 4C and D, and S8D–S8F).
Early embryonic neurogenesis treatments with P7C3-A20 improved deficit appearance in adult SOX11-deficient mice. A and B Analysis of PROX1 + granule cells in the Sox11 cHET mouse hippocampus treated with or without P7C3-A20 intervention in the adult (8–12 W), adolescence (P21–P49), or embryonic (E12.5-P0) stage (n = 4 mice; *p < 0.01, one-way ANOVA). Scale bar, 100 μm. Error bars, mean ± SEM. C PPI of SOX11-deficient mice treated with or without P7C3-A20 intervention during the embryonic stage (n = 13; *p < 0.01, one-way ANOVA). D Hippocampus-dependent memory test in the SOX11-deficient mice treated with P7C3-A20 intervention during the adult, adolescence or embryonic stage (n = 13; *p < 0.05, Student’s t test). E Elevated Plus maze test. Representative heat maps and quantification of the cumulative movement of the SOX11-deficient mouse after treatment with P7C3-A20 intervention during embryonic neurogenesis. Error bars, mean ± SEM, n = 13, *p < 0.05, (one-way ANOVA). F Quantification of mossy fiber connections to the hippocampal CA3-SL region in Sox11 cHET mouse mice after treatment with P7C3-A20 intervention during the adult, adolescence or embryonic stage. Error bars, mean ± SEM, n = 4 mice, *p < 0.05, (one-way ANOVA)
To examine whether these improvements in P7C3-A20-treated Sox11cHET mice were related to mossy fiber connections, we performed immunostaining. While abnormal mossy fiber projections in Sox11cHET mice did not change much after treatment in the adolescent or adult period (Fig. 4F), intervention in the embryonic brain significantly corrected abnormal mossy fiber connections, thereby reducing the wrong targets from 55% to close to 30% (Fig. 4F). Notably, mossy fiber connections have an average 20% of wrong targets in wild-type mice. Sox11cKO mice did not show any response to the treatment (Fig. S8G). Collectively, these data indicated that activation of neurogenesis in the embryonic brain could improve mossy fiber connection, attenuate PPI, and alleviate behavioral disorders in Sox11cHET mice.
Discussion
Starting with iPSCs-derived NPCs from patients with schizophrenia, we found that a high-risk allele at SNP rs1686406 directly downregulated SOX11 expression in the patients’ NPCs, and such NPCs exhibited abnormal polarity due to SOX11 deficiency. In the mouse brain, knockout SOX11 caused abnormal distribution of DG granule cells in the hippocampus. In turn, these disturbed granule cells formed disrupted mossy fiber connections to CA3 neurons from the very beginning, first observed in the P7 hippocampus. Moreover, abnormal mossy fiber connections were resistant to neural circuit remodeling. It is interesting to note that the chemical activation of neurogenesis in the embryonic brain, but not in the postnatal brain, significantly reduced the percentage of abnormal mossy fiber connections, alleviated PPI, and attenuated schizophrenia-like behaviors in Sox11cHET mice. Therefore, disrupted mossy fiber connections in the hippocampus were genetically determined and occurred before the onset of disordered behaviors in Sox11cHET mice. Therefore, abnormal mossy fiber connections may reflect pathological substrate in SOX11-associated schizophrenia.
In addition to the hippocampus, SOX11 is also expressed in the cerebral cortex. It has been reported that SOX11 may have a specific function in the generation of deep-layer neurons in the cerebral cortex [51]. Based on this Emx1-CRE-mediated knockout study, Chen and colleagues concluded that SOX11 deficiency led to an increase in SOX2 + NPCs at SVZ, but a significant reduction in CTIP2 + deep-layer neurons detected at E14.5. Controversially, Shim reported that, in the P0 neocortex, the number of CTIP2 + deep-layer neurons did not differ between Sox11cko mice and normal controls [34]. In our study, we analyzed dendritic complexity and neuronal distribution in the cortex and the hippocampus. We did not detect obvious differences in the cortex layers between the control and mutant mice in terms of neuronal distribution and dendritic complexity of pyramidal neurons (Fig. S5A–S5D). In contrast, in SOX11 mutant mice, neurons in the hippocampal DG and CA3 regions exhibited irregular distribution and significant reduction in complexity (Fig. 2H–J and S5F). These results are supported by the report that SOX11 expression in the hippocampal NPCs was significantly higher than that in the cortex NPCs [25] (Fig. S7J). More importantly, we found that SOX11 deficiency did not obviously affect cortical differentiation (Fig. S7E), whereas it significantly affected hippocampal development (Fig. 3G–K). Therefore, compared with the cortex, which appeared to be minutely affected, the hippocampus was severely altered in SOX11-deficient mice.
Most medicines currently used to treat schizophrenia target the dopamine system [52]. These drugs cannot prevent the onset of schizophrenia; they only temporarily attenuate the symptoms of the disease. The reason for the limited prevention drugs is the lack of understanding the molecular mechanism of the etiology of schizophrenia. In the present study, we showed that the SOX11 deficiency led to abnormal mossy fiber connections to the wrong target lamina in CA3, leading to abnormal electrophysiological activity in the DG–CA3 system. Interestingly, the activation of neurogenesis in the adult brain neither corrected abnormal mossy fiber connections nor improved PPI in the SOX11-mutant mice. In contrast, intervention neurogenesis in the embryonic brain significantly corrected abnormal mossy fiber connections (Fig. 4F) and improved schizophrenia-associated behaviors (Fig. 4C and E) in Sox11cHET mice. These data indicated that mossy fiber mis-pathfinding (Fig. 3E) occurred before the onset of behavioral disorders in the SOX11-deficient mice (Fig. S7K). Collectively, these findings suggest that abnormal mossy fiber connections are genetically determined and strongly correlated with abnormal behaviors in SOX11-deficient mice. This abnormality may reflect the pathological substrate of SOX11-associated schizophrenia. One important implication of this study is that an early intervention in abnormal embryonic neurogenesis might represent a viable path toward preventing disease onset in individuals who are genetically at risk.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request. The accession numbers for the RNA-seq data reported in this paper are GEO: GSE158777. To review GEO accession GSE158777: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE158777. Enter token <wrybgymedjopvmr> into the box.
References
McTeague LM et al (2020) Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry 177(5):411–421
Kolomeets NS, Orlovskaya DD, Uranova NA (2007) Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse 61(8):615–621
Goldsmith SK, Joyce JN (1995) Alterations in hippocampal mossy fiber pathway in schizophrenia and Alzheimer’s disease. Biol Psychiatry 37(2):122–126
Murray KD et al (2020) Age-related changes in synaptic plasticity associated with mossy fiber terminal integration during adult neurogenesis. eNeuro. https://doi.org/10.1523/ENEURO.0030-20.2020
Nakahara S et al (2018) Hippocampal pathophysiology: commonality shared by temporal lobe epilepsy and psychiatric disorders. Neurosci J 2018:4852359
Klein R (2010) Topography in hippocampal mossy fiber plasticity. Neuron 65(5):580–582
Urban N, Guillemot F (2014) Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 8:396
Berg DA et al (2019) A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell 177(3):654–668 e15
Iwano T et al (2012) Prox1 postmitotically defines dentate gyrus cells by specifying granule cell identity over CA3 pyramidal cell fate in the hippocampus. Development 139(16):3051–3062
Kerr AM, Jonas P (2008) The two sides of hippocampal mossy fiber plasticity. Neuron 57(1):5–7
Katzel D et al (2020) Hippocampal hyperactivity as a druggable circuit-level origin of aberrant salience in schizophrenia. Front Pharmacol 11:486811
Wilke SA et al (2012) NeuroD2 regulates the development of hippocampal mossy fiber synapses. Neural Dev 7:9
Tawarayama H et al (2018) The chemorepellent draxin is involved in hippocampal mossy fiber projection. Biochem Biophys Res Commun 500(2):217–223
Nakahara S et al (2015) Mossy fiber mis-pathfinding and semaphorin reduction in the hippocampus of alpha-CaMKII hKO mice. Neurosci Lett 598:47–51
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195(1):51–86
Ramirez-Amaya V et al (2001) Spatial long-term memory is related to mossy fiber synaptogenesis. J Neurosci 21(18):7340–7348
Wilke SA et al (2014) Specific disruption of hippocampal mossy fiber synapses in a mouse model of familial Alzheimer’s disease. PLoS ONE 9(1):e84349
Segev A et al (2020) Reduced GluN1 in mouse dentate gyrus is associated with CA3 hyperactivity and psychosis-like behaviors. Mol Psychiatry 25(11):2832–2843
Sun CP et al (2020) Association of SOX11 polymorphisms in distal 3ʹUTR with susceptibility for schizophrenia. J Clin Lab Anal 34(8):e23306
Schepers GE, Teasdale RD, Koopman P (2002) Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell 3(2):167–170
Mu L et al (2012) SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J Neurosci 32(9):3067–3080
Turan S et al (2019) A novel human stem cell model for Coffin-Siris syndrome-like syndrome reveals the importance of SOX11 dosage for neuronal differentiation and survival. Hum Mol Genet 28(15):2589–2599
Tsurusaki Y et al (2014) De novo SOX11 mutations cause Coffin–Siris syndrome. Nat Commun 5:4011
Di Nuovo SF et al (2004) Psychopathology and mental retardation: a study using the Rorschach Inkblot Test. Psychol Rep 94(3 Pt 2):1313–1321
Zhong S et al (2020) Decoding the development of the human hippocampus. Nature 577(7791):531–536
Rasanen N et al (2022) The iPSC perspective on schizophrenia. Trends Neurosci 45(1):8–26
Yu DX et al (2014) Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Reports 2(3):295–310
Zhang C et al (2021) Altered expression of Par3, aPKC-lambda, and Lgl1 in hippocampus in kainic acid-induced status epilepticus rat model. Front Neurol 12:780042
Okita K et al (2013) An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31(3):458–466
Elkabetz Y et al (2008) Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev 22(2):152–165
Yoon KJ et al (2014) Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 15(1):79–91
Arai Y, Taverna E (2017) Neural progenitor cell polarity and cortical development. Front Cell Neurosci 11:384
Wang Y et al (2013) Transcription factor Sox11 is essential for both embryonic and adult neurogenesis. Dev Dyn 242(6):638–653
Shim S et al (2012) Cis-regulatory control of corticospinal system development and evolution. Nature 486(7401):74–79
Ma DK et al (2009) Adult neural stem cells in the mammalian central nervous system. Cell Res 19(6):672–682
Sawangjit A et al (2018) The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature 564(7734):109–113
Nakazawa K et al (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297(5579):211–218
Kreutz MR, Sala C (2012) Postsynaptic molecular mechanisms. Preface Adv Exp Med Biol 970:v–vi
Hauser J et al (2005) Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psychiatry 10(2):201–207
Nakahara S, Matsumoto M, van Erp TGM (2018) Hippocampal subregion abnormalities in schizophrenia: a systematic review of structural and physiological imaging studies. Neuropsychopharmacol Rep 38(4):156–166
Swerdlow NR et al (2014) Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophr Res 152(2–3):503–512
Green MF et al (2018) Social disconnection in schizophrenia and the general community. Schizophr Bull 44(2):242–249
Hall J (2017) Schizophrenia—an anxiety disorder? Br J Psychiatry 211(5):262–263
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2):322–328
Ricceri L, Moles A, Crawley J (2007) Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res 176(1):40–52
Miyamoto Y, Sakane F, Hashimoto K (2015) N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adh Migr 9(3):183–192
Goncalves JT, Schafer ST, Gage FH (2016) Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167(4):897–914
Loris ZB, Pieper AA, Dietrich WD (2017) The neuroprotective compound P7C3-A20 promotes neurogenesis and improves cognitive function after ischemic stroke. Exp Neurol 290:63–73
Rolando C, Taylor V (2014) Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol 107:183–206
Larsen B, Luna B (2018) Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94:179–195
Chen C et al (2015) Orchestration of neuronal differentiation and progenitor pool expansion in the developing cortex by SoxC genes. J Neurosci 35(29):10629–10642
Miyamoto S et al (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10(1):79–104
Funding
We thank Byron Lee (MIT), Zhenge Luo (Shanghai Tech), Qiwu Xu and Wenyuan Wang (Institute of Neuroscience, CAS) for the critical comments and technical help. This work was supported by the National Natural Science Foundation of China (31721003, 81630035, 31820103009, 31701154). We thank LetPub (www.letpub.com) for its assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
XA, SG and LL conceived the study. XA and AW did most of the experiments with the help of HZ, RG and HS, JC and XA generated iPSCs. XA, AW, SG and LL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no competing interests.
Ethical statement and consent to participate
All animal protocols are in accordance with the University of Health Guide for the Care and Use of Laboratory Animals were approved by the Biological Research Ethics Committee of Tongji University. Protocols for Generation of human iPSC lines were previously approved by the Tongji University Institutional Review Board and informed consent was obtained from all subjects.
Consent for publication
All authors have been involved in writing the manuscript and consented to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abulaiti, X., Wang, A., Zhang, H. et al. Disrupted mossy fiber connections from defective embryonic neurogenesis contribute to SOX11-associated schizophrenia. Cell. Mol. Life Sci. 79, 180 (2022). https://doi.org/10.1007/s00018-022-04206-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04206-4